Abstract
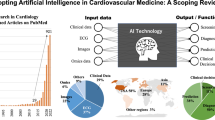
Artificial intelligence (AI) is transforming echocardiography, ushering in an era of improved diagnostic precision, efficiency and patient care. In this Review, we present an in-depth exploration of AI applications in echocardiography, highlighting the latest advances, practical implementations and future directions. We discuss the integration of AI throughout the echocardiographic workflow, from image acquisition and analysis to interpretation. We outline the potential of AI to automate routine measurements and calculations, enable task shifting, recognize disease-specific patterns and uncover new phenogroups that might surpass current diagnostic classifications. Moreover, we address the aspects needed to create trustworthy AI systems, through careful validation, navigating regulatory requirements and upholding ethical standards, thereby presenting a balanced perspective on the advantages and limitations of this rapidly evolving technology. Through an examination of current AI applications, clinical studies and technological breakthroughs, we offer a comprehensive understanding of the evolving role of AI in the future of echocardiography and its capacity to advance cardiovascular care, while also acknowledging the current limitations of the widespread clinical implementation of AI-supported echocardiography.
Key points
-
Echocardiography is a crucial tool in cardiology, but is time intensive, produces variable results and requires technical expertise, and current workforce shortages worldwide create delays in patient care.
-
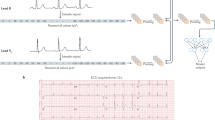
Artificial intelligence (AI) technologies offer transformative solutions for echocardiography, allowing automated image classification, segmentation and measurement.
-
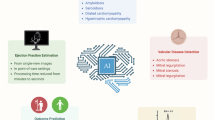
AI methods have shown potential in guiding image acquisition, automating routine echocardiographic measurements such as left ventricular function and detecting cardiovascular diseases such as cardiac amyloidosis, hypertrophic cardiomyopathy and valvular heart disease.
-
Beyond improving workflows and standardization in echocardiography laboratories, potential clinical applications include screening and monitoring for cardiovascular diseases, improving efficacy in clinical trials, task shifting and the expanded use of mobile devices.
-
AI-enhanced echocardiography is advancing rapidly and being integrated into clinical practice at leading centres, but further research is warranted to address its limitations.
-
Developers of AI-enhanced echocardiography should prioritize creating trustworthy applications that support the implementation of user-friendly AI to improve patient care.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1–39.e14 (2015).
Thorstensen, A., Dalen, H., Amundsen, B. H., Aase, S. A. & Stoylen, A. Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur. J. Echocardiogr. 11, 149–156 (2010).
Nagueh, S. F. et al. Interobserver variability in applying American Society of Echocardiography/European Association of Cardiovascular Imaging 2016 guidelines for estimation of left ventricular filling pressure. Circ. Cardiovasc. Imaging 12, e008122 (2019).
Won, D. et al. Sound the alarm: the sonographer shortage is echoing across healthcare. J. Ultrasound Med. 43, 1289–1301 (2024).
Hanneman, K. et al. Value creation through artificial intelligence and cardiovascular imaging: a scientific statement from the American Heart Association. Circulation 149, e296–e311 (2024).
Jordan, M. I. & Mitchell, T. M. Machine learning: trends, perspectives, and prospects. Science 349, 255–260 (2015).
Carneiro, G., Nascimento, J. C. & Freitas, A. The segmentation of the left ventricle of the heart from ultrasound data using deep learning architectures and derivative-based search methods. IEEE Trans. Image Process. 21, 968–982 (2012).
Abdi, A. H. et al. Automatic quality assessment of echocardiograms using convolutional neural networks: feasibility on the apical four-chamber view. IEEE Trans. Med. Imaging 36, 1221–1230 (2017).
Madani, A., Arnaout, R., Mofrad, M. & Arnaout, R. Fast and accurate view classification of echocardiograms using deep learning. npj Digit. Med. 1, 6 (2018).
Østvik, A., Smistad, E., Aase, S. A., Haugen, B. O. & Lovstakken, L. Real-time standard view classification in transthoracic echocardiography using convolutional neural networks. Ultrasound Med. Biol. 45, 374–384 (2019).
Zhang, J. et al. Fully automated echocardiogram interpretation in clinical practice. Circulation 138, 1623–1635 (2018).
Siontis, K. C., Noseworthy, P. A., Attia, Z. I. & Friedman, P. A. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat. Rev. Cardiol. 18, 465–478 (2021).
