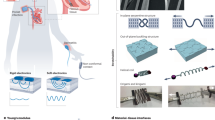
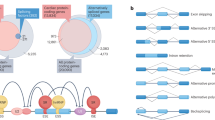
Abstract
The miniaturization of implantable sensors and actuators, combined with advances in interactive modelling and high-resolution imaging, is propelling the use of medical devices for counteracting impaired neural control of the cardiovascular system. In this Review, we discuss the current effectiveness of this technology for modulating autonomic activity in numerous cardiovascular conditions, including high blood pressure, heart failure and cardiac arrhythmias. We advocate for smarter closed-loop bionic devices fitted with feedback from multiple sensors to allow adaptive, state-dependent control, and discuss how the adoption of artificial intelligence technology would facilitate auto-personalization to meet the needs of patients. We also describe how transcriptomics of autonomic circuits can guide device-based approaches. Finally, the use of stem cell therapies to target sympathetic circuits more precisely will help to optimize the therapeutic effects of autonomic modulation for the treatment of arrhythmia. For bioelectronic medicine to achieve clinical utility in neurocardiology, these innovations must demonstrate improved efficacy beyond that offered by contemporary interventions.
Key points
-
Emerging evidence suggests that bioelectronic strategies that involve the site-specific targeting of the autonomic circuit could be used to treat cardiovascular diseases, including arrhythmia, heart failure and neurogenic hypertension.
-
Advances in implantable sensor technology and device miniaturization, together with the design of closed-loop bioelectronics linked to multi-feedback sensors, should contribute to the development of therapies to modulate autonomic nervous system activity.
-
Combining artificial intelligence and machine learning technologies with novel neuroceutical devices could result in optimized and personalized parameter set points that respond to physiological feedback within a closed-loop system, thereby enabling dynamic state-dependent adjustment.
-
Advances in Bluetooth technology might facilitate real-time device readout, effectiveness and feedback dosing of neuroceutical devices.
-
The use of transcriptomics to understand whether visceral reflex pathways are associated with distinct phenotypes might enable highly selective functional neuromodulation in device-based medicine.
-
The autografting of novel biomaterials into the autonomic nervous system or to the end organ, such as the heart, to alter excitability with closed-loop bioelectronics is promising for the treatment of arrhythmias.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Zhu, C. et al. High-resolution structure-function mapping of intact hearts reveals altered sympathetic control of infarct border zones. JCI Insight 7, e153913 (2022).
Zhou, L. et al. Wireless self-powered optogenetic system for long-term cardiac neuromodulation to improve post-MI cardiac remodeling and malignant arrhythmia. Adv. Sci. 10, e2205551 (2023).
Sharma, K. et al. Long-term follow-up of patients with heart failure and reduced ejection fraction receiving autonomic regulation therapy in the ANTHEM-HF pilot study. Int. J. Cardiol. 323, 175–178 (2021).
Schwartz, P. J. & Ackerman, M. J. Cardiac sympathetic denervation in the prevention of genetically mediated life-threatening ventricular arrhythmias. Eur. Heart J. 43, 2096–2102 (2022).
La Rovere, M. T., Porta, A. & Schwartz, P. J. Autonomic control of the heart and its clinical impact. a personal perspective. Front. Physiol. 11, 582 (2020).
Horn, C. C., Ardell, J. L. & Fisher, L. E. Electroceutical targeting of the autonomic nervous system. Physiology 34, 150–162 (2019).
Herring, N., Kalla, M. & Paterson, D. J. The autonomic nervous system and cardiac arrhythmias: current concepts and emerging therapies. Nat. Rev. Cardiol. 16, 707–726 (2019).
Hanna, P. et al. Neuroscientific therapies for atrial fibrillation. Cardiovasc. Res. 117, 1732–1745 (2021).
Hadaya, J. & Ardell, J. L. Autonomic modulation for cardiovascular disease. Front. Physiol. 11, 617459 (2020).
Tonko, J. B. & Lambiase, P. D. The proarrhythmogenic role of autonomics and emerging neuromodulation approaches to prevent sudden death in cardiac ion channelopathies. Cardiovasc. Res. 120, 114–131 (2024).
Stimulating Peripheral Activity to Relieve Conditions (SPARC). National Institutes of Health, and Office of Strategic Coordination-The Common Fund https://commonfund.nih.gov/sparc (2025).
University of Minnesota awarded $21M to lead research revealing effects of vagus nerve stimulation in humans. University of Minnesota https://twin-cities.umn.edu/news-events/university-minnesota-awarded-21m-lead-research-revealing-effects-vagus-nerve (2022).
Paterson, D. J. & Shivkumar, K. Bioelectronics for neurocardiology: diagnosis and therapeutics. Eur. Heart J. 44, 4822–4825 (2023).
Habecker, B. A. et al. Molecular and cellular neurocardiology in heart disease. J. Physiol. 603, 1689–1728 (2025).
Herring, N. et al. Neurocardiology: translational advancements and potential. J. Physiol. 603, 1729–1779 (2025).
Mori, S. & Shivkumar, K. Atlas of Cardiac Anatomy (Cardiotext, 2022).
Osanlouy, M. et al. The SPARC DRC: building a resource for the autonomic nervous system community. Front. Physiol. 12, 693735 (2021).
Zera, T., Moraes, D. J. A., da Silva, M. P., Fisher, J. P. & Paton, J. F. R. The logic of carotid body connectivity to the brain. Physiology 34, 264–282 (2019).
Moss, A. et al. A single cell transcriptomics map of paracrine networks in the intrinsic cardiac nervous system. iScience 24, 102713 (2021).
Gorky, J., Moss, A., Balycheva, M., Vadigepalli, R. & Schwaber, J. S. Input–output signal processing plasticity of vagal motor neurons in response to cardiac ischemic injury. iScience 24, 102143 (2021).
Gee, M. M. et al. Unpacking the multimodal, multi-scale data of the fast and slow lanes of the cardiac vagus through computational modelling. Exp. Physiol. 109, 1994–2000 (2023).
Ran, C., Boettcher, J. C., Kaye, J. A., Gallori, C. E. & Liberles, S. D. A brainstem map for visceral sensations. Nature 609, 320–326 (2022).
Bin, N. R. et al. An airway-to-brain sensory pathway mediates influenza-induced sickness. Nature 615, 660–667 (2023).
Dvoryanchikov, G. et al. Transcriptomes and neurotransmitter profiles of classes of gustatory and somatosensory neurons in the geniculate ganglion. Nat. Commun. 8, 760 (2017).
Kupari, J., Häring, M., Agirre, E., Castelo-Branco, G. & Ernfors, P. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 27, 2508–2523 (2019).
Prescott, S. L., Umans, B. D., Williams, E. K., Brust, R. D. & Liberles, S. D. An airway protection program revealed by sweeping genetic control of vagal afferents. Cell 181, 574–589.e514 (2020).
Zheng, Y. et al. Deep sequencing of somatosensory neurons reveals molecular determinants of intrinsic physiological properties. Neuron 103, 598–616.e597 (2019).
Mazzone, S. B. et al. Transcriptional profiling of individual airway projecting vagal sensory neurons. Mol. Neurobiol. 57, 949–963 (2020).
Brierley, D. I. & de Lartigue, G. Reappraising the role of the vagus nerve in GLP-1-mediated regulation of eating. Br. J. Pharmacol. 179, 584–599 (2022).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143.e1123 (2019).
Moss, A., Kuttippurathu, L., Srivastava, A., Schwaber, J. S. & Vadigepalli, R. Dynamic dysregulation of transcriptomic networks in brainstem autonomic nuclei during hypertension development in the female spontaneously hypertensive rat. Physiol. Genom. 56, 283–300 (2024).
