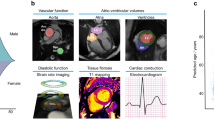
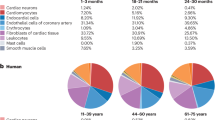
Abstract
The global rise in life expectancy underscores the urgent need to extend healthspan and prevent age-related diseases. Cardiovascular disease is the leading cause of death worldwide, with ageing as a major non-modifiable risk factor. Ageing drives progressive vascular dysfunction and cardiac decline, including heart failure with preserved or reduced ejection fraction. Vascular cells are particularly vulnerable to ageing, resulting in structural and functional deterioration of the microvasculature and macrovasculature. Emerging evidence highlights that ageing also disrupts the neurovascular interface — an intricate axis between the nervous and vascular systems that governs cardiac function. Alterations to the neurovascular unit in the heart contribute to impaired autonomic regulation, increasing the risk of arrhythmias and heart failure. In this Review, we examine how neurovascular ageing shapes cardiac dysfunction and explore the therapeutic potential of targeting the cardiac neurovascular unit to mitigate cardiovascular ageing and promote resilience in ageing populations.
Key points
-
The nervous and vascular systems form closely interconnected interfaces that coordinate tissue homeostasis, neuroimmune communication and vascular integrity in both health and disease.
-
Vascular ageing causes endothelial dysfunction, which affects neurovascular signalling, leading to reduced cardiac innervation and electrical instability; these changes can be reversed by senolytic therapies targeting senescent cells.
-
The cardiac neurovascular unit mirrors mechanisms in the central nervous system, where nerves and blood vessels align and co-develop, regulated by neural activity and endothelial signals, to maintain cardiac function.
-
Heart–brain communication mediated by neurovascular and neuronal pathways regulates cardiac autonomic control, linking central neurodegenerative processes to peripheral cardiac innervation and function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
The Global Cardiovascular Risk Consortium. Global effect of modifiable risk factors on cardiovascular disease and mortality. N. Engl. J. Med. 389, 1273–1285 (2023).
Liberale, L. et al. Roadmap for alleviating the manifestations of ageing in the cardiovascular system. Nat. Rev. Cardiol. 22, 577–605 (2025).
Iadecola, C. et al. The neurovasculome: key roles in brain health and cognitive impairment: a scientific statement from the American Heart Association/American Stroke Association. Stroke 54, e251–e271 (2023).
Anversa, P., Olivetti, G., Melissari, M. & Loud, A. V. Stereological measurement of cellular and subcellular hypertrophy and hyperplasia in the papillary muscle of adult rat. J. Mol. Cell Cardiol. 12, 781–795 (1980).
Wagner, J. U. G. & Dimmeler, S. The endothelial niche in heart failure: from development to regeneration. Eur. Heart J. 42, 4277–4279 (2021).
Wagner, J. U. G. & Dimmeler, S. Cellular cross-talks in the diseased and aging heart. J. Mol. Cell Cardiol. 138, 136–146 (2020).
Bassat, E. et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184 (2017).
Dalkara, T., Østergaard, L., Heusch, G. & Attwell, D. Pericytes in the brain and heart: functional roles and response to ischaemia and reperfusion. Cardiovasc. Res. 120, 2336–2348 (2024).
Vågesjö, E. et al. Perivascular macrophages regulate blood flow following tissue damage. Circ. Res. 128, 1694–1707 (2021).
He, H. et al. Perivascular macrophages limit permeability. Arterioscler. Thromb. Vasc. Biol. 36, 2203–2212 (2016).
Farbehi, N. et al. Single-cell expression profiling reveals dynamic flux of cardiac stromal, vascular and immune cells in health and injury. eLife 8, e43882 (2019).
Litviňuková, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Majesky, M. W. Vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 36, e82–e86 (2016).
Valenza, G., Matić, Z. & Catrambone, V. The brain–heart axis: integrative cooperation of neural, mechanical and biochemical pathways. Nat. Rev. Cardiol. 22, 537–550 (2025).
Pauza, D. H., Skripka, V., Pauziene, N. & Stropus, R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat. Rec. 259, 353–382 (2000).
