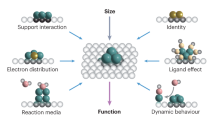
Abstract
Oxides are integral to heterogeneous catalysis, serving critical roles such as catalyst supports, active materials and electrodes. A highly ordered subset, single-crystalline oxides, have traditionally been used as model catalyst supports in fundamental surface science studies. However, advancements in bulk synthesis have rendered their general use more feasible for real-world applications. In this review, we explore the efficiency of single-crystalline oxides as active metals and supports across a wide range of heterogeneous processes, often performing exceptionally well. Beginning with synthetic methods, we discuss the advantages of single-crystalline oxides in thermo-, electro- and photocatalysis. Previously held conventions about catalytic activity, deactivation and surface–adsorbate interactions are re-evaluated by understanding how these ordered materials behave during the respective reactions. Last, we assess advances in characterization techniques and their impact on designing the next generation of catalysts based on single-crystalline oxides.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Chen, S., Xiong, F. & Huang, W. Surface chemistry and catalysis of oxide model catalysts from single crystals to nanocrystals. Surf. Sci. Rep. 74, 100471 (2019).
Goodman, D. W. Catalysis: from single crystals to the ‘real world’. Surf. Sci. 299-300, 837–848 (1994).
Oosterbeek, H. Bridging the pressure and material gap in heterogeneous catalysis: cobalt Fischer–Tropsch catalysts from surface science to industrial application. Phys. Chem. Chem. Phys. 9, 3570–3576 (2007).
Esposito, D. Mind the gap. Nat. Catal. 1, 807–808 (2018).
Huang, W. X. Oxide nanocrystal model catalysts. Acc. Chem. Res. 49, 520–527 (2016).
Somorjai, G. A. & Park, J. Y. Molecular surface chemistry by metal single crystals and nanoparticles from vacuum to high pressure. Chem. Soc. Rev. 37, 2155–2162 (2008).
Liu, L. C. & Corma, A. Structural transformations of solid electrocatalysts and photocatalysts. Nat. Rev. Chem. 5, 256–276 (2021).
Zaera, F. Shape-controlled nanostructures in heterogeneous catalysis. ChemSusChem 6, 1797–1820 (2013).
Liang, S. X., Zhang, L. C., Reichenberger, S. & Barcikowski, S. Design and perspective of amorphous metal nanoparticles from laser synthesis and processing. Phys. Chem. Chem. Phys. 23, 11121–11154 (2021).
Song, Y. et al. Dry reforming of methane by stable Ni-Mo nanocatalysts on single-crystalline MgO. Science 367, 777–781 (2020). This paper introduces the new nanocatalysts on single crystal edges (NOSCE) technique in heterogeneous catalysis.
Briega-Martos, V. & Yang, Y. Single-crystal metals and oxides as atomically precise energy materials platforms for fundamental electrocatalysis. Acc. Mater. Res. 5, 518–522 (2024).
Fu, C. et al. Spontaneous bulk-surface charge separation of TiO2-{001} nanocrystals leads to high activity in photocatalytic methane combustion. ACS Catal. 12, 6457–6463 (2022).
Berry, T., Ng, N. & McQueen, T. M. Tools and tricks for single crystal growth. Chem. Mater. 36, 4929–4944 (2024).
Jin, S. & Ruoff, R. S. Preparation and uses of large area single crystal metal foils. Apl. Mater. 7, 100905 (2019).
Birks, L. S., Hurley, J. W. & Sweeney, W. E. Perfection of ruby laser crystals. J. Appl. Phys. 36, 3562–3565 (1965).
Müller, G. The czochralski method — where we are 90 years after Jan Czochralski’s invention. Cryst. Res. Technol. 42, 1150–1161 (2007).
Kato, H., Kobayashi, M., Hara, M. & Kakihana, M. Fabrication of SrTiO3 exposing characteristic facets using molten salt flux and mprovement of photocatalytic activity for water splitting. Catal. Sci. Technol. 3, 1733–1738 (2013).
Lyu, S. C., Zhang, Y., Lee, C. J., Ruh, H. & Lee, H. J. Low-temperature growth of ZnO nanowire array by a simple physical vapor-deposition method. Chem. Mater. 15, 3294–3299 (2003).