Tromp, J. et al. Automated interpretation of systolic and diastolic function on the echocardiogram: a multicohort study. Lancet Digit. Health 4, e46–e54 (2022).
Hirata, Y., Nomura, Y., Saijo, Y., Sata, M. & Kusunose, K. Reducing echocardiographic examination time through routine use of fully automated software: a comparative study of measurement and report creation time. J. Echocardiogr. 22, 162–170 (2024).
Olaisen, S. et al. Automatic measurements of left ventricular volumes and ejection fraction by artificial intelligence: clinical validation in real time and large databases. Eur. Heart J. Cardiovasc. Imaging 25, 383–395 (2024).
Krittanawong, C. et al. Deep learning for echocardiography: introduction for clinicians and future vision: state-of-the-art review. Life 13, 1029 (2023).
Ostvik, A. et al. Myocardial function imaging in echocardiography using deep learning. IEEE Trans. Med. Imaging 40, 1340–1351 (2021).
Tromp, J. et al. A formal validation of a deep learning-based automated workflow for the interpretation of the echocardiogram. Nat. Commun. 13, 6776 (2022).
Myhre, P. L. et al. External validation of a deep learning algorithm for automated echocardiographic strain measurements. Eur. Heart J. Digit. Health 5, 60–68 (2024).
Cai, Q. et al. Automated echocardiographic diastolic function grading: a hybrid multi-task deep learning and machine learning approach. Int. J. Cardiol. 416, 132504 (2024).
He, B. et al. Blinded, randomized trial of sonographer versus AI cardiac function assessment. Nature 616, 520–524 (2023).
Balagopalan, A. et al. Machine learning for healthcare that matters: reorienting from technical novelty to equitable impact. PLoS Digit. Health 3, e0000474 (2024).
Ouyang, D. et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature 580, 252–256 (2020).
Kusunose, K. et al. A deep learning approach for assessment of regional wall motion abnormality from echocardiographic images. JACC Cardiovasc. Imaging 13, 374–381 (2020).
Asch, F. M. et al. Automated echocardiographic quantification of left ventricular ejection fraction without volume measurements using a machine learning algorithm mimicking a human expert. Circ. Cardiovasc. Imaging 12, e009303 (2019).
Asch, F. M. et al. Deep learning-based automated echocardiographic quantification of left ventricular ejection fraction: a point-of-care solution. Circ. Cardiovasc. Imaging 14, e012293 (2021).
Myhre, P. L. et al. Concordance of left ventricular volumes and function measurements between two human readers, a fully automated AI algorithm, and the 3D heart model. Front. Cardiovasc. Med. 11, 1400333 (2024).
Lang, R. M. et al. Use of machine learning to improve echocardiographic image interpretation workflow: a disruptive paradigm change? J. Am. Soc. Echocardiogr. 34, 443–445 (2021).
Leclerc, S. et al. Deep learning for segmentation using an open large-scale dataset in 2D echocardiography. IEEE Trans. Med. Imaging 38, 2198–2210 (2019).
Smistad, E. et al. Real-time automatic ejection fraction and foreshortening detection using deep learning. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 67, 2595–2604 (2020).
Ghorbani, A. et al. Deep learning interpretation of echocardiograms. npj Digit. Med. 3, 10 (2020).
Alvén, J., Hagberg, E., Hagerman, D., Petersen, R. & Hjelmgren, O. A deep multi-stream model for robust prediction of left ventricular ejection fraction in 2D echocardiography. Sci. Rep. 14, 2104 (2024).
Salte, I. M. et al. Artificial intelligence for automatic measurement of left ventricular strain in echocardiography. JACC Cardiovasc. Imaging 14, 1918–1928 (2021).
Salte, I. M. et al. Deep learning for improved precision and reproducibility of left ventricular strain in echocardiography: a test–retest study. J. Am. Soc. Echocardiogr. 36, 788–799 (2023).
Nyberg, J. et al. Deep learning improves test-retest reproducibility of regional strain in echocardiography. Eur. Heart J. Imaging Methods Pract. 2, qyae092 (2024).
Stowell, C. C. et al. 2-dimensional echocardiographic global longitudinal strain with artificial intelligence using open data from a UK-wide collaborative. JACC Cardiovasc. Imaging 17, 865–876 (2024).
Kwan, A. C. et al. Deep learning-derived myocardial strain. JACC Cardiovasc. Imaging 17, 715–725 (2024).
Schneider, M. et al. A machine learning algorithm supports ultrasound-naïve novices in the acquisition of diagnostic echocardiography loops and provides accurate estimation of LVEF. Int. J. Cardiovasc. Imaging 37, 577–586 (2021).