Rysevaite, K. et al. Immunohistochemical characterization of the intrinsic cardiac neural plexus in whole-mount mouse heart preparations. Heart Rhythm. 8, 731–738 (2011).
Vincentz, J. W., Rubart, M. & Firulli, A. B. Ontogeny of cardiac sympathetic innervation and its implications for cardiac disease. Pediatric Cardiol. 33, 923–928 (2012).
Kawashima, T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat. Embryol. 209, 425–438 (2005).
Wu, D. et al. The blood–brain barrier: structure, regulation and drug delivery. Signal. Transduct. Target. Ther. 8, 217 (2023).
Wagner, J. U. G. et al. Aging impairs the neurovascular interface in the heart. Science 381, 897–906 (2023).
Schaeffer, S. & Iadecola, C. Revisiting the neurovascular unit. Nat. Neurosci. 24, 1198–1209 (2021).
Manousiouthakis, E., Mendez, M., Garner, M. C., Exertier, P. & Makita, T. Venous endothelin guides sympathetic innervation of the developing mouse heart. Nat. Commun. 5, 3918 (2014).
Manickam, N. et al. Beneficial effects of vascular endothelial growth factor B gene transfer in the aged heart. Cardiovasc. Res. 121, 1594–1608 (2025).
Nam, J. et al. Coronary veins determine the pattern of sympathetic innervation in the developing heart. Development 140, 1475–1485 (2013).
Ketch, T., Biaggioni, I., Robertson, R. & Robertson, D. Four faces of baroreflex failure. Circulation 105, 2518–2523 (2002).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Circulation 107, 139–146 (2003).
Song, R. et al. Associations between cardiovascular risk, structural brain changes, and cognitive decline. J. Am. Coll. Cardiol. 75, 2525–2534 (2020).
Rakusan, K. & Nagai, J. Morphometry of arterioles and capillaries in hearts of senescent mice. Cardiovasc. Res. 28, 969–972 (1994).
Tamiato, A. et al. Age-dependent RGS5 loss in pericytes induces cardiac dysfunction and fibrosis. Circ. Res. 134, 1240–1255 (2024).
Grootaert, M. O. J. et al. Mural cell dysfunction contributes to diastolic heart failure by promoting endothelial dysfunction and vessel remodelling. Cardiovasc. Diabetol. 24, 62 (2025).
Simmonds, S. J. et al. Pericyte loss initiates microvascular dysfunction in the development of diastolic dysfunction. Eur. Heart J. Open 4, oead129 (2024).
Gates, P. E., Strain, W. D. & Shore, A. C. Human endothelial function and microvascular ageing. Exp. Physiol. 94, 311–316 (2009).
Vasa, M., Breitschopf, K., Zeiher, A. M. & Dimmeler, S. Nitric oxide activates telomerase and delays endothelial cell senescence. Circ. Res. 87, 540–542 (2000).
Li, Z., Froehlich, J., Galis, Z. S. & Lakatta, E. G. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension 33, 116–123 (1999).
Ungvari, Z. et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 15, 555–565 (2018).
Zhou, X., Perez, F., Han, K. & Jurivich, D. A. Clonal senescence alters endothelial ICAM-1 function. Mech. Ageing Dev. 127, 779–785 (2006).
Liu, Z. et al. Insights gained from single-cell RNA analysis of murine endothelial cells in aging hearts. Heliyon 9, e18324 (2023).
Rodriguez Morales, D. et al. Vascular niches are the primary hotspots for aging within the multicellular architecture of cardiac tissue. Circ. Res. 137, 1353–1367 (2025).
Hall, I. F., Kishta, F., Xu, Y., Baker, A. H. & Kovacic, J. C. Endothelial to mesenchymal transition: at the axis of cardiovascular health and disease. Cardiovasc. Res. 120, 223–236 (2024).
Kovacic, J. C. et al. Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 190–209 (2019).
Damon, D. H. Sympathetic innervation promotes vascular smooth muscle differentiation. Am. J. Physiol. Heart Circ. Physiol. 288, H2785–H2791 (2005).
Vidal, R. et al. Transcriptional heterogeneity of fibroblasts is a hallmark of the aging heart. JCI Insight 4, e131092 (2019).
Kortlever, R. M., Higgins, P. J. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8, 877–884 (2006).