Li, W.-N., Yuan, J., Gomez-Mower, S., Sithambaram, S. & Suib, S. L. Synthesis of single crystal manganese oxide octahedral molecular sieve (OMS) nanostructures with tunable tunnels and shapes. J. Phys. Chem. B 110, 3066–3070 (2006).
Zhang, L. H., Wu, J. J., Liao, H. B., Hou, Y. L. & Gao, S. Octahedral Fe3O4 nanoparticles and their assembled structures. Chem. Commun. 4378–4380 (2009).
Wang, X. et al. Synthesis of single-crystalline Co3O4 octahedral cages with tunable surface aperture and their lithium storage properties. J. Phys. Chem. C. 113, 15553–15558 (2009).
Corbett, J. D. in Survey of Progress in Chemistry Vol. 2 (ed. Scott, A. F.) 91–154 (Elsevier, 1964).
Fisher, I. R., Shapiro, M. C. & Analytis, J. G. Principles of crystal growth of intermetallic and oxide compounds from molten solutions. Phil. Mag. 92, 2401–2435 (2012).
Voronkova, V. I., Yanovskii, V. K., Vodolazskaya, I. V. & Shubentsova, E. S. in Growth of Crystals (eds Givargizov, E. I. & Grinberg, S. A.) 111–127 (Springer, 1993).
Boltersdorf, J., King, N. & Maggard, P. A. Flux-mediated crystal growth of metal oxides: synthetic tunability of particle morphologies, sizes, and surface features for photocatalysis research. CrystEngComm 17, 2225–2241 (2015).
Gupta, S. K. & Mao, Y. A review on molten salt synthesis of metal oxide nanomaterials: status, opportunity, and challenge. Prog. Mater. Sci. 117, 100734 (2021).
Jiang, Z.-Y. et al. Molten salt route toward the growth of ZnO nanowires in unusual growth directions. J. Phys. Chem. B 109, 23269–23273 (2005).
Ke, X. et al. Molten salt synthesis of single-crystal Co3O4 nanorods. Mater. Lett. 61, 3901–3903 (2007).
Rockett, A. in The Materials Science of Semiconductors (ed. Rockett, A.) 505–572 (Springer, 2008).
Pan, Z. W., Dai, Z. R. & Wang, Z. L. Nanobelts of semiconducting oxides. Science 291, 1947–1949 (2001).
Sandana, V. E. et al. Comparison of ZnO nanostructures grown using pulsed laser deposition, metal organic chemical vapor deposition, and physical vapor transport. J. Vac. Sci. Technol. B 27, 1678–1683 (2009).
Muth, J. F., Kolbas, R. M., Sharma, A. K., Oktyabrsky, S. & Narayan, J. Excitonic structure and absorption coefficient measurements of ZnO single crystal epitaxial films deposited by pulsed laser deposition. J. Appl. Phys. 85, 7884–7887 (1999).
Wu, J.-J. & Liu, S.-C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 14, 215–218 (2002).
Mathur, S., Barth, S., Werner, U., Hernandez-Ramirez, F. & Romano-Rodriguez, A. Chemical vapor growth of one-dimensional magnetite nanostructures. Adv. Mater. 20, 1550–1554 (2008).
Haddad, K. et al. Growth of single crystal, oriented SnO2 nanocolumn arrays by aerosol chemical vapour deposition. CrystEngComm 18, 7544–7553 (2016).
Whittingham, M. S. Hydrothermal synthesis of transition metal oxides under mild conditions. Curr. Opin. Solid. State Mater. Sci. 1, 227–232 (1996).
Rabenau, A. The role of hydrothermal synthesis in preparative chemistry. Angew. Chem. Int. Ed. Engl. 24, 1026–1040 (1985).
Beck, J. S. et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 114, 10834–10843 (1992).
Cundy, C. S. & Cox, P. A. The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem. Rev. 103, 663–702 (2003).
Kardash, T. Y. et al. The evolution of the M1 local structure during preparation of VMoNbTeO catalysts for ethane oxidative dehydrogenation to ethylene. RSC Adv. 8, 35903–35916 (2018).