Huang, W. et al. Point-of-care AI-enhanced novice echocardiography for screening heart failure (PANES-HF). Sci. Rep. 14, 13503 (2024).
Narang, A. et al. Utility of a deep-learning algorithm to guide novices to acquire echocardiograms for limited diagnostic use. JAMA Cardiol. 6, 624–632 (2021).
Berg, E. A. R. et al. Fully automatic estimation of global left ventricular systolic function using deep learning in transoesophageal echocardiography. Eur. Heart J. Imaging Methods Pract. 1, qyad007 (2023).
Yu, J. et al. Automatic assessment of left ventricular function for hemodynamic monitoring using artificial intelligence and transesophageal echocardiography. J. Clin. Monit. Comput. https://doi.org/10.1007/s10877-023-01118-x (2024).
Lau, E. S. et al. Deep learning-enabled assessment of left heart structure and function predicts cardiovascular outcomes. J. Am. Coll. Cardiol. 82, 1936–1948 (2023).
Howard, J. P. et al. Automated left ventricular dimension assessment using artificial intelligence developed and validated by a UK-wide collaborative. Circ.: Cardiovasc. Imaging 14, e011951 (2021).
Duffy, G. et al. High-throughput precision phenotyping of left ventricular hypertrophy with cardiovascular deep learning. JAMA Cardiol. 7, 386–395 (2022).
Gilbert, A. et al. User-intended doppler measurement type prediction combining CNNs with smart post-processing. IEEE J. Biomed. Health Inform. 25, 2113–2124 (2021).
Jeon, J. et al. A unified approach for comprehensive analysis of various spectral and tissue doppler echocardiography. in 2024 IEEE International Symposium on Biomedical Imaging (ISBI) https://doi.org/10.1109/ISBI56570.2024.10635387 (IEEE, 2024).
Jevsikov, J. et al. Automated mitral inflow Doppler peak velocity measurement using deep learning. Comput. Biol. Med. 171, 108192 (2024).
Chen, X. et al. Artificial intelligence-assisted left ventricular diastolic function assessment and grading: multiview versus single view. J. Am. Soc. Echocardiogr. 36, 1064–1078 (2023).
Park, J. et al. Artificial intelligence-enhanced automation of left ventricular diastolic assessment: a pilot study for feasibility, diagnostic validation, and outcome prediction. Cardiovasc. Diagn. Ther. 14, 352–366 (2024).
O’Neill, T., Kang, P., Hagendorff, A. & Tayal, B. The clinical applications of left atrial strain: a comprehensive review. Medicina 60, 693 (2024).
Ferkh, A., Clark, A. & Thomas, L. Left atrial phasic function: physiology, clinical assessment and prognostic value. Heart 109, 1661–1669 (2023).
Yaku, H., Komtebedde, J., Silvestry, F. E. & Shah, S. J. Deep learning-based automated measurements of echocardiographic estimators of invasive pulmonary capillary wedge pressure perform equally to core lab measurements: results from reduce LAP-HF II. J. Am. Coll. Cardiol. https://doi.org/10.1016/S0735-1097%2824%2902306-4 (2024).
Konstam, M. A. et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation 137, e578–e622 (2018).
Tokodi, M. et al. Deep learning-based prediction of right ventricular ejection fraction using 2D echocardiograms. JACC Cardiovasc. Imaging 16, 1005–1018 (2023).
Murayama, M. et al. Deep learning to assess right ventricular ejection fraction from two-dimensional echocardiograms in precapillary pulmonary hypertension. Echocardiography 41, e15812 (2024).
Chernyshov, A. et al. Automated segmentation and quantification of the right ventricle in 2-D echocardiography. Ultrasound Med. Biol. 50, 540–548 (2024).
Arbelo, E. et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur. Heart J. 44, 3503–3626 (2023).
Alwan, L. et al. Current and evolving multimodality cardiac imaging in managing transthyretin amyloid cardiomyopathy. JACC Cardiovasc. Imaging 17, 195–211 (2024).
Yu, X. et al. Using deep learning method to identify left ventricular hypertrophy on echocardiography. Int. J. Cardiovasc. Imaging 38, 759–769 (2022).
Kamel, M. A. et al. How artificial intelligence can enhance the diagnosis of cardiac amyloidosis: a review of recent advances and challenges. J. Cardiovasc. Dev. Dis. 11, 118 (2024).