Ngo, H. B. et al. A chemogenetic tool that enables functional neural circuit analysis. Cell Rep. 32, 108139 (2020).
Zhang, Y. et al. A microscale soft ionic power source modulates neuronal network activity. Nature 620, 1001–1006 (2023).
Liu, J. et al. Enzyme-enabled droplet biobattery for powering synthetic tissues. Angew. Chem. Int. Ed. Engl. 63, e202408665 (2024).
Wu, H. F., Hamilton, C., Porritt, H., Winbo, A. & Zeltner, N. Modelling neurocardiac physiology and diseases using human pluripotent stem cells: current progress and future prospects. J. Physiol. 603, 1865–1885 (2024).
Li, N. et al. Human induced pluripotent stem cell-derived cardiac myocytes and sympathetic neurons in disease modelling. Phil. Trans. R. Soc. Lond. B 378, 20220173 (2023).
Paton, J. F., Boscan, P., Pickering, A. E. & Nalivaiko, E. The yin and yang of cardiac autonomic control: vago-sympathetic interactions revisited. Brain Res. Brain Res. Rev. 49, 555–565 (2005).
Fisher, J. P., Zera, T. & Paton, J. F. R. Respiratory-cardiovascular interactions. Handb. Clin. Neurol. 188, 279–308 (2022).
Bardsley, E. N. & Paterson, D. J. Neurocardiac regulation: from cardiac mechanisms to novel therapeutic approaches. J. Physiol. 598, 2957–2976 (2020).
Guyenet, P. G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 7, 335–346 (2006).
Moore, J. P. Interoceptive signals from the heart and coronary circulation in health and disease. Auton. Neurosci. 253, 103180 (2024).
Floras, J. S. & Ponikowski, P. The sympathetic/parasympathetic imbalance in heart failure with reduced ejection fraction. Eur. Heart J. 36, 1974–1982b (2015).
Felippe, I. S. A., Río, R. D., Schultz, H., Machado, B. H. & Paton, J. F. R. Commonalities and differences in carotid body dysfunction in hypertension and heart failure. J. Physiol. 601, 5527–5551 (2023).
Danson, E. J., Li, D., Wang, L., Dawson, T. A. & Paterson, D. J. Targeting cardiac sympatho-vagal imbalance using gene transfer of nitric oxide synthase. J. Mol. Cell Cardiol. 46, 482–489 (2009).
Zucker, I. H., Xiao, L. & Haack, K. K. The central renin-angiotensin system and sympathetic nerve activity in chronic heart failure. Clin. Sci. 126, 695–706 (2014).
Schwartz, P. J. Cardiac sympathetic denervation to prevent life-threatening arrhythmias. Nat. Rev. Cardiol. 11, 346–353 (2014).
Savastano, S. & Schwartz, P. J. Blocking nerves and saving lives: left stellate ganglion block for electrical storms. HeartRhythm 20, 1039–1047 (2023).
Sridharan, A., Bradfield, J. S., Shivkumar, K. & Ajijola, O. A. Autonomic nervous system and arrhythmias in structural heart disease. Auton. Neurosci. 243, 103037 (2022).
La Rovere, M. T. et al. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation 103, 2072–2077 (2001).
Tse, R. et al. Sudden cardiac deaths have higher proportion of left stellate ganglionitis. Forensic Sci. Med. Pathol. 18, 156–164 (2022).
Tobert, K. E., Bos, J. M., Moir, C., Polites, S. F. & Ackerman, M. J. Bilateral cardiac sympathetic denervation in patients with congenital long QT syndrome. HeartRhythm 20, 1033–1038 (2023).
Qassim, H. et al. Deep brain stimulation for chronic facial pain: an individual participant data (IPD) meta-analysis. Brain Sci. 13, 492 (2023).
Owen, S. L. F., Green, A. L., Stein, J. F. & Aziz, T. Z. Deep brain stimulation for the alleviation of post-stroke neuropathic pain. Pain 120, 202–206 (2006).
Bittar, R. G. et al. Deep brain stimulation for pain relief: a meta-analysis. J. Clin. Neurosci. 12, 515–519 (2005).
Singleton, W. G. B., Ashida, R. & Patel, N. K. Deep brain stimulation for facial pain. Prog. Neurol. Surg. 35, 141–161 (2020).
Moran, C. H. et al. Clinical outcome of “asleep” deep brain stimulation for Parkinson disease using robot-assisted delivery and anatomic targeting of the subthalamic nucleus: a series of 152 patients. Neurosurgery 88, 165–173 (2020).
Little, S. et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 74, 449–457 (2013).
Sagalow, E. S., Ananth, A., Alapati, R., Fares, E. & Fast, Z. Transvenous phrenic nerve stimulation for central sleep apnea. Am. J. Cardiol. 180, 155–162 (2022).
Angeli, T. R. et al. Acute slow wave responses to high-frequency gastric electrical stimulation in patients with gastroparesis defined by high-resolution mapping. Neuromodulation 19, 864–871 (2016).
Mastoris, I. et al. Emerging implantable-device technology for patients at the intersection of electrophysiology and heart failure interdisciplinary care. J. Card. Fail. 28, 991–1015 (2022).
Bekfani, T. et al. A current and future outlook on upcoming technologies in remote monitoring of patients with heart failure. Eur. J. Heart Fail. 23, 175–185 (2021).
Pereira, E. A. et al. Sustained reduction of hypertension by deep brain stimulation. J. Clin. Neurosci. 17, 124–127 (2010).
Heusser, K. et al. Acute response to unilateral unipolar electrical carotid sinus stimulation in patients with resistant arterial hypertension. Hypertension 67, 585–591 (2016).
McMurray, J. J. & Pfeffer, M. A. Heart failure. Lancet 365, 1877–1889 (2005).
McKee, P. A., Castelli, W. P., McNamara, P. M. & Kannel, W. B. The natural history of congestive heart failure: the Framingham study. N. Engl. J. Med. 285, 1441–1446 (1971).
Lloyd-Jones, D. M. et al. Lifetime risk for developing congestive heart failure: the Framingham heart study. Circulation 106, 3068–3072 (2002).
Mosterd, A. & Hoes, A. W. Clinical epidemiology of heart failure. Heart 93, 1137–1146 (2007).
Ziaeian, B. & Fonarow, G. C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 13, 368–378 (2016).
GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet 403, 2162–2203 (2024).
Fuchs, F. D. & Whelton, P. K. High blood pressure and cardiovascular disease. Hypertension 75, 285–292 (2020).
Zhou, B., Perel, P., Mensah, G. A. & Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 18, 785–802 (2021).
Kario, K., Okura, A., Hoshide, S. & Mogi, M. The WHO Global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens. Res. 47, 1099–1102 (2024).
Lawlor, D. A. et al. Survival with treated and well-controlled blood pressure: findings from a prospective cohort study. PLoS ONE 6, e17792 (2011).
Almgren, T., Persson, B., Wilhelmsen, L., Rosengren, A. & Andersson, O. K. Stroke and coronary heart disease in treated hypertension — a prospective cohort study over three decades. J. Intern. Med. 257, 496–502 (2005).
Brown, R. E., Riddell, M. C., Macpherson, A. K., Canning, K. L. & Kuk, J. L. The joint association of physical activity, blood-pressure control, and pharmacologic treatment of hypertension for all-cause mortality risk. Am. J. Hypertens. 26, 1005–1010 (2013).
Voora, R. & Hinderliter, A. L. Modulation of sympathetic overactivity to treat resistant hypertension. Curr. Hypertens. Rep. 20, 92 (2018).
Grassi, G. Sympathomodulatory effects of antihypertensive drug treatment. Am. J. Hypertens. 29, 665–675 (2016).