Dordevic, T., Wittwer, A., Jaglicic, Z. & Djerdj, I. Hydrothermal synthesis of single crystal CoAs2O4 and NiAs2O4 compounds and their magnetic properties. RSC Adv. 5, 18280–18287 (2015).
Zhang, S. W. & Chen, G. Z. Manganese oxide based materials for supercapacitors. Energy Mater. 3, 186–200 (2008).
Yuan, Z.-Y., Zhang, Z., Du, G., Ren, T.-Z. & Su, B.-L. A simple method to synthesise single-crystalline manganese oxide nanowires. Chem. Phys. Lett. 378, 349–353 (2003).
Wang, X. & Li, Y. Selected-control hydrothermal synthesis of α- and β-MnO2 single crystal nanowires. J. Am. Chem. Soc. 124, 2880–2881 (2002).
Fang, K.-M. et al. Gelatin-assisted hydrothermal synthesis of single crystalline zinc oxide nanostars and their photocatalytic properties. J. Colloid Interface Sci. 402, 68–74 (2013).
Feng, X. et al. Vertically aligned single crystal TiO2 nanowire arrays grown directly on transparent conducting oxide coated glass: synthesis details and applications. Nano Lett. 8, 3781–3786 (2008).
Mulinari, T. A. et al. Microwave-hydrothermal synthesis of single-crystalline Co3O4 spinel nanocubes. CrystEngComm 15, 7443–7449 (2013).
Hench, L. L. & West, J. K. The sol-gel process. Chem. Rev. 90, 33–72 (1990).
Li, G.-R. et al. Electrochemical synthesis of nanostructured materials for electrochemical energy conversion and storage. Nanoscale 5, 4056–4069 (2013).
Dong, H., Chen, Y. C. & Feldmann, C. Polyol synthesis of nanoparticles: status and options regarding metals, oxides, chalcogenides, and non-metal elements. Green Chem. 17, 4107–4132 (2015).
Kumar, A., Kuang, Y., Liang, Z. & Sun, X. Microwave chemistry, recent advancements, and eco-friendly microwave-assisted synthesis of nanoarchitectures and their applications: a review. Mater. Today Nano 11, 100076 (2020).
Patzke, G. R., Zhou, Y., Kontic, R. & Conrad, F. Oxide nanomaterials: synthetic developments, mechanistic studies, and technological innovations. Angew. Chem. Int. Ed. Engl. 50, 826–859 (2011).
Zitoun, D., Pinna, N., Frolet, N. & Belin, C. Single crystal manganese oxide multipods by oriented attachment. J. Am. Chem. Soc. 127, 15034–15035 (2005).
Kim, M. H., Lim, B., Lee, E. P. & Xia, Y. Polyol synthesis of Cu2O nanoparticles: use of chloride to promote the formation of a cubic morphology. J. Mater. Chem. 18, 4069–4073 (2008).
Mourdikoudis, S. & Liz-Marzán, L. M. Oleylamine in nanoparticle synthesis. Chem. Mater. 25, 1465–1476 (2013).
Mourdikoudis, S. et al. Oleic acid/oleylamine ligand pair: a versatile combination in the synthesis of colloidal nanoparticles. Nanoscale Horiz. 7, 941–1015 (2022).
Srinivas, B., Pandit, M. A. & Muralidharan, K. Importance of clean surfaces on the catalyst: SnS2 nanorings for environmental remediation. ACS Omega 4, 14970–14980 (2019).
Qiu, C. W. et al. Visualising Co nanoparticle aggregation and encapsulation in Co/TiO2 catalysts and its mitigation through surfactant residues. J. Catal. 419, 58–67 (2023).
Devi, N., Sahoo, S., Kumar, R. & Singh, R. K. A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 13, 11679–11711 (2021).
Joshi, U. A., Jang, J. S., Borse, P. H. & Lee, J. S. Microwave synthesis of single-crystalline perovskite BiFeO3 nanocubes for photoelectrode and photocatalytic applications. Appl. Phys. Lett. 92, 242106 (2008).