Goto, S. et al. Artificial intelligence-enabled fully automated detection of cardiac amyloidosis using electrocardiograms and echocardiograms. Nat. Commun. 12, 2726 (2021).
Oikonomou, E. K. et al. Artificial intelligence-enabled electrocardiography and echocardiography to track preclinical progression of transthyretin amyloid cardiomyopathy. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehaf450 (2025).
Narula, S., Shameer, K., Salem Omar, A. M., Dudley, J. T. & Sengupta, P. P. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J. Am. Coll. Cardiol. 68, 2287–2295 (2016).
Krishna, H. et al. Fully automated artificial intelligence assessment of aortic stenosis by echocardiography. J. Am. Soc. Echocardiogr. 36, 769–777 (2023).
Holste, G., Oikonomou, E. K., Mortazavi, B. J., Wang, Z. & Khera, R. Efficient deep learning-based automated diagnosis from echocardiography with contrastive self-supervised learning. Commun. Med. 4, 1–10 (2024).
Holste, G. et al. Severe aortic stenosis detection by deep learning applied to echocardiography. Eur. Heart J. 44, 4592–4604 (2023).
Wessler, B. S. et al. Automated detection of aortic stenosis using machine learning. J. Am. Soc. Echocardiogr. 36, 411–420 (2023).
Long, A. et al. Deep learning for echo analysis, tracking, and evaluation of mitral regurgitation (DELINEATE-MR). Circulation https://doi.org/10.1161/CIRCULATIONAHA.124.068996 (2024).
Vrudhula, A. et al. High-throughput deep learning detection of mitral regurgitation. Circulation 150, 923–933 (2024).
Wifstad, S. V. et al. EasyPISA: automatic integrated PISA measurements of mitral regurgitation from 2-D color-doppler using deep learning. Ultrasound Med. Biol. https://doi.org/10.1016/j.ultrasmedbio.2024.06.008 (2024).
Sadeghpour, A. et al. An automated machine learning-based quantitative multiparametric approach for mitral regurgitation severity grading. JACC Cardiovasc. Imaging https://doi.org/10.1016/j.jcmg.2024.06.011 (2024).
Bernard, J. et al. Integrating echocardiography parameters with explainable artificial intelligence for data-driven clustering of primary mitral regurgitation phenotypes. JACC Cardiovasc. Imaging 16, 1253–1267 (2023).
Nedadur, R., Wang, B. & Tsang, W. Artificial intelligence for the echocardiographic assessment of valvular heart disease. Heart 108, 1592–1599 (2022).
Bozkurt, B. et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. https://doi.org/10.1016/j.cardfail.2021.01.022 (2021).
Akerman, A. P. et al. Automated echocardiographic detection of heart failure with preserved ejection fraction using artificial intelligence. JACC Adv. https://doi.org/10.1016/j.jacadv.2023.100452 (2023).
Cassianni, C. et al. Automated echocardiographic detection of heart failure with preserved ejection fraction using artificial intelligence is associated with cardiac mortality and heart failure hospitalization. J. Am. Soc. Echocardiogr. 37, 914–916 (2024).
Lin, X. et al. Echocardiography-based AI detection of regional wall motion abnormalities and quantification of cardiac function in myocardial infarction. Front. Cardiovasc. Med. 9, 903660 (2022).
Slivnick, J. A. et al. Echocardiographic detection of regional wall motion abnormalities using artificial intelligence compared to human readers. J. Am. Soc. Echocardiogr. 37, 655–663 (2024).
European Society of Cardiology. PROTEUS trial suggests AI for heart scans may benefit decision making for less-experienced clinicians (ESC, 2024); https://www.escardio.org/The-ESC/Press-Office/Press-releases/PROTEUS-Trial-suggests-AI-for-heart-scans-may-benefit-decision-making-for-less-experienced-clinicians.
Laumer, F. et al. Assessment of artificial intelligence in echocardiography diagnostics in differentiating Takotsubo syndrome from myocardial infarction. JAMA Cardiol. 7, 494–503 (2022).
Liao, Z. et al. Automatic echocardiographic evaluation of the probability of pulmonary hypertension using machine learning. Pulm. Circ. 13, e12272 (2023).
Hirata, Y., Tsuji, T., Kotoku, J., Sata, M. & Kusunose, K. Echocardiographic artificial intelligence for pulmonary hypertension classification. Heart 110, 586–593 (2024).