Hasan, W. et al. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res. 1124, 142–154 (2006).
Mias, C. et al. Cardiac fibroblasts regulate sympathetic nerve sprouting and neurocardiac synapse stability. PLoS ONE 8, e79068 (2013).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Pirzgalska, R. M. et al. Sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017).
Wolf, Y. et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 18, 665–674 (2017).
Moura Silva, H. et al. c-MAF-dependent perivascular macrophages regulate diet-induced metabolic syndrome. Sci. Immunol. 6, eabg7506 (2021).
Viola, M. F. et al. Dedicated macrophages organize and maintain the enteric nervous system. Nature 618, 818–826 (2023).
Matheis, F. et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180, 64–78.e16 (2020).
Wernli, G., Hasan, W., Bhattacherjee, A., van Rooijen, N. & Smith, P. G. Macrophage depletion suppresses sympathetic hyperinnervation following myocardial infarction. Basic Res. Cardiol. 104, 681–693 (2009).
Hulsmans, M. et al. Macrophages facilitate electrical conduction in the heart. Cell 169, 510–522.e20 (2017).
Elliott, H. L., Sumner, D. J., McLean, K. & Reid, J. L. Effect of age on the responsiveness of vascular α-adrenoceptors in man. J. Cardiovasc. Pharmacol. 4, 388–392 (1982).
Pinckard, J. et al. Functional ultrasound as a quantitative approach for measuring functional hyperemia in aging models. Neuroimage 316, 121313 (2025).
Bristow, M. R. et al. Decreased catecholamine sensitivity and β-adrenergic-receptor density in failing human hearts. N. Engl. J. Med. 307, 205–211 (1982).
Lakatta, E. G. & Sollott, S. J. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 132, 699–721 (2002).
Xiao, R. P., Spurgeon, H. A., O’Connor, F. & Lakatta, E. G. Age-associated changes in beta-adrenergic modulation on rat cardiac excitation-contraction coupling. J. Clin. Invest. 94, 2051–2059 (1994).
Perez, S. D. et al. Chronically lowering sympathetic activity protects sympathetic nerves in spleens from aging F344 rats. J. Neuroimmunol. 247, 38–51 (2012).
Mahmoud, A. I. et al. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev. Cell 34, 387–399 (2015).
White, I. A., Gordon, J., Balkan, W. & Hare, J. M. Sympathetic reinnervation is required for mammalian cardiac regeneration. Circ. Res. 117, 990–994 (2015).
Tahsili-Fahadan, P. & Geocadin, R. G. Heart–brain axis. Circ. Res. 120, 559–572 (2017).
Nolte, C. H. et al. Type 1 myocardial infarction in patients with acute ischemic stroke. JAMA Neurol. 81, 703–711 (2024).
Singh, T. et al. Takotsubo syndrome: pathophysiology, emerging concepts, and clinical implications. Circulation 145, 1002–1019 (2022).
Kwaśniak-Butowska, M. et al. Cardiovascular dysautonomia and cognition in Parkinson’s disease — a possible relationship. Neurol. Neurochir. Pol. 55, 525–535 (2021).
Allan, L. M. et al. Autonomic dysfunction in dementia. J. Neurol. Neurosurg. Psychiatry 78, 671 (2007).
Sanna, G. D. et al. Cardiac abnormalities in Alzheimer disease: clinical relevance beyond pathophysiological rationale and instrumental findings? JACC Heart Fail. 7, 121–128 (2019).
Guendelman, S., Kaltwasser, L., Bayer, M., Gallese, V. & Dziobek, I. Brain mechanisms underlying the modulation of heart rate variability when accepting and reappraising emotions. Sci. Rep. 14, 18756 (2024).
Hsueh, B. et al. Cardiogenic control of affective behavioural state. Nature 615, 292–299 (2023).
Huang, L.-Y. et al. Associations of cardiovascular risk factors and lifestyle behaviors with neurodegenerative disease: a Mendelian randomization study. Transl. Psychiatry 13, 267 (2023).
Dampney, R. A. L. Central neural control of the cardiovascular system: current perspectives. Adv. Physiol. Educ. 40, 283–296 (2016).