Kaur, A., Bajaj, B., Kaushik, A., Saini, A. & Sud, D. A review on template assisted synthesis of multi-functional metal oxide nanostructures: status and prospects. Mater. Sci. Eng. B 286, 116005 (2022).
Ajayan, P. M., Stephan, O., Redlich, P. & Colliex, C. Carbon nanotubes as removable templates for metal oxide nanocomposites and nanostructures. Nature 375, 564–567 (1995).
Wang, X., Yu, L., Hu, P. & Yuan, F. Synthesis of single-crystalline hollow octahedral NiO. Cryst. Growth Des. 7, 2415–2418 (2007).
Yang, H. et al. One-step nanocasting synthesis of highly ordered single crystalline indium oxide nanowire arrays from mesostructured frameworks. J. Am. Chem. Soc. 125, 4724–4725 (2003).
Yue, Zhou Synthesis of porous single crystals of metal oxides via a solid−liquid route. Chem. Mater. 19, 2359–2363 (2007).
Zheng, X. et al. Close-packed colloidal SiO2 as a nanoreactor: generalized synthesis of metal oxide mesoporous single crystals and mesocrystals. Chem. Mater. 26, 5700–5709 (2014).
Varma, A., Mukasyan, A. S., Rogachev, A. S. & Manukyan, K. V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 116, 14493–14586 (2016).
Nguyen, T.-S. et al. Ultrastable iridium–ceria nanopowders synthesized in one step by solution combustion for catalytic hydrogen production. J. Mater. Chem. A 2, 19822–19832 (2014).
Cai, Z. & Li, J. Facile synthesis of single crystalline SnO2 nanowires. Ceram. Int. 39, 377–382 (2013).
Merchan-Merchan, W., Saveliev, A. V. & Desai, M. Volumetric flame synthesis of well-defined molybdenum oxide nanocrystals. Nanotechnology 20, 475601 (2009).
Dong, Z., Al-Sharab, J. F., Kear, B. H. & Tse, S. D. Combined flame and electrodeposition synthesis of energetic coaxial tungsten-oxide/aluminum nanowire arrays. Nano Lett. 13, 4346–4350 (2013).
Teoh, W. Y., Amal, R. & Mädler, L. Flame spray pyrolysis: an enabling technology for nanoparticles design and fabrication. Nanoscale 2, 1324–1347 (2010).
Yan, J. K. et al. Advances in the synthesis of halide perovskite single crystals for optoelectronic applications. Chem. Mater. 35, 2683–2712 (2023).
Yun, Q. B. et al. Recent progress on phase engineering of nanomaterials. Chem. Rev. 123, 13489–13692 (2023).
Leybo, D. et al. Metal–support interactions in metal oxide-supported atomic, cluster, and nanoparticle catalysis. Chem. Soc. Rev. 53, 10450–10490 (2024).
Liu, L. C. et al. Crystal-plane effects on the catalytic properties of Au/TiO2. ACS Catal. 3, 2768–2775 (2013).
Zhang, Y. S. et al. Structure sensitivity of Au-TiO2 strong metal-support interactions. Angew. Chem. Int. Ed. Engl. 60, 12074–12081 (2021).
Lang, R. et al. Non defect-stabilized thermally stable single-atom catalyst. Nat. Commun. 10, 234 (2019).
Cheng, F. Y., Duan, X. Y. & Xie, K. Dry reforming of CH4/CO2 by stable Ni nanocrystals on porous single-crystalline mgo monoliths at reduced temperature. Angew. Chem. Int. Ed. Engl. 60, 18792–18799 (2021).
Li, W., Zhao, Z. & Wang, G. Modulating morphology and textural properties of ZrO2 for supported Ni catalysts toward dry reforming of methane. AIChE J. 63, 2900–2915 (2017).
Xiao, Y. C. & Xie, K. Active exsolved metal-oxide interfaces in porous single-crystalline ceria monoliths for efficient and durable CH4/CO2 reforming. Angew. Chem. Int. Ed. Engl. 61, e202113079 (2021).
Song, Y.-Q., He, D.-H. & Xu, B.-Q. Effects of preparation methods of ZrO2 support on catalytic performances of Ni/ZrO2 catalysts in methane partial oxidation to syngas. Appl. Catal. A Gen. 337, 19–28 (2008).