Mor-Avi, V. et al. Real-time artificial intelligence–based guidance of echocardiographic imaging by novices: image quality and suitability for diagnostic interpretation and quantitative analysis. Circ. Cardiovasc. Imaging 16, e015569 (2023).
Pasdeloup, D. et al. Real-time echocardiography guidance for optimized apical standard views. Ultrasound Med. Biol. 49, 333–346 (2023).
Sabo, S. et al. Real-time guidance by deep learning of experienced operators to improve the standardization of echocardiographic acquisitions. Eur. Heart J. Imaging Methods Pract. 1, qyad040 (2023).
Sabo, S. et al. Real-time guiding by deep learning during echocardiography to reduce left ventricular foreshortening and measurement variability. Eur. Heart J. Imaging Methods Pract. 1, qyad012 (2023).
Luong, C. et al. Automated estimation of echocardiogram image quality in hospitalized patients. Int. J. Cardiovasc. Imaging 37, 229–239 (2021).
Huang, K.-C. et al. Artificial intelligence aids cardiac image quality assessment for improving precision in strain measurements. JACC Cardiovasc. Imaging 14, 335–345 (2021).
Van De Vyver, G. et al. Regional image quality scoring for 2-D echocardiography using deep learning. Ultrasound Med. Biol. 51, 638–649 (2025).
Liu, Z. et al. Automated deep neural network-based identification, localization, and tracking of cardiac structures for ultrasound-guided interventional surgery. J. Thorac. Dis. 15, 2129–2140 (2023).
Baum, E. et al. Acquisition of cardiac point-of-care ultrasound images with deep learning: a randomized trial for educational outcomes with novices. CHEST Pulm. 1, 100023 (2023).
Li, K., Li, A., Xu, Y., Xiong, H. & Meng, M. Q.-H. RL-TEE: autonomous probe guidance for transesophageal echocardiography based on attention-augmented deep reinforcement learning. IEEE Trans. Autom. Sci. Eng. 21, 1526–1538 (2024).
Steffner, K. R. et al. Deep learning for transesophageal echocardiography view classification. Sci. Rep. 14, 11 (2024).
Kagiyama, N. Artificial intelligence-based automated echocardiographic measurements and the workflow of sonographers: randomized crossover trial (AI-Echo RCT) (American Heart Association, 2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05919342 (2023).
Motazedian, P. et al. Diagnostic accuracy of point-of-care ultrasound with artificial intelligence-assisted assessment of left ventricular ejection fraction. npj Digit. Med. 6, 201 (2023).
Kagiyama, N. et al. Multicenter validation study for automated left ventricular ejection fraction assessment using a handheld ultrasound with artificial intelligence. Sci. Rep. 14, 15359 (2024).
Oikonomou, E. K. et al. Artificial intelligence-guided detection of under-recognised cardiomyopathies on point-of-care cardiac ultrasonography: a multicentre study. Lancet Digit. Health 7, e113–e123 (2025).
Campell, R. OPERA - AI — reporting of handheld echocardiography in suspected heart failure (European Society of Cardiology Congress, 2023).
Tromp, J. et al. Nurse-led home-based detection of cardiac dysfunction by ultrasound: results of the CUMIN pilot study. Eur. Heart J. Digit. Health 5, 163–169 (2024).
Peck, D. et al. The use of artificial intelligence guidance for rheumatic heart disease screening by novices. J. Am. Soc. Echocardiogr. 36, 724–732 (2023).
Lyon, A. R. et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur. Heart J. Cardiovasc. Imaging 23, e333–e465 (2022).
Papadopoulou, S.-L. et al. Artificial intelligence-assisted evaluation of cardiac function by oncology staff in chemotherapy patients. Eur. Heart J. Digit. Health 5, 278–287 (2024).
Oikonomou, E. K. et al. A multimodal video-based AI biomarker for aortic stenosis development and progression. JAMA Cardiol. 9, 534–544 (2024).
Oo, M. M. et al. Artificial intelligence-assisted automated heart failure detection and classification from electronic health records. ESC Heart Fail. 11, 2769–2777 (2024).
Pandey, A. et al. Deep-learning models for the echocardiographic assessment of diastolic dysfunction. JACC Cardiovasc. Imaging 14, 1887–1900 (2021).
Sengupta, P. P. et al. A machine-learning framework to identify distinct phenotypes of aortic stenosis severity. JACC Cardiovasc. Imaging 14, 1707–1720 (2021).
Sánchez-Puente, A. et al. Machine learning to optimize the echocardiographic follow-up of aortic stenosis. JACC Cardiovasc. Imaging 16, 733–744 (2023).