Sakata, K., Shirotani, M., Yoshida, H. & Kurata, C. Comparison of effects of enalapril and nitrendipine on cardiac sympathetic nervous system in essential hypertension. J. Am. Coll. Cardiol. 32, 438–443 (1998).
Ohbayashi, Y. et al. Effect of an angiotensin II type 1 receptor blocker, valsartan, on neurohumoral factors in patients with hypertension: comparison with a long-acting calcium channel antagonist, amlodipine. J. Cardiovasc. Pharmacol. 42, S71–S74 (2003).
Bardsley, E. N., Davis, H., Buckler, K. J. & Paterson, D. J. Neurotransmitter switching coupled to β-adrenergic signaling in sympathetic neurons in prehypertensive states. Hypertension 71, 1226–1238 (2018).
Mancia, G. et al. Individualized beta-blocker treatment for high blood pressure dictated by medical comorbidities: indications beyond the 2018 European Society of Cardiology/European Society Of Hypertension guidelines. Hypertension 79, 1153–1166 (2022).
Grassi, G. et al. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J. Hypertens. 21, 1761–1769 (2003).
Neumann, J. et al. Sympathetic hyperactivity in hypertensive chronic kidney disease patients is reduced during standard treatment. Hypertension 49, 506–510 (2007).
Grassi, G. Sympathetic modulation as a goal of antihypertensive treatment: from drugs to devices. J. Hypertens. 41, 1688–1695 (2023).
Quarti-Trevano, F. et al. Failure of antihypertensive treatment to restore normal sympathetic activity. Hypertension 82, 1024–1034 (2025).
Kario, K. et al. Additional impact of morning haemostatic risk factors and morning blood pressure surge on stroke risk in older Japanese hypertensive patients. Eur. Heart J. 32, 574–580 (2011).
Malan, L. et al. Facilitated defensive coping, silent ischaemia and ECG left-ventricular hypertrophy: the SABPA study. J. Hypertens. 30, 543–550 (2012).
Renna, N. F. et al. Morning blood pressure surge as a predictor of cardiovascular events in patients with hypertension. Blood Press. Monit. 28, 149–157 (2023).
van den Born, B. H. et al. ESC Council on Hypertension position document on the management of hypertensive emergencies. Eur. Heart J. Cardiovasc. Pharmacother. 5, 37–46 (2019).
Webb, A. J. & Rothwell, P. M. The effect of antihypertensive treatment on headache and blood pressure variability in randomized controlled trials: a systematic review. J. Neurol. 259, 1781–1787 (2012).
Chant, B. et al. Antihypertensive treatment fails to control blood pressure during exercise. Hypertension 72, 102–109 (2018).
Sica, D. A. Centrally acting antihypertensive agents: an update. J. Clin. Hypertens. 9, 399–405 (2007).
Vongpatanasin, W., Kario, K., Atlas, S. A. & Victor, R. G. Central sympatholytic drugs. J. Clin. Hypertens. 13, 658–661 (2011).
Shahim, B., Kapelios, C. J., Savarese, G. & Lund, L. H. Global public health burden of heart failure: an updated review. Card. Fail. Rev. 9, e11 (2023).
Taylor, C. J. et al. Survival following a diagnosis of heart failure in primary care. Fam. Pract. 34, 161–168 (2017).
McDonagh, T. A. et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 44, 3627–3639 (2023).
Heidenreich, P. A. et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College Of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 145, e895–e1032 (2022).
Jasinska-Piadlo, A. & Campbell, P. Management of patients with heart failure and preserved ejection fraction. Heart 109, 874–883 (2023).
Anker, S. D. et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 385, 1451–1461 (2021).
Solomon, S. D. et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 387, 1089–1098 (2022).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Floras, J. S. The 2021 Carl Ludwig lecture. unsympathetic autonomic regulation in heart failure: patient-inspired insights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 321, R338–r351 (2021).
Kalla, M. et al. The cardiac sympathetic co-transmitter neuropeptide Y is pro-arrhythmic following ST-elevation myocardial infarction despite beta-blockade. Eur. Heart J. 41, 2168–2179 (2020).
Schwartz, P. J. The rationale and the role of left stellectomy for the prevention of malignant arrhythmias. Ann. NY Acad. Sci. 427, 199–221 (1984).
Elliott, I. A. et al. Minimally invasive bilateral stellate ganglionectomy for refractory ventricular tachycardia. Ann. Thor. Surg. 111, e295–e296 (2021).
Dusi, V., De Ferrari, G. M., Pugliese, L. & Schwartz, P. J. Cardiac sympathetic denervation in channelopathies. Front. Cardiovasc. Med. 6, 27 (2019).
Vrabec, T. et al. Bioelectronic block of stellate ganglia mitigates pacing-induced heterogeneous release of catecholamine and neuropeptide Y in the infarcted pig heart. J. Physiol. 603, 2071–2088 (2024).
Muir, J., Anguiano, M. & Kim, C. K. Neuromodulator and neuropeptide sensors and probes for precise circuit interrogation in vivo. Science 385, eadn6671 (2024).
Nelson, A. J., Pagidipati, N. J. & Bosworth, H. B. Improving medication adherence in cardiovascular disease. Nat. Rev. Cardiol. 21, 417–429 (2024).
Martinez-Sanchez, N. et al. The sympathetic nervous system in the 21st century: neuroimmune interactions in metabolic homeostasis and obesity. Neuron 110, 3597–3626 (2022).
Zhu, Y. et al. Sympathetic neuropeptide Y protects from obesity by sustaining thermogenic fat. Nature 634, 243–250 (2024).
Vanoli, E. et al. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ. Res. 68, 1471–1481 (1991).
Cole, C. R., Blackstone, E. H., Pashkow, F. J., Snader, C. E. & Lauer, M. S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 341, 1351–1357 (1999).
Hernesniemi, J. A. et al. Cardiorespiratory fitness and heart rate recovery predict sudden cardiac death independent of ejection fraction. Heart 106, 434–440 (2020).
De Ferrari, G. M. et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur. Heart J. 32, 847–855 (2011).
Hilz, M. J. Transcutaneous vagus nerve stimulation — a brief introduction and overview. Auton. Neurosci. 243, 103038 (2022).
Schwartz, P. J. et al. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur. J. Heart Fail. 10, 884–891 (2008).
Premchand, R. K. et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J. Card. Fail. 20, 808–816 (2014).
Premchand, R. K. et al. Extended follow-up of patients with heart failure receiving autonomic regulation therapy in the ANTHEM-HF study. J. Card. Fail. 22, 639–642 (2016).
Gold, M. R. et al. Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF trial. J. Am. Coll. Cardiol. 68, 149–158 (2016).
Zannad, F. et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural cardiac TherApy foR heart failure (NECTAR-HF) randomized controlled trial. Eur. Heart J. 36, 425–433 (2015).
Konstam, M. et al. Autonomic regulation therapy to improve symptoms and clinical outcomes in patients with heart failure and reduced ejection fraction (ANTHEM-HFrEF) pivotal study results. J. Card. Fail. 30, 313 (2024).
Kronsteiner, B. et al. Characterization, number, and spatial organization of nerve fibers in the human cervical vagus nerve and its superior cardiac branch. Brain Stimul. 17, 510–524 (2024).
Lewis, M. E. et al. Vagus nerve stimulation decreases left ventricular contractility in vivo in the human and pig heart. J. Physiol. 534, 547–552 (2001).
Muppidi, S., Gupta, P. K. & Vernino, S. Reversible right vagal neuropathy. Neurology 77, 1577–1579 (2011).