Rodriguez, J. A., Liu, P., Hrbek, J., Evans, J. & Pérez, M. Water gas shift reaction on Cu and Au nanoparticles supported on CeO2(111) and ZnO(000-1): intrinsic activity and importance of support interactions. Angew. Chem. Int. Ed. Engl. 46, 1329–1332 (2007).
Si, R. & Flytzani-Stephanopoulos, M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water–gas shift reaction. Angew. Chem. Int. Ed. Engl. 47, 2884–2887 (2008). This paper shows how thermochemical catalyst performance can be tuned by the single-crystal shape.
Qiao, Z.-A., Wu, Z. & Dai, S. Shape-controlled ceria-based nanostructures for catalysis applications. ChemSusChem 6, 1821–1833 (2013).
Jomjaree, T. et al. Catalytic performance of Ni catalysts supported on CeO2 with different morphologies for low-temperature CO2 methanation. Catal. Today 375, 234–244 (2021).
Xiao, Y. C., Li, H. & Xie, K. Activating lattice oxygen at the twisted surface in a mesoporous CeO2 single crystal for efficient and durable catalytic CO oxidation. Angew. Chem. Int. Ed. Engl. 60, 5240–5244 (2021).
Luo, W. et al. Morphology and crystal-plane dependence of CeO2-TiO2 catalysts: activity and mechanism for the selective catalytic reduction of NOx with NH3. Chem. Eng. J. 444, 136488 (2022).
Kwok, K. M., Ong, S. W. D., Chen, L. & Zeng, H. C. Transformation of stöber silica spheres to hollow hierarchical single-crystal ZSM-5 zeolites with encapsulated metal nanocatalysts for selective catalysis. ACS Appl. Mater. Interfaces 11, 14774–14785 (2019).
Sun, M.-H. et al. Hierarchical zeolite single-crystal reactor for excellent catalytic efficiency. Matter 3, 1226–1245 (2020).
Qin, Z. et al. Preparation of single-crystal ‘House-of-Cards’-like ZSM-5 and their performance in ethanol-to-hydrocarbon conversion. Chem. Mater. 31, 4639–4648 (2019). This paper presents research on selectively increasing desired catalytic activity by introducing porosity into a single crystal.
Zhang, Q. et al. High-quality single-crystalline MFI-type nanozeolites: a facile synthetic strategy and MTP catalytic studies. Chem. Mater. 30, 2750–2758 (2018).
Over, H. Fundamental studies of planar single-crystalline oxide model electrodes (RuO2, IrO2) for acidic water splitting. ACS Catal. 11, 8848–8871 (2021).
Gayen, P., Saha, S. & Ramani, V. Pyrochlores for advanced oxygen electrocatalysis. Acc. Chem. Res. 55, 2191–2200 (2022).
Park, J. et al. Single crystalline pyrochlore nanoparticles with metallic conduction as efficient bi-functional oxygen electrocatalysts for Zn–air batteries. Energy Environ. Sci. 10, 129–136 (2017).
Tung, C.-W. et al. Reversible adapting layer produces robust single-crystal electrocatalyst for oxygen evolution. Nat. Commun. 6, 8106 (2015). This paper experimentally demonstrated the advantages of single-crystal catalyst supports compared with polycrystals in electrochemical catalysis reactions.
Ling, T. et al. Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis. Nat. Commun. 7, 12876 (2016). This paper explained that single-crystal catalysts exhibit better electrochemical activity than polycrystal catalysts.
Nong, S. et al. Well-dispersed ruthenium in mesoporous crystal TiO2 as an advanced electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 140, 5719–5727 (2018).
Liang, Y. et al. Crystal plane dependent electrocatalytic performance of NiS2 nanocrystals for hydrogen evolution reaction. J. Catal. 381, 63–69 (2020). This paper shows that electrochemical catalyst performance can be tuned depending on the single-crystal shape.
Gu, Z. et al. Oxygen vacancy tuning toward efficient electrocatalytic CO2 reduction to C2H4. Small Methods 3, 1800449 (2019).