Sengupta, P. P., Dey, D., Davies, R. H., Duchateau, N. & Yanamala, N. Challenges for augmenting intelligence in cardiac imaging. Lancet Digit. Health 6, e739–e748 (2024).
Díaz-Rodríguez, N. et al. Connecting the dots in trustworthy artificial intelligence: from AI principles, ethics, and key requirements to responsible AI systems and regulation. Inf. Fusion 99, 101896 (2023).
Lüscher, T. F., Wenzl, F. A., D’Ascenzo, F., Friedman, P. A. & Antoniades, C. Artificial intelligence in cardiovascular medicine: clinical applications. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehae465 (2024).
Khera, R. et al. Transforming cardiovascular care with artificial intelligence: from discovery to practice: JACC state-of-the-art review. J. Am. Coll. Cardiol. 84, 97–114 (2024).
Tseng, A. S., Lopez-Jimenez, F. & Pellikka, P. A. Future guidelines for artificial intelligence in echocardiography. J. Am. Soc. Echocardiogr. 35, 878–882 (2022).
Vrudhula, A., Kwan, A. C., Ouyang, D. & Cheng, S. Machine learning and bias in medical imaging: opportunities and challenges. Circ. Cardiovasc. Imaging 17, e015495 (2024).
Mihan, A., Pandey, A. & Spall, H. G. V. Mitigating the risk of artificial intelligence bias in cardiovascular care. Lancet Digit. Health 6, e749–e754 (2024).
Seyyed-Kalantari, L., Zhang, H., McDermott, M. B. A., Chen, I. Y. & Ghassemi, M. Underdiagnosis bias of artificial intelligence algorithms applied to chest radiographs in under-served patient populations. Nat. Med. 27, 2176–2182 (2021).
Duffy, G. et al. Confounders mediate AI prediction of demographics in medical imaging. npj Digit. Med. 5, 188 (2022).
Brown, A. et al. Detecting shortcut learning for fair medical AI using shortcut testing. Nat. Commun. 14, 4314 (2023).
Banerjee, I. et al. “Shortcuts” causing bias in radiology artificial intelligence: causes, evaluation, and mitigation. J. Am. Coll. Radiol. 20, 842–851 (2023).
Yang, Y., Zhang, H., Gichoya, J. W., Katabi, D. & Ghassemi, M. The limits of fair medical imaging AI in real-world generalization. Nat. Med. https://doi.org/10.1038/s41591-024-03113-4 (2024).
Dohare, S. et al. Loss of plasticity in deep continual learning. Nature 632, 768–774 (2024).
Gama, F. et al. Implementation frameworks for artificial intelligence translation into health care practice: scoping review. J. Med. Internet Res. 24, e32215 (2022).
Rajpurkar, P., Chen, E., Banerjee, O. & Topol, E. J. AI in health and medicine. Nat. Med. 28, 31–38 (2022).
European Parliament. Directorate general for parliamentary research services. artificial intelligence in healthcare: applications, risks, and ethical and societal impacts (2022); https://data.europa.eu/doi/10.2861/568473.
Khera, R., Simon, M. A. & Ross, J. S. Automation bias and assistive AI: risk of harm from AI-driven clinical decision support. JAMA 330, 2255–2257 (2023).
Wang, F. & Beecy, A. Implementing AI models in clinical workflows: a roadmap. BMJ Evid. Based Med. https://doi.org/10.1136/bmjebm-2023-112727 (2024).
Gill, S. K. et al. Artificial intelligence to enhance clinical value across the spectrum of cardiovascular healthcare. Eur. Heart J. 44, 713–725 (2023).
Christensen, M., Vukadinovic, M., Yuan, N. & Ouyang, D. Vision–language foundation model for echocardiogram interpretation. Nat. Med. 30, 1481–1488 (2024).
Barros-Gomes, S. et al. Characteristics and consequences of work-related musculoskeletal pain among cardiac sonographers compared with peer employees: a multisite cross-sectional study. J. Am. Soc. Echocardiogr. 32, 1138–1146 (2019).
Li, K. et al. Autonomous navigation of an ultrasound probe towards standard scan planes with deep reinforcement learning. in 2021 IEEE International Conference on Robotics and Automation (ICRA) https://doi.org/10.1109/ICRA48506.2021.9561295 (IEEE, 2021).
Soemantoro, R., Kardos, A., Tang, G. & Zhao, Y. An AI-powered navigation framework to achieve an automated acquisition of cardiac ultrasound images. Sci. Rep. 13, 15008 (2023).