O’Callaghan, E. L. et al. Utility of a novel biofeedback device for within-breath modulation of heart rate in rats: a quantitative comparison of vagus nerve vs. right atrial pacing. Front. Physiol. 7, 27 (2016).
Nogaret, A. et al. Silicon central pattern generators for cardiac diseases. J. Physiol. 593, 763–774 (2015).
De Ferrari, G. M. & Schwartz, P. J. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail. Rev. 16, 195–203 (2011).
Thompson, N. et al. Towards spatially selective efferent neuromodulation: anatomical and functional organization of cardiac fibres in the porcine cervical vagus nerve. J. Physiol. 603, 1983–2004 (2024).
Fitchett, A., Mastitskaya, S. & Aristovich, K. Selective neuromodulation of the vagus nerve. Front. Neurosci. 15, 685872 (2021).
Ravagli, E., Ardell, J., Holder, D. & Aristovich, K. A combined cuff electrode array for organ-specific selective stimulation of vagus nerve enabled by electrical impedance tomography. Front. Med. Technol. 5, 1122016 (2023).
Ardell, J. L. et al. Defining the neural fulcrum for chronic vagus nerve stimulation: implications for integrated cardiac control. J. Physiol. 595, 6887–6903 (2017).
Butt, M. F., Albusoda, A., Farmer, A. D. & Aziz, Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J. Anat. 236, 588–611 (2020).
Krahl, S. E., Senanayake, S. S. & Handforth, A. Destruction of peripheral C-fibers does not alter subsequent vagus nerve stimulation-induced seizure suppression in rats. Epilepsia 42, 586–589 (2001).
Yu, L. et al. Chronic intermittent low-level stimulation of tragus reduces cardiac autonomic remodeling and ventricular arrhythmia inducibility in a post-infarction canine model. JACC Clin. Electrophysiol. 2, 330–339 (2016).
Yu, L. et al. Low-level tragus stimulation for the treatment of ischemia and reperfusion injury in patients with ST-segment elevation myocardial infarction: a proof-of-concept study. JACC Cardiovasc. Interv. 10, 1511–1520 (2017).
Stavrakis, S. et al. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J. Am. Coll. Cardiol. 65, 867–875 (2015).
Stavrakis, S. et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): a randomized clinical trial. JACC Clin. Electrophysiol. 6, 282–291 (2020).
Stavrakis, S. et al. Noninvasive vagus nerve stimulation in postural tachycardia syndrome: a randomized clinical trial. JACC Clin. Electrophysiol. 10, 346–355 (2024).
Antonino, D. et al. Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: a randomized placebo-controlled trial. Brain Stimul. 10, 875–881 (2017).
Sinkovec, M., Trobec, R. & Meglic, B. Cardiovascular responses to low-level transcutaneous vagus nerve stimulation. Auton. Neurosci. 236, 102851 (2021).
Aaronson, S. T. et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 6, 631–640 (2013).
Huang, F. et al. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement. Altern. Med. 14, 203 (2014).
Jacobs, H. I., Riphagen, J. M., Razat, C. M., Wiese, S. & Sack, A. T. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol. Aging 36, 1860–1867 (2015).
Huston, J. M. et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 35, 2762–2768 (2007).
Schwartz, P. J., Foreman, R. D., Stone, H. L. & Brown, A. M. Effect of dorsal root section on the arrhythmias associated with coronary occlusion. Am. J. Physiol. 231, 923–928 (1976).
Rock, A. K., Truong, H., Park, Y. L. & Pilitsis, J. G. Spinal cord stimulation. Neurosurg. Clin. N. Am. 30, 169–194 (2019).
Sdrulla, A. D., Guan, Y. & Raja, S. N. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 18, 1048–1067 (2018).
Sverrisdottir, Y. B. et al. Human dorsal root ganglion stimulation reduces sympathetic outflow and long-term blood pressure. JACC Basic Transl. Sci. 5, 973–985 (2020).
Howard-Quijano, K. et al. Spinal cord stimulation reduces ventricular arrhythmias during acute ischemia by attenuation of regional myocardial excitability. Am. J. Physiol. Heart Circ. Physiol. 313, H421–H431 (2017).
Kuwabara, Y. et al. Neuromodulation with thoracic dorsal root ganglion stimulation reduces ventricular arrhythmogenicity. Front. Physiol. https://doi.org/10.3389/fphys.2021.713717 (2021).
Southerland, E. M. et al. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am. J. Physiol. Heart Circ. Physiol. 292, H311–H317 (2007).
Lopshire, J. C. et al. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation 120, 286–294 (2009).
Squair, J. W. et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature 590, 308–314 (2021).
Sarikhani, P. et al. Reinforcement learning for closed-loop regulation of cardiovascular system with vagus nerve stimulation: a computational study. J. Neural Eng. 21, 036027 (2024).
Bender, S. A. et al. Closed-loop multimodal neuromodulation of vagus nerve for control of heart rate. Proc. Am. Control. Conf. 2024, 4536–4541 (2024).
Solinsky, R., Burns, K., Tuthill, C., Hamner, J. W. & Taylor, J. A. Transcutaneous spinal cord stimulation and its impact on cardiovascular autonomic regulation after spinal cord injury. Am. J. Physiol. Heart Circ. Physiol. 326, H116–H122 (2024).
Lenarczyk, R. et al. Management of patients with an electrical storm or clustered ventricular arrhythmias: a clinical consensus statement of the European Heart Rhythm Association of the ESC-endorsed by the Asia-Pacific Heart Rhythm Society, Heart Rhythm Society, and Latin-American Heart Rhythm Society. Europace 26, euae049 (2024).
Herring, N. & Paterson, D. J. Neuromodulators of peripheral cardiac sympatho-vagal balance. Exp. Physiol. 94, 46–53 (2009).
Tan, C. M. J. et al. The role of neuropeptide Y in cardiovascular health and disease. Front. Physiol. 9, 1281 (2018).
Hashmonai, M. The history of sympathetic surgery. Thorac. Surg. Clin. 26, 383–388 (2016).
Jonnesco, T. Traitment chirurgical de I’angine de poitrine par la resection du sympathetique cervicothoracique. Presse Méd. 29, 193–195 (1921).
Zipes, D. P. et al. Treatment of ventricular arrhythmia by permanent atrial pacemaker and cardiac sympathectomy. Ann. Intern. Med. 68, 591–597 (1968).
Schwartz, P. J. & Malliani, A. Electrical alternation of the T-wave: clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long Q–T syndrome. Am. Heart J. 89, 45–50 (1975).
Xiong, L. et al. Targeted ablation of cardiac sympathetic neurons attenuates adverse postinfarction remodelling and left ventricular dysfunction. Exp. Physiol. 103, 1221–1229 (2018).
Ziegler, K. A. et al. Local sympathetic denervation attenuates myocardial inflammation and improves cardiac function after myocardial infarction in mice. Cardiovasc. Res. 114, 291–299 (2018).
Yang, X., Zhang, L., Liu, H., Shao, Y. & Zhang, S. Cardiac sympathetic denervation suppresses atrial fibrillation and blood pressure in a chronic intermittent hypoxia rat model of obstructive sleep apnea. J. Am. Heart Assoc. 8, e010254 (2019).
Surman, T. L., Stuklis, R. G. & Chan, J. C. Thoracoscopic sympathectomy for long QT syndrome. Literature review and case study. Heart Lung Circ. 28, 486–494 (2019).
Chihara, R. K., Chan, E. Y., Meisenbach, L. M. & Kim, M. P. Surgical cardiac sympathetic denervation for ventricular arrhythmias: a systematic review. Methodist Debakey Cardiovasc. J. 17, 24–35 (2021).