Ruiz Vargas, E., Sörös, P., Shoemaker, J. K. & Hachinski, V. Human cerebral circuitry related to cardiac control: a neuroimaging meta-analysis. Ann. Neurol. 79, 709–716 (2016).
Karim, S., Chahal, A., Khanji, M. Y., Petersen, S. E. & Somers, V. K. Autonomic cardiovascular control in health and disease. Compr. Physiol. 13, 4493–4511 (2023).
Harrison, N. A., Cooper, E., Voon, V., Miles, K. & Critchley, H. D. Central autonomic network mediates cardiovascular responses to acute inflammation: relevance to increased cardiovascular risk in depression? Brain Behav. Immun. 31, 189–196 (2013).
Dyavanapalli, J. Novel approaches to restore parasympathetic activity to the heart in cardiorespiratory diseases. Am. J. Physiol. Heart Circ. Physiol. 319, H1153–H1161 (2020).
Liu, J. et al. Heart–brain interaction in cardiogenic dementia: pathophysiology and therapeutic potential. Front. Cardiovasc. Med. 11, 1304864 (2024).
Zhang, D. et al. Correlation of ventricular arrhythmogenesis with neuronal remodeling of cardiac postganglionic parasympathetic neurons in the late stage of heart failure after myocardial infarction. Front. Neurosci. 11, 252 (2017).
Ziegler, K. A. et al. Immune-mediated denervation of the pineal gland underlies sleep disturbance in cardiac disease. Science 381, 285–290 (2023).
Martín Giménez, V. M. et al. Melatonin as an anti-aging therapy for age-related cardiovascular and neurodegenerative diseases. Front. Aging Neurosci. 14, 888292 (2022).
Reiter, R. J., Rosales-Corral, S. & Sharma, R. Circadian disruption, melatonin rhythm perturbations and their contributions to chaotic physiology. Adv. Med. Sci. 65, 394–402 (2020).
Li, H. et al. Pharmacological targeting of endothelial nitric oxide synthase dysfunction and nitric oxide replacement therapy. Free Radic. Biol. Med. 237, 455–472 (2025).
Yu, J. et al. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl Acad. Sci. USA 102, 10999–11004 (2005).
Daiber, A. et al. New therapeutic implications of endothelial nitric oxide synthase (eNOS) function/dysfunction in cardiovascular disease. Int. J. Mol. Sci. 20, 187 (2019).
Gao, J., Pan, X., Li, G., Chatterjee, E. & Xiao, J. Physical exercise protects against endothelial dysfunction in cardiovascular and metabolic diseases. J. Cardiovasc. Transl. Res. 15, 604–620 (2022).
Dookun, E., Passos, J. F., Arthur, H. M. & Richardson, G. D. Therapeutic potential of senolytics in cardiovascular disease. Cardiovasc. Drugs Ther. 36, 187–196 (2022).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Csik, B. et al. Senescent endothelial cells in cerebral microcirculation are key drivers of age-related blood–brain barrier disruption, microvascular rarefaction, and neurovascular coupling impairment in mice. Aging Cell 24, e70048 (2025).
Tarantini, S. et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience 43, 2427–2440 (2021).
Aratani, S. & Nakanishi, M. Recent advances in senolysis for age-related diseases. Physiology 38, 205–216 (2023).
Gonzales, M. M. et al. Senolytic therapy in mild Alzheimer’s disease: a phase 1 feasibility trial. Nat. Med. 29, 2481–2488 (2023).
Wissler Gerdes, E. O., Misra, A., Netto, J. M. E., Tchkonia, T. & Kirkland, J. L. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 200, 111591 (2021).
Grunewald, M. et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science 373, eabc8479 (2025).
Pius-Sadowska, E. & Machaliński, B. BDNF — a key player in cardiovascular system. J. Mol. Cell Cardiol. 110, 54–60 (2017).
Story, G. M. et al. Inactivation of one copy of the mouse neurotrophin-3 gene induces cardiac sympathetic deficits. Physiol. Genomics 2, 129–136 (2000).
Miwa, K. et al. Glial cell line-derived neurotrophic factor (GDNF) enhances sympathetic neurite growth in rat hearts at early developmental stages. Biomed. Res. 31, 353–361 (2010).