Wang, X. et al. Morphology and mechanism of highly selective Cu(II) oxide nanosheet catalysts for carbon dioxide electroreduction. Nat. Commun. 12, 794 (2021).
Liu, H. et al. Highly efficient CO2 electrolysis within a wide operation window using octahedral tin oxide single crystals. J. Mater. Chem. A 9, 7848–7856 (2021).
Ye, L. T., Shang, Z. B. & Xie, K. Selective oxidative coupling of methane to ethylene in a solid oxide electrolyser based on porous single-crystalline CeO2 monoliths. Angew. Chem. Int. Ed. Engl. 61, e202207211 (2022).
Dong, C. Y. et al. Size-dependent activity and selectivity of carbon dioxide photocatalytic reduction over platinum nanoparticles. Nat. Commun. 9, 1252 (2018).
Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 48, 53–229 (2003).
Zeng, J. H., Jin, B. B. & Wang, Y. F. Facet enhanced photocatalytic effect with uniform single-crystalline zinc oxide nanodisks. Chem. Phys. Lett. 472, 90–95 (2009).
Debroye, E. et al. Facet-dependent photoreduction on single ZnO crystals. J. Phys. Chem. Lett. 8, 340–346 (2017).
Wu, N. et al. Shape-enhanced photocatalytic activity of single-crystalline anatase TiO2 (101) nanobelts. J. Am. Chem. Soc. 132, 6679–6685 (2010).
Gordon, T. R. et al. Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 134, 6751–6761 (2012). This paper investigates photocatalytic activity of single-crystal surfaces with various shapes through changing their synthetic protocols.
Xing, M. Y. et al. Enhanced photocatalysis by Au nanoparticle loading on TiO2 single-crystal (001) and (110) facets. J. Phys. Chem. Lett. 4, 3910–3917 (2013). This paper introduces research on enhancing photocatalytic activity by placing active metals on single crystals.
Parzinger, E. et al. Photocatalytic stability of single- and few-layer MoS2. ACS Nano 9, 11302–11309 (2015).
Kislov, N. et al. Photocatalytic degradation of methyl orange over single crystalline ZnO: orientation dependence of photoactivity and photostability of ZnO. Langmuir 25, 3310–3315 (2009).
Liu, J. et al. 2D ZnO mesoporous single-crystal nanosheets with exposed {0001} polar facets for the depollution of cationic dye molecules by highly selective adsorption and photocatalytic decomposition. Appl. Catal. B Environ. 181, 138–145 (2016).
Wu, W., Hao, R., Liu, F., Su, X. & Hou, Y. Single-crystalline α-Fe2O3 nanostructures: controlled synthesis and high-index plane-enhanced photodegradation by visible light. J. Mater. Chem. A 1, 6888–6894 (2013).
Lu, Y. et al. Facile synthesis of graphene-like copper oxide nanofilms with enhanced electrochemical and photocatalytic properties in energy and environmental applications. ACS Appl. Mater. Interfaces 7, 9682–9690 (2015).
Yin, G. et al. Photocatalytic carbon dioxide reduction by copper oxide nanocluster-grafted niobate nanosheets. ACS Nano 9, 2111–2119 (2015).
Li, S. et al. Ferroelectric polarization and thin-layered structure synergistically promoting CO2 photoreduction of Bi2MoO6. J. Mater. Chem. A 8, 9268–9277 (2020).
Han, Q. et al. Convincing synthesis of atomically thin, single-crystalline InVO4 sheets toward promoting highly selective and efficient solar conversion of CO2 into CO. J. Am. Chem. Soc. 141, 4209–4213 (2019).
Chen, S. et al. Facet-engineered surface and interface design of monoclinic scheelite bismuth vanadate for enhanced photocatalytic performance. ACS Catal. 10, 1024–1059 (2020).
Li, R. G. et al. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 4, 1432 (2013).
Yan, J. et al. Tungsten oxide single crystal nanosheets for enhanced multichannel solar light harvesting. Adv. Mater. 27, 1580–1586 (2015).
Pastor, E. et al. Complementary probes for the electrochemical interface. Nat. Rev. Chem. 8, 159–178 (2024).