Dusi, V. et al. Left cardiac sympathetic denervation for long QT syndrome: 50 years’ experience provides guidance for management. JACC Clin. Electrophysiol. 8, 281–294 (2022).
Wilde, A. A. et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N. Engl. J. Med. 358, 2024–2029 (2008).
Li, Y. L. et al. Stellate ganglia: a key therapeutic target for malignant ventricular arrhythmia in heart disease. Circulation Res. 136, 1049–1069 (2025).
Do, D. H. et al. Thoracic epidural anesthesia can be effective for the short-term management of ventricular tachycardia storm. J. Am. Heart Assoc. 6, e007080 (2017).
Savastano, S. et al. Electrical storm treatment by percutaneous stellate ganglion block: the STAR study. Eur. Heart J. 45, 823–833 (2024).
Ali, R., Ciccone, J. & Tseng, V. Cervical sympathetic blockade for the management of electrical storm. J. Clin. Anesth. 36, 47–50 (2017).
Przybylski, A., Romanek, J., Chlebuś, M., Deręgowska, B. & Kuźniar, J. Percutaneous stellate ganglion block as an adjunctive therapy in the treatment of incessant ventricular tachycardia. Kardiol. Pol. 76, 1018–1020 (2018).
Schwartz, P. J. & Stone, H. L. Left stellectomy in the prevention of ventricular fibrillation caused by acute myocardial ischemia in conscious dogs with anterior myocardial infarction. Circulation 62, 1256–1265 (1980).
Schwartz, P. J., Stone, H. L. & Brown, A. M. Effects of unilateral stellate ganglion blockade on the arrhythmias associated with coronary occlusion. Am. Heart J. 92, 589–599 (1976).
Perlow, S. & Vehe, K. L. Variations in the gross anatomy of the stellate and lumbar sympathetic ganglia. Am. J. Surg. 30, 454–458 (1935).
Song, Z. F., Sun, M. M., Wu, Z. Y. & Xia, C. L. Anatomical study and clinical significance of the rami communicantes between cervicothoracic ganglion and brachial plexus. Clin. Anat. 23, 811–814 (2010).
Tubbs, R. S. et al. The vertebral nerve revisited. Clin. Anat. 20, 644–647 (2007).
Krum, H. et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373, 1275–1281 (2009).
Böhm, M. et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet 395, 1444–1451 (2020).
Mahfoud, F. et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet 399, 1401–1410 (2022).
Dibona, G. F. Differentiation of vasoactive renal sympathetic nerve fibres. Acta Physiol. Scand. 168, 195–200 (2000).
Marfurt, C. F. & Echtenkamp, S. F. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J. Comp. Neurol. 311, 389–404 (1991).
Tyshynsky, R. et al. Periglomerular afferent innervation of the mouse renal cortex. Front. Neurosci. 17, 974197 (2023).
Ong, J. et al. Renal sensory nerves increase sympathetic nerve activity and blood pressure in 2-kidney 1-clip hypertensive mice. J. Neurophysiol. 122, 358–367 (2019).
Vongpatanasin, W. & Addo, T. The next chapter of renal denervation after US Food And Drug Administration approval. Circulation 149, 760–763 (2024).
Foss, J. D. et al. Role of afferent and efferent renal nerves in the development of AngII-salt hypertension in rats. Physiol. Rep. 6, e13602 (2018).
Osborn, J. W., Tyshynsky, R. & Vulchanova, L. Function of renal nerves in kidney physiology and pathophysiology. Annu. Rev. Physiol. 83, 429–450 (2021).
Burchell, A. E. et al. Controversies surrounding renal denervation: lessons learned from real-world experience in two United Kingdom centers. J. Clin. Hypertens. 18, 585–592 (2016).
Burchell, A. Renal denervation for resistant hypertension: predictors of procedural response and efficacy. PhD thesis, University of Bristol. https://research-information.bris.ac.uk/en/studentTheses/renal-denervation-for-resistant-hypertension (2019).
Plunkett, M. J., Paton, J. F. R. & Fisher, J. P. Autonomic control of the pulmonary circulation: implications for pulmonary hypertension. Exp. Physiol. 110, 42–57 (2024).
Zhang, H. et al. Pulmonary artery denervation for pulmonary arterial hypertension: a sham-controlled randomized PADN-CFDA Trial. JACC Cardiovasc. Interv. 15, 2412–2423 (2022).
Chen, S. L. et al. Pulmonary artery denervation to treat pulmonary arterial hypertension: the single-center, prospective, first-in-man PADN-1 study (first-in-man pulmonary artery denervation for treatment of pulmonary artery hypertension). J. Am. Coll. Cardiol. 62, 1092–1100 (2013).
Velez-Roa, S. et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 110, 1308–1312 (2004).
Ciarka, A., Doan, V., Velez-Roa, S., Naeije, R. & van de Borne, P. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am. J. Resp. Crit. Care Med. 181, 1269–1275 (2010).
Simpson, L. L. et al. Evidence for a physiological role of pulmonary arterial baroreceptors in sympathetic neural activation in healthy humans. J. Physiol. 598, 955–965 (2020).
Zhang, H. et al. Pulmonary artery denervation significantly increases 6-min walk distance for patients with combined pre- and post-capillary pulmonary hypertension associated with left heart failure: the PADN-5 study. JACC Cardiovasc. Inter. 12, 274–284 (2019).
Witkowski, A. et al. Transcatheter pulmonary denervation in patients with left heart failure with reduced ejection fraction and combined precapillary and postcapillary pulmonary hypertension: a prospective single center experience. Catheter Cardiovasc. Interv. 98, 588–594 (2021).
Zheng, M. et al. Pulmonary artery denervation inhibits left stellate ganglion stimulation-induced ventricular arrhythmias originating from the RVOT. JACC Clin. Electrophysiol. 9, 1354–1367 (2023).
Emans, T. W. et al. Forgotten circulation: reduced mesenteric venous capacitance in hypertensive rats is improved by decreasing sympathetic activity. Hypertension 81, 823–835 (2024).
Kittipibul, V. et al. Splanchnic nerve modulation effects on surrogate measures of venous capacitance. J. Am. Heart Assoc. 12, e028780 (2023).
Fudim, M. et al. Endovascular ablation of the right greater splanchnic nerve in heart failure with preserved ejection fraction: early results of the REBALANCE-HF trial roll-in cohort. Eur. J. Heart Fail. 24, 1410–1414 (2022).
Green, A. L. & Paterson, D. J. Using deep brain stimulation to unravel the mysteries of cardiorespiratory control. Compr. Physiol. 10, 1085–1104 (2020).
Kringelbach, M. L., Jenkinson, N., Owen, S. L. & Aziz, T. Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 8, 623–635 (2007).
Boccard, S. G., Pereira, E. A., Moir, L., Aziz, T. Z. & Green, A. L. Long-term outcomes of deep brain stimulation for neuropathic pain. Neurosurgery 72, 221–230 (2013).
McDowell, K. et al. Neuropeptide Y is elevated in heart failure and is an independent predictor of outcomes. Eur. J. Heart Fail. 26, 107–116 (2024).
Gibbs, T. et al. Neuropeptide-Y levels in ST-segment-elevation myocardial infarction: relationship with coronary microvascular function, heart failure, and mortality. J. Am. Heart Assoc. 11, e024850 (2022).
Januzzi, J. L. et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the international collaborative of NT-proBNP study. Eur. Heart J. 27, 330–337 (2006).
Cohn, J. N. et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N. Engl. J. Med. 311, 819–823 (1984).
Rennert, R. C. et al. A histological and mechanical analysis of the cardiac lead-tissue interface: implications for lead extraction. Acta Biomater. 10, 2200–2208 (2014).
