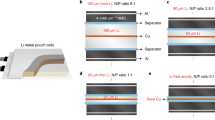
Abstract
The combination of Li-rich layered oxide cathodes and lithium metal anodes enables lithium metal batteries (LMBs) to achieve specific energies exceeding 600 Wh kg−1, which is a crucial threshold requiring the activation of anionic oxygen-redox of cathode. The specific energy is attained owing to oxygen-redox reactions at the cathode and reversible Li plating–stripping at the anode, but these processes also induce distinct failure mechanisms. Structural destabilization at the cathode and anodic dendrite growth cause cell-level failures that impact the lifespan of LMBs more profoundly than material degradation alone. Moreover, the presence of lithium metal anodes obscures the detection of active Li loss, often leading to misinterpretations related to capacity fading and cycle life. This Review examines the progress in realizing 600 Wh kg−1 LMBs and understanding their lifespan failure mechanisms. We discuss the challenges in accurately assessing the lifespan and Li loss pathways of LMBs, and we elucidate the fundamental chemistry mechanisms driving both material-level and cell-level failures. In particular, the electrochemical implications of cell parameters, cell assembly and operating conditions on the lifespan are highlighted. We also outline the gaps in knowledge and advanced techniques required to decipher detailed failure modes for LMBs with oxygen-redox reactions and Li plating–stripping.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Li, Q., Yu, X., Li, H. & Chen, L. The road towards high-energy-density batteries. Innov. Energy 1, 100005 (2024).
Choi, J. W. & Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 1, 1–16 (2016).
Shi, Z. et al. Self-regulatory lean-electrolyte flow for building 600 Wh kg−1-level rechargeable lithium batteries. Adv. Mater. 37, 2419377 (2025).
Qiu, B. et al. Negative thermal expansion and oxygen-redox electrochemistry. Nature 640, 941–946 (2025).
Fang, C., Wang, X. & Meng, Y. S. Key issues hindering a practical lithium-metal anode. Trends Chem. 1, 152–158 (2019).
Zhang, M. et al. Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage. Nat. Rev. Mater. 7, 522–540 (2022).
Qiu, B., Qiao, Y., Li, B. & Liu, Z. Next-generation cathode materials for ultrahigh-energy batteries. Next Mater. 1, 100034 (2023).
Rinkel, B. L., Hall, D. S., Temprano, I. & Grey, C. P. Electrolyte oxidation pathways in lithium-ion batteries. J. Am. Chem. Soc. 142, 15058–15074 (2020).
He, X. et al. The passivity of lithium electrodes in liquid electrolytes for secondary batteries. Nat. Rev. Mater. 6, 1036–1052 (2021).
Hobold, G. M. et al. Moving beyond 99.9% coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951–960 (2021).
Yang, Y. et al. High-efficiency lithium-metal anode enabled by liquefied gas electrolytes. Joule 3, 1986–2000 (2019). This work reports an average CELi of 99.9%.
Yang, W., Chen, A., He, P. & Zhou, H. Advancing lithium metal electrode beyond 99.9% coulombic efficiency via super-saturated electrolyte with compressed solvation structure. Nat. Commun. 16, 1–12 (2025).
Liu, J. et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 4, 180–186 (2019). This perspective highlights the need to integrate key material and cell design principles in LMB research and evaluation.
Hatzell, K. et al. Aligning lithium metal battery research and development across academia and industry. Joule 8, 1550–1555 (2024). This commentary highlights the need to align LMB academic research with practical industrial development.
Zhang, K. et al. A high-performance lithium metal battery with ion-selective nanofluidic transport in a conjugated microporous polymer protective layer. Adv. Mater. 33, 2006323 (2021).
Zhao, P. et al. Constructing self-adapting electrostatic interface on lithium metal anode for stable 400 Wh kg−1 pouch cells. Adv. Energy Mater. 12, 2200568 (2022).