Miwa, K. et al. Axon guidance of sympathetic neurons to cardiomyocytes by glial cell line-derived neurotrophic factor (GDNF). PLoS ONE 8, e65202 (2013).
Rossi, D. et al. Reelin reverts biochemical, physiological and cognitive alterations in mouse models of tauopathy. Prog. Neurobiol. 186, 101743 (2020).
Liu, X. et al. Lymphoangiocrine signals promote cardiac growth and repair. Nature 588, 705–711 (2020).
Sonntag, W. E. et al. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J. Anat. 197, 575–585 (2000).
Xu, X. et al. IGF-1 Provides protective role in arteriosclerotic cerebral small vessel disease. Hypertension 82, 1137–1150 (2025).
Zaman, R. et al. Selective loss of resident macrophage-derived insulin-like growth factor-1 abolishes adaptive cardiac growth to stress. Immunity 54, 2057–2071.e6 (2021).
Perrotta, S. et al. A heart-brain-spleen axis controls cardiac remodeling to hypertensive stress. Immunity 58, 648–665.e7 (2025).
Cao, J.-M. et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101, 1960–1969 (2000).
Dajani, A. H. J. et al. Heterogeneous cardiac sympathetic innervation gradients promote arrhythmogenesis in murine dilated cardiomyopathy. JCI Insight 8, e157956 (2023).
Ziegler, K. A. et al. Neural mechanisms in cardiovascular health and disease. Circ. Res. 136, 1233–1261 (2025).
Hawley, J. A., Hargreaves, M., Joyner, M. J. & Zierath, J. R. Integrative biology of exercise. Cell 159, 738–749 (2014).
Wichi, R. B., De Angelis, K., Jones, L. & Irigoyen, M. C. A brief review of chronic exercise intervention to prevent autonomic nervous system changes during the aging process. Clinics 64, 253–258 (2009).
Roh, J., Rhee, J., Chaudhari, V. & Rosenzweig, A. The role of exercise in cardiac aging. Circ. Res. 118, 279–295 (2016).
Iemitsu, M., Maeda, S., Jesmin, S., Otsuki, T. & Miyauchi, T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am. J. Physiol. Heart Circ. Physiol. 291, H1290–H1298 (2006).
Lerchenmüller, C. et al. Restoration of cardiomyogenesis in aged mouse hearts by voluntary exercise. Circulation 146, 412–426 (2022).
Fukuda, K., Kanazawa, H., Aizawa, Y., Ardell, J. L. & Shivkumar, K. Cardiac innervation and sudden cardiac death. Circ. Res. 116, 2005–2019 (2015).
Ieda, M. et al. Nerve growth factor is critical for cardiac sensory innervation and rescues neuropathy in diabetic hearts. Circulation 114, 2351–2363 (2006).
Kiss, T. et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 42, 527–546 (2020).
Szarvas, Z. et al. Effects of NAD+ supplementation with oral nicotinamide riboside on vascular health and cognitive function in older adults with peripheral artery disease: results from a pilot 4-week open-label clinical trial. J. Pharmacol. Exp. Ther. 392, 103607 (2025).
Dierickx, P. et al. Circadian REV-ERBs repress E4bp4 to activate NAMPT-dependent NAD+ biosynthesis and sustain cardiac function. Nat. Cardiovascular Res. 1, 45–58 (2022).
Weiss, E. P. & Fontana, L. Caloric restriction: powerful protection for the aging heart and vasculature. Am. J. Physiol. Heart Circ. Physiol. 301, H1205–H1219 (2011).
Anderson, R. M., Shanmuganayagam, D. & Weindruch, R. Caloric restriction and aging: studies in mice and monkeys. Toxicol. Pathol. 3, 47–51 (2009).
Barzilai, N., Banerjee, S., Hawkins, M., Chen, W. & Rossetti, L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J. Clin. Invest. 101, 1353–1361 (1998).
Crandall, D. L., Lai, F. M., Huggins, F. J., Tanikella, T. K. & Cervoni, P. Effect of caloric restriction on cardiac reactivity and beta-adrenoceptor concentration. Am. J. Physiol. Heart Circ. Physiol. 244, H444–H448 (1983).
Loh, J. S. et al. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal. Transduct. Target. Ther. 9, 37 (2024).