Bilal, M., Syed, N. N., Jamil, Y., Tariq, A. & Khan, H. R. Powering the future: exploring self-charging cardiac implantable electronic devices and the Qi revolution. Pacing Clin. Electrophysiol. 47, 542–550 (2024).
Somani, S. & Rogers, A. J. Advances in cardiac pacing with leadless pacemakers and conduction system pacing. Curr. Opin. Cardiol. 39, 1–5 (2024).
Knops, R. E. et al. A dual-chamber leadless pacemaker. N. Engl. J. Med. 388, 2360–2370 (2023).
Knops, R. E. et al. A modular communicative leadless pacing-defibrillator system. N. Engl. J. Med. 391, 1402–1412 (2024).
O’Callaghan, E. L. et al. Enhancing respiratory sinus arrhythmia increases cardiac output in rats with left ventricular dysfunction. J. Physiol. 598, 455–471 (2020).
Shanks, J. et al. Reverse re-modelling chronic heart failure by reinstating heart rate variability. Basic Res. Cardiol. 117, 4 (2022).
Azizi, M. et al. Patient-level pooled analysis of endovascular ultrasound renal denervation or a sham procedure 6 months after medication escalation: the RADIANCE clinical trial program. Circulation 149, 747–759 (2024).
Spiering, W. et al. Endovascular baroreflex amplification for resistant hypertension: a safety and proof-of-principle clinical study. Lancet 390, 2655–2661 (2017).
Seravalle, G., Dell’Oro, R. & Grassi, G. Baroreflex activation therapy systems: current status and future prospects. Expert Rev. Med. Devices 16, 1025–1033 (2019).
Patel, N. K. et al. Deep brain stimulation relieves refractory hypertension. Neurology 76, 405–407 (2011).
O’Callaghan, E. L. et al. Deep brain stimulation for the treatment of resistant hypertension. Curr. Hypertens. Rep. 16, 493 (2014).
Esler, M. & Kaye, D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J. Cardiovasc. Pharmacol. 35, S1–S7 (2000).
Seagard, J. L., Hopp, F. A., Drummond, H. A. & Van Wynsberghe, D. M. Selective contribution of two types of carotid sinus baroreceptors to the control of blood pressure. Circ. Res. 72, 1011–1022 (1993).
Thrasher, T. N. Arterial baroreceptor input contributes to long-term control of blood pressure. Curr. Hypertens. Rep. 8, 249–254 (2006).
Thrasher, T. N. Unloading arterial baroreceptors causes neurogenic hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1044–R1053 (2002).
Thoren, P., Andresen, M. C. & Brown, A. M. Resetting of aortic baroreceptors with non-myelinated afferent fibers in spontaneously hypertensive rats. Acta Physiol. Scand. 117, 91–97 (1983).
Andresen, M. C. & Brown, A. M. Baroreceptor function in spontaneously hypertensive rats. effect of preventing hypertension. Circ. Res. 47, 829–834 (1980).
van Kleef, M. et al. Treatment of resistant hypertension with endovascular baroreflex amplification: 3-year results from the CALM-FIM study. JACC Cardiovasc. Interv. 15, 321–332 (2022).
van Kleef, M. et al. Endovascular baroreflex amplification and the effect on sympathetic nerve activity in patients with resistant hypertension: a proof-of-principle study. PLoS ONE 16, e0259826 (2021).
Heusser, K. et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension 55, 619–626 (2010).
Scheffers, I. J. et al. Novel baroreflex activation therapy in resistant hypertension: results of a European multi-center feasibility study. J. Am. Coll. Cardiol. 56, 1254–1258 (2010).
Bakris, G. L. et al. Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long-term follow-up in the rheos pivotal trial. J. Am. Soc. Hypertens. 6, 152–158 (2012).
Bloch, M. J. & Basile, J. N. The Rheos Pivotol trial evaluating baroreflex activation therapy fails to meet efficacy and safety end points in resistant hypertension: back to the drawing board. J. Clin. Hypertens. 14, 184–186 (2012).
Hoppe, U. C. et al. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim Neo trial. J. Am. Soc. Hypertens. 6, 270–276 (2012).
Wallbach, M. et al. Effects of baroreflex activation therapy on ambulatory blood pressure in patients with resistant hypertension. Hypertension 67, 701–709 (2016).
Abraham, W. T. et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail. 3, 487–496 (2015).
Neuzil, P. et al. Pacemaker-mediated programmable hypertension control therapy. J. Am. Heart Assoc. 6, e006974 (2017).
Kalarus, Z. et al. Pacemaker-based cardiac neuromodulation therapy in patients with hypertension: a pilot study. J. Am. Heart Assoc. 10, e020492 (2021).
Manisty, C. H. et al. The acute effects of changes to AV delay on BP and stroke volume: potential implications for design of pacemaker optimization protocols. Circ. Arrhythm. Electrophysiol. 5, 122–130 (2012).
Evaluation of sympathetic activity effects by the backbeat medical cardiac neuromodulation therapy (CNT). ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05462405 (2024).
Marcus, N. J., Del Rio, R., Schultz, E. P., Xia, X. H. & Schultz, H. D. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J. Physiol. 592, 391–408 (2014).
Abdala, A. P. et al. Hypertension is critically dependent on the carotid body input in the spontaneously hypertensive rat. J. Physiol. 590, 4269–4277 (2012).
Izdebska, E., Izdebski, J. & Trzebski, A. Hemodynamic responses to brief hyperoxia in healthy and in mild hypertensive human subjects in rest and during dynamic exercise. J. Physiol. Pharmacol. 47, 243–256 (1996).
Ponikowski, P. et al. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation 104, 544–549 (2001).
Siński, M. et al. Tonic activity of carotid body chemoreceptors contributes to the increased sympathetic drive in essential hypertension. Hypertens. Res. 35, 487–491 (2012).
Tymoteusz, Ż. et al. Translating physiology of the arterial chemoreflex into novel therapeutic interventions targeting carotid bodies in cardiometabolic disorders. J. Physiol. 603, 2487–2516 (2025).
Narkiewicz, K. et al. Unilateral carotid body resection in resistant hypertension: a safety and feasibility trial. JACC Basic Transl. Sci. 1, 313–324 (2016).
Niewinski, P. et al. Carotid body resection for sympathetic modulation in systolic heart failure: results from first-in-man study. Eur. J. Heart Fail. 19, 391–400 (2017).
Lobo, M. D. In Renal Denervation: Treatment and Device-Based Neuromodulation (eds Heuser, R. R. et al.) 193–198 (Springer International, 2023).
Neuzil, P. et al. Long term effect of transvenous carotid body ablation in the treatment of patients with resistant hypertension. Eur. Heart J. 38, ehx504.4123 (2017).
Schlaich, M. et al. Transvenous carotid body ablation for resistant hypertension: main results of a multicentre safety and proof-of-principle cohort study. Eur. Heart J. 39, ehy565.1416 (2018).
Kulej-Lyko, K., Niewinski, P., Tubek, S. & Ponikowski, P. Contribution of peripheral chemoreceptors to exercise intolerance in heart failure. Front. Physiol. 13, 878363 (2022).
Kulej-Lyko, K. et al. Inhibition of peripheral chemoreceptors improves ventilatory efficiency during exercise in heart failure with preserved ejection fraction — a role of tonic activity and acute reflex response. Front. Physiol. 13, 911636 (2022).
Bernard, C. Lectures on the Phenomena of Life Common to Animals and Plants (1872, 1873) (transl. Hoff, H. E. et al.) (Charles C. Thomas, 1974).
Cannon, W. B. The Wisdom of the Body (W. W. Norton & Company, 1932).
Hypertension. World Health Organization https://www.who.int/news-room/fact-sheets/detail/hypertension (2023).