Qiu, S. et al. Strategies for the synthesis of large zeolite single crystals. Micro. Meso. Mater. 21, 245–251 (1998).
Hauptman, H. A. The phase problem of X-ray crystallography. Rep. Prog. Phys. 54, 1427 (1991).
Brouwer, D. H., Darton, R. J., Morris, R. E. & Levitt, M. H. A solid-state NMR method for solution of zeolite crystal structures. J. Am. Chem. Soc. 127, 10365–10370 (2005).
Munn, J., Barnes, P., Häusermann, D., Axon, S. A. & Klinowski, J. In-situ studies of the hydrothermal synthesis of zeolites using synchrotron energy-dispersive X-ray diffraction. Phase Transit. 39, 129–134 (1992).
Harlow, G. S., Lundgren, E. & Escudero-Escribano, M. Recent advances in surface x-ray diffraction and the potential for determining structure-sensitivity relations in single-crystal electrocatalysis. Curr. Opin. Electrochem. 23, 162–173 (2020).
Rao, R. R. et al. Operando identification of site-dependent water oxidation activity on ruthenium dioxide single-crystal surfaces. Nat. Catal. 3, 516–525 (2020).
Thomas, A. G. et al. Comparison of the electronic structure of anatase and rutile TiO2 single-crystal surfaces using resonant photoemission and X-ray absorption spectroscopy. Phys. Rev. B 75, 035105 (2007).
Tepavcevic, S. et al. Fundamental insights from a single-crystal sodium iridate battery. Adv. Energ. Mater. 10, 1903128 (2020).
Addiego, C., Gao, W. P., Huyan, H. & Pan, X. Q. Probing charge density in materials with atomic resolution in real space. Nat. Rev. Phys. 5, 117–132 (2023).
Fang, S. et al. Atomic electrostatic maps of 1D channels in 2D semiconductors using 4D scanning transmission electron microscopy. Nat. Commun. 10, 1127 (2019).
Shibata, N. et al. Differential phase-contrast microscopy at atomic resolution. Nat. Phys. 8, 611–615 (2012).
Kohno, Y., Seki, T., Findlay, S. D., Ikuhara, Y. & Shibata, N. Real-space visualization of intrinsic magnetic fields of an antiferromagnet. Nature 602, 234–239 (2022).
Nan, P. et al. Visualizing the Mg atoms in Mg3Sb2 thermoelectrics using advanced iDPC-STEM technique. Mater. Today Phys. 21, 100524 (2021).
Wang, C. L. et al. CO-induced dimer decay responsible for gem-dicarbonyl formation on a model single-atom catalyst. Angew. Chem. Int. Ed. Engl. 63, e202317347 (2024).
Kraushofer, F. et al. Surface reduction state determines stabilization and incorporation of Rh on α-Fe2O3(11-02). Adv. Mater. Interfaces 8, 2001908 (2021).
Nguyen, L., Tao, F. F., Tang, Y., Dou, J. & Bao, X.-J. Understanding catalyst surfaces during catalysis through near ambient pressure X-ray photoelectron spectroscopy. Chem. Rev. 119, 6822–6905 (2019).
Tao, F. Design of an in-house ambient pressure AP-XPS using a bench-top X-ray source and the surface chemistry of ceria under reaction conditions. Chem. Commun. 48, 3812–3814 (2012).
Shah, S. A. & Baldelli, S. Chemical imaging of surfaces with sum frequency generation vibrational spectroscopy. Acc. Chem. Res. 53, 1139–1150 (2020).
Rupprechter, G. Surface vibrational spectroscopy from ultrahigh vacuum to atmospheric pressure: adsorption and reactions on single crystals and nanoparticle model catalysts monitored by sum frequency generation spectroscopy. Phys. Chem. Chem. Phys. 3, 4621–4632 (2001).
Zilli, A. et al. Frequency tripling via sum-frequency generation at the nanoscale. ACS Photonics 8, 1175–1182 (2021).
Yamaguchi, S., Suzuki, Y., Nojima, Y. & Otosu, T. Perspective on sum frequency generation spectroscopy of ice surfaces and interfaces. Chem. Phys. 522, 199–210 (2019).