Polster, S. P. et al. Permissive microbiome characterizes human subjects with a neurovascular disease cavernous angioma. Nat. Commun. 11, 2659 (2020).
Lin, L. et al. The brain-protective mechanism of fecal microbiota transplantation from young donor mice in the natural aging process via exosome, gut microbiota, and metabolomics analyses. Pharmacol. Res. 207, 107323 (2024).
Kim, C.-S. et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. A Biol. Sci. Med. Sci. 76, 32–40 (2021).
Zhang, C., Wang, Y., Wang, D., Zhang, J. & Zhang, F. NSAID exposure and risk of Alzheimer’s disease: an updated meta-analysis from cohort studies. Front. Aging Neurosci. 10, 83 (2018).
Everett, B. M. et al. Inhibition of Interleukin-1β and reduction in atherothrombotic cardiovascular events in the CANTOS trial. J. Am. Coll. Cardiol. 76, 1660–1670 (2020).
Finocchiaro, S. et al. Anti-inflammatory pharmacotherapy in patients with cardiovascular disease. Eur. Heart J. Cardiovasc. Pharmacother. 19, pvaf058 (2025).
Koopman, F. A. et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 113, 8284–8289 (2016).
Tynan, A., Brines, M. & Chavan, S. S. Control of inflammation using non-invasive neuromodulation: past, present and promise. Int. Immunol. 34, 119–128 (2022).
Zandstra, T. E. et al. Asymmetry and heterogeneity: part and parcel in cardiac autonomic innervation and function. Front. Physiol. 12, 665298 (2021).
Nyúl-Tóth, Á. et al. Novel intravital approaches to quantify deep vascular structure and perfusion in the aging mouse brain using ultrasound localization microscopy (ULM). J. Cereb. Blood Flow Metab. 44, 1378–1396 (2024).
Vinegoni, C., Aguirre, A. D., Lee, S. & Weissleder, R. Imaging the beating heart in the mouse using intravital microscopy techniques. Nat. Protoc. 10, 1802–1819 (2015).
Ahn, S., Yoon, J. & Kim, P. Intravital imaging of cardiac tissue utilizing tissue-stabilized heart window chamber in live animal model. Eur. Heart J. Imaging Methods Pract. 12, qyae062 (2024).
Borrelli, C., Bengel, F. M. & Gimelli, A. Integrated catecholamine imaging in heart and lung across different cardiopulmonary disorders: a systems-based pilot analysis. Int. J. Cardiol. 390, 131208 (2023).
Mitsui, J. et al. Pathology of the sympathetic nervous system corresponding to the decreased cardiac uptake in 123I-metaiodobenzylguanidine (MIBG) scintigraphy in a patient with Parkinson disease. J. Neurol. Sci. 243, 101–104 (2006).
Orimo, S., Yogo, M., Nakamura, T., Suzuki, M. & Watanabe, H. 123I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in α-synucleinopathies. Ageing Res. Rev. 30, 122–133 (2016).
Acknowledgements
We thank H. Kujundzic (Goethe University Frankfurt, Germany) for providing fluorescence images of the cardiac neurovasculome. J.U.G.W. is supported by the Dr Rolf-M.-Schwiete Foundation (2021-002). J.U.G.W. and S.D. are supported by the German Research Foundation (SFB1366 No. 394046768). S.D. is supported by the European Research Council (GAP – 101053352, Neuroheart) and the German Research Foundation (SFB1531 No. 456687919, the Cluster of Excellence Cardiopulmonary Institute Exc2026/1).
Author information
Authors and Affiliations
Contributions
Both authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Stefano Tarantini and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Cardiac neurovascular unit
-
A microscale concept describing the intimate structural and functional association between neurons, vascular cells (endothelial cells, pericytes and vascular smooth muscle cells) and the extracellular matrix at a given microvascular segment (arteriole, capillary or venule) in the heart.
- Cardiac neurovasculome
-
A macroscale systems concept denoting the totality of all neurovascular units, including all vascular and nervous structures, in the heart.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wagner, J.U.G., Dimmeler, S. Neurovascular interactions in the ageing heart. Nat Rev Cardiol (2026). https://doi.org/10.1038/s41569-026-01260-4
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41569-026-01260-4