High blood pressure and older adults. National Heart, Lung, and Blood Institute https://www.nia.nih.gov/health/high-blood-pressure/high-blood-pressure-and-older-adults (2022).
McEvoy, J. W. et al. 2024 ESC guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 45, 3912–4018 (2024).
Paton, J. F., Dickinson, C. J. & Mitchell, G. Harvey Cushing and the regulation of blood pressure in giraffe, rat and man: introducing ‘Cushing’s mechanism’. Exp. Physiol. 94, 11–17 (2009).
Potts, J. T. et al. Contraction-sensitive skeletal muscle afferents inhibit arterial baroreceptor signalling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience 119, 201–214 (2003).
Ottaviani, M. M., Wright, L., Dawood, T. & Macefield, V. G. In vivo recordings from the human vagus nerve using ultrasound-guided microneurography. J. Physiol. 598, 3569–3576 (2020).
Incognito, A. V. et al. Arterial baroreflex regulation of muscle sympathetic nerve activity at rest and during stress. J. Physiol. 597, 4729–4741 (2019).
Barrett, C. J., Navakatikyan, M. A. & Malpas, S. C. Long-term control of renal blood flow: what is the role of the renal nerves? Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1534–R1545 (2001).
Briant, L. J., O’Callaghan, E. L., Champneys, A. R. & Paton, J. F. Respiratory modulated sympathetic activity: a putative mechanism for developing vascular resistance? J. Physiol. 593, 5341–5360 (2015).
D’Souza, A. W. et al. Age- and sex-related differences in sympathetic vascular transduction and neurohemodynamic balance in humans. Am. J. Physiol. Heart Circ. Physiol. 325, H917–H932 (2023).
Stehlin, E. et al. Chronic measurement of left ventricular pressure in freely moving rats. J. Appl. Physiol. 115, 1672–1682 (2013).
Hawryluk, G. W. J. et al. Intracranial pressure: current perspectives on physiology and monitoring. Intensive Care Med. 48, 1471–1481 (2022).
Abraham, W. T. et al. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet 387, 453–461 (2016).
Dabbour, A. H. et al. The safety of micro-implants for the brain. Front. Neurosci. 15, 796203 (2021).
Osswald, S. et al. Closed-loop stimulation using intracardiac impedance as a sensor principle: correlation of right ventricular dP/dtmax and intracardiac impedance during dobutamine stress test. Pacing Clin. Electrophysiol. 23, 1502–1508 (2000).
Griesbach, L. et al. Clinical performance of automatic closed-loop stimulation systems. Pacing Clin. Electrophysiol. 26, 1432–1437 (2003).
Prabhu, S., Rangarajan, S. & Kothare, M. Data-driven discovery of sparse dynamical model of cardiovascular system for model predictive control. Comput. Biol. Med. 166, 107513 (2023).
Gee, M. M., Lenhoff, A. M., Schwaber, J. S., Ogunnaike, B. A. & Vadigepalli, R. Closed-loop modeling of central and intrinsic cardiac nervous system circuits underlying cardiovascular control. AIChE J. 69, e18033 (2023).
Thompson, N. et al. Organotopic organization of the porcine mid-cervical vagus nerve. Front. Neurosci. 17, 963503 (2023).
Shanks, J., Pachen, M., Chang, J. W., George, B. & Ramchandra, R. Cardiac vagal nerve activity increases during exercise to enhance coronary blood flow. Circ. Res. 133, 559–571 (2023).
Wang, L. et al. Neuronal nitric oxide synthase gene transfer decreases [Ca2+]i in cardiac sympathetic neurons. J. Mol. Cell Cardiol. 43, 717–725 (2007).
Mohan, R. M. et al. Neuronal nitric oxide synthase gene transfer promotes cardiac vagal gain of function. Circ. Res. 91, 1089–1091 (2002).
Heaton, D. A. et al. Gene transfer of neuronal nitric oxide synthase into intracardiac ganglia reverses vagal impairment in hypertensive rats. Hypertension 49, 380–388 (2007).
Park, J. H., Gorky, J., Ogunnaike, B., Vadigepalli, R. & Schwaber, J. S. Investigating the effects of brainstem neuronal adaptation on cardiovascular homeostasis. Front. Neurosci. 14, 470 (2020).
Park, J. et al. Inputs drive cell phenotype variability. Genome Res. 24, 930–941 (2014).
Staehle, M. M. et al. Diurnal patterns of gene expression in the dorsal vagal complex and the central nucleus of the amygdala — non-rhythm-generating brain regions. Front. Neurosci. 14, 375 (2020).
Hornung, E. et al. Neuromodulatory co-expression in cardiac vagal motor neurons of the dorsal motor nucleus of the vagus. iScience 27, 110549 (2024).
Roth, E. et al. Effects of PACAP and preconditioning against ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro. Ann. NY Acad. Sci. 1163, 512–516 (2009).
Lee, E. H. & Seo, S. R. Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep. 47, 369–375 (2014).
Liu, D. M., Cuevas, J. & Adams, D. J. VIP and PACAP potentiation of nicotinic ACh-evoked currents in rat parasympathetic neurons is mediated by G-protein activation. Eur. J. Neurosci. 12, 2243–2251 (2000).
Mao, P. et al. Mitochondrial mechanism of neuroprotection by CART. Eur. J. Neurosci. 26, 624–632 (2007).
Machhada, A. et al. Optogenetic stimulation of vagal efferent activity preserves left ventricular function in experimental heart failure. JACC Basic Transl. Sci. 5, 799–810 (2020).
Hanna, P. et al. Innervation and neuronal control of the mammalian sinoatrial node a comprehensive atlas. Circ. Res. 128, 1279–1296 (2021).
Achanta, S. et al. A comprehensive integrated anatomical and molecular atlas of rat intrinsic cardiac nervous system. iScience 23, 101140 (2020).
Leung, C. et al. 3D single cell scale anatomical map of sex-dependent variability of the rat intrinsic cardiac nervous system. iScience 24, 102795 (2021).
Cheng, Z., Zhang, H., Guo, S. Z., Wurster, R. & Gozal, D. Differential control over postganglionic neurons in rat cardiac ganglia by NA and DmnX neurons: anatomical evidence. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R625–R633 (2004).
Kellett, D. O. et al. Transcriptional response of the heart to vagus nerve stimulation. Physiol. Genom. 56, 167–178 (2024).
Winbo, A. & Paterson, D. J. The brain–heart connection in sympathetically triggered inherited arrhythmia syndromes. Heart Lung Circ. 29, 529–537 (2020).
Zeppenfeld, K. et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 43, 3997–4126 (2022).
Priori, S. G. et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. HeartRhythm 10, 1932–1963 (2013).
Wu, H. F. et al. Parasympathetic neurons derived from human pluripotent stem cells model human diseases and development. Cell Stem Cell 31, 734–753.e738 (2024).
Zaglia, T. & Mongillo, M. Cardiac sympathetic innervation, from a different point of (re)view. J. Physiol. 595, 3919–3930 (2017).
Shivkumar, K. & Ardell, J. L. Cardiac autonomic control in health and disease. J. Physiol. 594, 3851–3852 (2016).
Bardsley, E. N. et al. RNA sequencing reveals novel transcripts from sympathetic stellate ganglia during cardiac sympathetic hyperactivity. Sci. Rep. 8, 8633 (2018).
Larsen, H. E., Lefkimmiatis, K. & Paterson, D. J. Sympathetic neurons are a powerful driver of myocyte function in cardiovascular disease. Sci. Rep. 6, 38898 (2016).