Kennedy, G., Baker, L. R. & Somorjai, G. A. Selective amplification of C = O bond hydrogenation on Pt/TiO2: catalytic reaction and sum- frequency generation vibrational spectroscopy studies of crotonaldehyde hydrogenation. Angew. Chem. Int. Ed. Engl. 53, 3405–3408 (2014).
Neri, G., Walsh, J. J., Teobaldi, G., Donaldson, P. M. & Cowan, A. J. Detection of catalytic intermediates at an electrode surface during carbon dioxide reduction by an earth-abundant catalyst. Nat. Catal. 1, 952–959 (2018).
Gardner, A. M. et al. Potential dependent reorientation controlling activity of a molecular electrocatalyst. J. Am. Chem. Soc. 146, 7130–7134 (2024).
Campbell, C. T. The energetics of supported metal nanoparticles: relationships to sintering rates and catalytic activity. Acc. Chem. Res. 46, 1712–1719 (2013).
Luo, L., Hernandez, R., Zhou, X.-D. & Yan, H. Heterogeneous catalysis at metal-oxide interfaces using in situ and operando spectroscopy: from nanoparticles to single-atom sites. Appl. Catal. A Gen. 624, 118330 (2021).
Zhou, Y., Doronkin, D. E., Chen, M. L., Wei, S. Q. & Grunwaldt, J. D. Interplay of Pt and crystal facets of TiO2: CO oxidation activity and XAS/DRIFTS studies. ACS Catal. 6, 7799–7809 (2016).
Zeinalipour-Yazdi, C. D., Willock, D. J., Thomas, L., Wilson, K. & Lee, A. F. CO adsorption over Pd nanoparticles: a general framework for IR simulations on nanoparticles. Surf. Sci. 646, 210–220 (2016).
Fois, E. & Tabacchi, G. in Tailored Functional Oxide Nanomaterials (eds Maccato, C. & Barreca, D.) 111–136 (Wiley–VCH, 2022).
Rousseau, R., Glezakou, V. A. & Selloni, A. Theoretical insights into the surface physics and chemistry of redox-active oxides. Nat. Rev. Mater. 5, 460–475 (2020).
Grönbeck, H. in Metal Oxide Nanoparticles (eds Diwald, O. & Berger, T.) 693–710 (Wiley, 2021).
Demkov, A. A., Fredrickson, K. D., Seo, H. & O’Hara, A. in Handbook of Materials Modeling. Applications: Current and Emerging Materials (eds Andreoni, W. & Yip, S.) 1–30 (Springer, 2018).
Liu, L. C. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Abriata, J. P. & Laughlin, D. E. The third law of thermodynamics and low temperature phase stability. Prog. Mater. Sci. 49, 367–387 (2004).
Muhammad, P. et al. Defect engineering in nanocatalysts: from design and synthesis to applications. Adv. Func. Mater. 34, e202314686 (2024).
Milisavljevic, I. & Wu, Y. Current status of solid-state single crystal growth. BMC Mater. 2, 2 (2020).
Idriss, H. & Barteau, M. A. Active sites on oxides: from single crystals to catalysts. Adv. Catal. 45, 261–331 (2000).
Kim, J. H., Suh, D. J., Park, T. J. & Kim, K. L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni-alumina aerogel catalysts. Appl. Catal. A Gen. 197, 191–200 (2000).
Acknowledgements
This work is funded by King Abdullah University of Science and Technology (KAUST).
Author information
Authors and Affiliations
Contributions
S.-J.K. and R.V.M.-G. contributed equally and wrote the manuscript. P.B. and J.M. provided sections and editing. C.T.Y. conceived, wrote, edited, and supervised the review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, SJ., Maligal-Ganesh, R.V., Mahmood, J. et al. Structural control over single-crystalline oxides for heterogeneous catalysis. Nat Rev Chem 9, 397–414 (2025). https://doi.org/10.1038/s41570-025-00715-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41570-025-00715-5
This article is cited by
-
Dynamics in electrochemical organic oxidation reactions from in situ and operando techniques
Nature Reviews Chemistry (2025)