Amezcua, K.-L. et al. Design and testing of ultrasound probe adapters for a robotic imaging platform. Sci. Rep. 14, 5102 (2024).
Villalobos Lizardi, J. C. et al. A guide for assessment of myocardial stiffness in health and disease. Nat. Cardiovasc. Res. 1, 8–22 (2022).
Caenen, A. et al. Ultrasound shear wave elastography in cardiology. JACC Cardiovasc. Imaging 17, 314–329 (2024).
Hathaway, Q. A. et al. Ultrasonic texture features for assessing cardiac remodeling and dysfunction. J. Am. Coll. Cardiol. 80, 2187–2201 (2022).
Sengupta, P. P. & Chandrashekhar, Y. Advancing myocardial tissue analysis using echocardiography. JACC Cardiovasc. Imaging 17, 228–231 (2024).
Jamthikar, A. D. et al. Cardiac ultrasonic tissue characterization in myocardial infarction based on deep transfer learning and radiomics features. Preprint at medRxiv https://doi.org/10.1101/2024.03.29.24305067 (2024).
Amado-Rey, A., GonçalvesSeabra, A. & Stieglitz, T. Towards ultrasound wearable technology for cardiovascular monitoring: from device development to clinical validation. IEEE Rev. Biomed. Eng. https://doi.org/10.1109/RBME.2024.3410399 (2024).
Moisello, E. et al. PMUT and CMUT devices for biomedical applications: a review. IEEE Access 12, 18640–18657 (2024).
Hu, H. et al. A wearable cardiac ultrasound imager. Nature 613, 667–675 (2023).
Democratizing echocardiography with AI. Us2.ai https://us2.ai/publications/ai-echo-white-paper/ (2023).
Turing, A. M. On computable numbers, with an application to the entscheidungsproblem. Proc. Lond. Math. Soc. s2-42, 230–265 (1937).
Hunter, D. J. & Holmes, C. Where medical statistics meets artificial intelligence. N. Engl. J. Med. 389, 1211–1219 (2023).
Haug, C. J. & Drazen, J. M. Artificial intelligence and machine learning in clinical medicine. N. Engl. J. Med. 388, 1201–1208 (2023).
Shehab, M. et al. Machine learning in medical applications: a review of state-of-the-art methods. Comput. Biol. Med. 145, 105458 (2022).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Sidey-Gibbons, J. A. M. & Sidey-Gibbons, C. J. Machine learning in medicine: a practical introduction. BMC Med. Res. Methodol. 19, 64 (2019).
Reading Turchioe, M. et al. Systematic review of current natural language processing methods and applications in cardiology. Heart 108, 909–916 (2022).
Will ChatGPT transform healthcare? Nat. Med. 29, 505–506 (2023).
Boonstra, M. J., Weissenbacher, D., Moore, J. H., Gonzalez-Hernandez, G. & Asselbergs, F. W. Artificial intelligence: revolutionizing cardiology with large language models. Eur. Heart J. 45, 332–345 (2024).
Amadou, A. A. et al. EchoApex: a general-purpose vision foundation model for echocardiography. Preprint at https://doi.org/10.48550/arXiv.2410.11092 (2024).
Zhang, Z., Wu, Q., Ding, S., Wang, X. & Ye, J. Echo-vision-FM: a pre-training and fine-tuning framework for echocardiogram videos vision foundation model. Preprint at medRxiv https://doi.org/10.1101/2024.10.09.24315195 (2024).
Collins, G. S., Reitsma, J. B., Altman, D. G. & Moons, K. G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. BMC Med. 13, 1 (2015).
Liu, X., Cruz Rivera, S., Moher, D., Calvert, M. J. & Denniston, A. K. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat. Med. 26, 1364–1374 (2020).
Sengupta, P. P. et al. Proposed requirements for cardiovascular imaging-related machine learning evaluation (PRIME): a checklist. JACC Cardiovasc. Imaging 13, 2017–2035 (2020).
Lafitte, S. et al. Integrating artificial intelligence into an echocardiography department: feasibility and comparative study of automated versus human measurements in a high-volume clinical setting. Arch. Cardiovasc. Dis. https://doi.org/10.1016/j.acvd.2025.04.051 (2025).