Dokshokova, L. et al. Nerve growth factor transfer from cardiomyocytes to innervating sympathetic neurons activates TrkA receptors at the neuro-cardiac junction. J. Physiol. 600, 2853–2875 (2022).
Davis, H., Liu, K., Li, N., Li, D. & Paterson, D. J. Healthy cardiac myocytes can decrease sympathetic hyperexcitability in the early stages of hypertension. Front. Synaptic Neurosci. 14, 949150 (2022).
Berg, T. & Jensen, J. Simultaneous parasympathetic and sympathetic activation reveals altered autonomic control of heart rate, vascular tension, and epinephrine release in anesthetized hypertensive rats. Front. Neurol. 2, 71 (2011).
Landis, S. C. Rat sympathetic neurons and cardiac myocytes developing in microcultures: correlation of the fine structure of endings with neurotransmitter function in single neurons. Proc. Natl Acad. Sci. USA 73, 4220–4224 (1976).
Furshpan, E. J., MacLeish, P. R., O’Lague, P. H. & Potter, D. D. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc. Natl Acad. Sci. USA 73, 4225–4229 (1976).
Oh, Y. et al. Functional coupling with cardiac muscle promotes maturation of hPSC-derived sympathetic neurons. Cell Stem Cell 19, 95–106 (2016).
Luther, J. A. & Birren, S. J. Neurotrophins and target interactions in the development and regulation of sympathetic neuron electrical and synaptic properties. Auton. Neurosci. 151, 46–60 (2009).
Fagan, A. M. et al. TrkA, but not TrkC, receptors are essential for survival of sympathetic neurons in vivo. J. Neurosci. 16, 6208–6218 (1996).
Bengel, F. M. et al. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N. Engl. J. Med. 345, 731–738 (2001).
Takahashi, K. & Yamanaka, S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat. Rev. Mol. Cell Biol. 17, 183–193 (2016).
Ludwig, T. E. et al. 20 years of human pluripotent stem cell research: it all started with five lines. Cell Stem Cell 23, 644–648 (2018).
Moretti, A. et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 363, 1397–1409 (2010).
Wu, H. F. & Zeltner, N. Overview of methods to differentiate sympathetic neurons from human pluripotent stem cells. Curr. Protoc. Stem Cell Biol. 50, e92 (2019).
Wu, H. F. et al. Norepinephrine transporter defects lead to sympathetic hyperactivity in familial dysautonomia models. Nat. Commun. 13, 7032 (2022).
Takayama, Y. et al. Selective induction of human autonomic neurons enables precise control of cardiomyocyte beating. Sci. Rep. 10, 9464 (2020).
Narkar, A., Feaster, T. K., Casciola, M. & Blinova, K. Human in vitro neurocardiac coculture (ivNCC) assay development for evaluating cardiac contractility modulation. Physiol. Rep. 10, e15498 (2022).
Winbo, A. et al. Functional hyperactivity in long QT syndrome type 1 pluripotent stem cell-derived sympathetic neurons. Am. J. Physiol. Heart Circ. Physiol. 321, H217–h227 (2021).
Goldstein, R. S., Pomp, O., Brokhman, I. & Ziegler, L. Generation of neural crest cells and peripheral sensory neurons from human embryonic stem cells. Meth. Mol. Biol. 584, 283–300 (2010).
Neely, O. C., Domingos, A. I. & Paterson, D. J. Macrophages can drive sympathetic excitability in the early stages of hypertension. Front. Cardiovasc. Med. 8, 807904 (2021).
Zimmermann, C. J., Herson, P. S., Neeves, K. B. & Marr, D. W. M. Multimodal microwheel swarms for targeting in three-dimensional networks. Sci. Rep. 12, 5078 (2022).
Madonna, R. et al. ESC working group on cellular biology of the heart: position paper for cardiovascular research: tissue engineering strategies combined with cell therapies for cardiac repair in ischaemic heart disease and heart failure. Cardiovasc. Res. 115, 488–500 (2019).
Zimmermann, W. H. et al. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 90, 223–230 (2002).
Ergir, E. et al. Generation and maturation of human iPSC-derived 3D organotypic cardiac microtissues in long-term culture. Sci. Rep. 12, 17409 (2022).
Qian, T., Shusta, E. V. & Palecek, S. P. Advances in microfluidic platforms for analyzing and regulating human pluripotent stem cells. Curr. Opin. Genet. Dev. 34, 54–60 (2015).
Kolanowski, T. J. et al. Enhanced structural maturation of human induced pluripotent stem cell-derived cardiomyocytes under a controlled microenvironment in a microfluidic system. Acta Biomater. 102, 273–286 (2020).
Kolanowski, T. J., Antos, C. L. & Guan, K. Making human cardiomyocytes up to date: derivation, maturation state and perspectives. Int. J. Cardiol. 241, 379–386 (2017).
Zhang, Y. et al. Millimetre-scale bioresorbable optoelectronic systems for electrotherapy. Nature 640, 77–86 (2025).
Stark, C., Rytkin, E., Mircea, A. A. & Efimov, I. R. Advances in cardiac devices and bioelectronics augmented with artificial intelligence. J. Physiol. https://doi.org/10.1113/JP287135 (2025).
Brüning, J. et al. In-silico enhanced animal study of pulmonary artery pressure sensors: assessing hemodynamics using computational fluid dynamics. Front. Cardiovasc. Med. 10, 1193209 (2023).
Kushwah, C., Riesenhuber, M., Asmul, S., Gyöngyösi, M. & Nogaret, A. In-vivo blood pressure sensing with bi-filler nanocomposite. Biomater. Adv. 162, 213905 (2024).
Grinstein, J. et al. HVAD waveform analysis as a noninvasive marker of pulmonary capillary wedge pressure: a first step toward the development of a smart left ventricular assist device pump. ASAIO J. 64, 10–15 (2018).
Imamura, T. & Narang, N. Advances in hemodynamic monitoring in heart failure patients. Intern. Med. 60, 167–171 (2021).
Pijacka, W. et al. Purinergic receptors in the carotid body as a new drug target for controlling hypertension. Nat. Med. 22, 1151–1159 (2016).
Gold, O. M. S., Bardsley, E. N., Ponnampalam, A. P., Pauza, A. G. & Paton, J. F. R. Cellular basis of learning and memory in the carotid body. Front. Synaptic Neurosci. 14, 902319 (2022).
Acknowledgements
J.F.R.P. is funded by the Health Research Council, Marsden Fund, the Royal Society of New Zealand, the Sidney Taylor Trust and is an inaugural Partridge Family laureate. J.F.R.P. was supported by a visiting research fellowship at Merton College, Oxford, during the writing of this Review with D.J.P. R.V. is funded by the National Heart Lung and Blood Institute (R01 HL161696). N.H. is supported by a British Heart Foundation Senior Clinical Research Fellowship (FS/SCRF/20/32005). D.J.P. is supported by a BHF Special Project Grant (SP/F/22/150027) and a Leducq International Network of Excellence Award (23CVD04) on Bioelectronics for Neurocardiology (with N.H.).
Author information
Authors and Affiliations
Contributions
J.F.R.P., T.Z., N.H. and D.J.P. researched data and wrote the article. J.F.R.P., N.H. and D.J.P. contributed to the discussion of its content. All authors reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Gaetano De Ferrari, who co-reviewed with Veronica Dusi, and John Osborn for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
SPARC portal: https://sparc.science/apps/maps?type=ac
Rights and permissions
About this article
Cite this article
Paton, J.F.R., Żera, T., Vadigepalli, R. et al. Multimodal, device-based therapeutic targeting of the cardiovascular autonomic nervous system. Nat Rev Cardiol (2025). https://doi.org/10.1038/s41569-025-01212-4
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41569-025-01212-4