Acknowledgements
We would like to honour the memory of R.M. Lang, an extraordinary leader whose pioneering contributions to echocardiography have left an indelible mark on the field and inspired researchers and clinicians throughout his remarkable career. His passion for education and commitment to advancing medical knowledge will be profoundly missed, but his legacy will continue to inspire future generations of cardiologists and sonographers. We express our sincere gratitude to A. Østvik, L. Løvstakken, E. Smistad and H. Dalen (all from the Norwegian University of Science and Technology, Trondheim, Norway) for their invaluable contributions to the technical aspects related to AI technologies in this article.
Author information
Authors and Affiliations
Contributions
P.L.M., B.G. and C.S.P.L. researched data for the article. All authors contributed substantially to discussion of content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.L.M. has received research grants from AstraZeneca and consulting fees from Amarin, AmGen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Novartis, Novo Nordisk, Orion Pharma, Pharmacosmos, Vifor and Us2.ai. B.G. has received consultant or speaker fees from Bayer, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Novartis and Pfizer, and holds a position at the Centre for Innovative Ultrasound Solutions (CIUS), a Norwegian Research Council centre for research-based innovation, in which GE HealthCare is a consortium partner. F.M.A. reports institutional research contracts with Abbott, Ancora Heart, Boston Scientific, Croivalve, Edwards Lifesciences, egnite, GE HealthCare, InnovHeart, InterShunt, LAMINAR, Medtronic, Neovasc, TOMTEC, Tricares, Ultromics, Us2.ai, VDyne and Xeltis. V.D. received speaker fees from Abbott Structural, Edwards Lifesciences, GE HealthCare, JenaValve, Medtronic, Philips, Siemens and Products&Features, and received consulting fees from Edwards Lifesciences, MSD and Novo Nordisk. R.K. is an associate editor of JAMA; receives support from the National Heart, Lung, and Blood Institute of the National Institutes of Health (awards R01HL167858, R01AG089981 and K23HL153775), the Doris Duke Charitable Foundation (award 2022060) and the Blavatnik Family Foundation; receives research support through Yale University from BridgeBio, Bristol Myers Squibb and Novo Nordisk; is a coinventor of US pending patent applications WO2023230345A1, US20220336048A1, 63/346,610, 63/484,426, 63/508,315, 63/580,137, 63/606,203, 63/619,241, 63/562,335 and 18/813,882; and is a co-founder of Ensight-AI and Evidence2Health, which are health platforms to improve cardiovascular diagnosis and evidence-based cardiovascular care. P.A.P. is editor-in-chief of the Journal of the American Society of Echocardiography; is supported as the Betty Knight Scripps–George M. Gura, Jr., MD Professor of Cardiovascular Diseases Clinical Research at Mayo Clinic; and receives research support from the National Heart, Lung, and Blood Institute of the National Institutes of Health and through Mayo Clinic from Edwards Lifesciences and Ultromics. P.P.S. has received grants from the National Heart, Lung, and Blood Institute (award 1R01HL173998-01A1) and National Science Foundation (award 2125872). He has served on the Advisory Board of HeartSciences and RCE Technologies and holds stock options; has received grants or contracts from Butterfly, HeartSciences, MindMics and RCE Technologies; and holds patents with Mayo Clinic (US8328724B2), HeartSciences (US11445918B2) and Rutgers Health (62/864,771, US202163152686P, WO2022182603A1, US202163211829P, WO2022266288A1 and US202163212228P). S.V. reports grants and/or contracts from Abbott Vascular, the American College of Cardiology, the National Heart Lung and Blood Institute (R01HL168940-01A1, R01HL141213-03, U24HL165029-02 and U24HL171356-01A1), Boston Scientific, Cytokinetics, Edwards Lifesciences and the Food and Drug Administration, and has served as an advisory board member, consultant or speaker for Abbott Vascular, the American College of Physicians, AstraZeneca, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, HeartFlow, JenaValve, Ikon, Medtronic, Medscape and Total CME. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from Novo Nordisk and Roche Diagnostics; has served as consultant or on the advisory board, steering committee or executive committee for Alnylam Pharma, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Biopeutics, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Corteria, CPC Clinical Research, Eli Lilly, Impulse Dynamics, Intellia Therapeutics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Quidel Corporation, Radcliffe Group, Roche and Us2.ai; and serves as co-founder and non-executive director of Us2.ai (US patent no. 10,702, 247). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Andrea Barbieri, Kenya Kusunose and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Myhre, P.L., Grenne, B., Asch, F.M. et al. Artificial intelligence-enhanced echocardiography in cardiovascular disease management. Nat Rev Cardiol (2025). https://doi.org/10.1038/s41569-025-01197-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s41569-025-01197-0