Wang, Z. et al. High-performance localized high-concentration electrolytes by diluent design for long-cycling lithium metal batteries. Chin. Chem. Lett. 35, 108570 (2024).
Zhang, Q. K. et al. Reforming the uniformity of solid electrolyte interphase by nanoscale structure regulation for stable lithium metal batteries. Angew. Chem. Int. Ed. 135, e202306889 (2023).
Wang, Z. et al. Highly soluble organic nitrate additives for practical lithium metal batteries. Carbon Energy 5, e283 (2023).
Zhang, Y. et al. Enabling 420 Wh kg−1 stable lithium-metal pouch cells by lanthanum doping. Adv. Mater. 35, 2211032 (2023).
Zhang, Q.-K. et al. Homogeneous and mechanically stable solid–electrolyte interphase enabled by trioxane-modulated electrolytes for lithium metal batteries. Nat. Energy 8, 725–735 (2023).
Zhang, S. et al. Oscillatory solvation chemistry for a 500 Wh kg−1 Li-metal pouch cell. Nat. Energy 9, 1285–1296 (2024).
Guo, J. C. et al. A self-reconfigured, dual-layered artificial interphase toward high-current-density quasi-solid-state lithium metal batteries. Adv. Mater. 35, 2300350 (2023).
Ma, Q. et al. Formulating the electrolyte towards high-energy and safe rechargeable lithium–metal batteries. Angew. Chem. Int. Ed. 60, 16554–16560 (2021).
Deng, W. et al. Competitive solvation-induced concurrent protection on the anode and cathode toward a 400 Wh kg–1 lithium metal battery. ACS Energy Lett. 6, 115–123 (2020).
Qiao, R. et al. Non-fluorinated electrolytes with micelle-like solvation for ultra-high energy density lithium metal batteries. Chem 11, 102306 (2024).
Tang, T. et al. Long-lifespan 522 Wh kg−1 lithium metal pouch cell enabled by compound additives engineering. Angew. Chem. Int. Ed. 64, e202417471 (2025).
Jie, Y. et al. Towards long-life 500 Wh kg−1 lithium metal pouch cells via compact ion-pair aggregate electrolytes. Nat. Energy 9, 987–998 (2024).
Su, H. et al. Achieving practical high-energy-density lithium-metal batteries by a dual-anion regulated electrolyte. Adv. Mater. 35, 2301171 (2023).
Ji, H. et al. Liquid–liquid interfacial tension stabilized Li-metal batteries. Nature 643, 1255–1262 (2025).
Wei, Z. et al. Eliminating oxygen releasing of Li-rich layered cathodes by tuning the distribution of superlattice domain. Mater. Today Energy 27, 101039 (2022).
Luo, P. et al. Understanding and mitigating acidic species in all-fluorinated electrolytes for a stable 572 Wh kg−1 lithium metal battery (LMB). Energy Storage Mater. 78, 104234 (2025).
Liu, X. et al. 570 Wh kg−1-grade lithium metal pouch cell with 4.9 V highly Li+ conductive armor-like cathode electrolyte interphase via partially fluorinated electrolyte engineering. Adv. Mater. 36, 2401505 (2024).
Huang, H. et al. Delocalized electrolyte design enables 600 Wh kg−1 lithium metal pouch cells. Nature 644, 660–667 (2025).
He, Y. et al. Optimizing Li plating behavior via controlling areal capacity of a cathode for cycling stability on 600 Wh kg–1 lithium-metal batteries. ACS Appl. Mater. Interfaces 16, 33475–33484 (2024).
Li, Q., Yang, Y., Yu, X. & Li, H. A 700 W⋅h⋅kg−1 rechargeable pouch type lithium battery. Chin. Phys. Lett. 40, 048201 (2023). This work reports the 700 Wh kg−1LMB prototype by using LMA and LLOs.
He, M. et al. Industry needs for practical lithium-metal battery designs in electric vehicles. Nat. Energy 9, 1199–1205 (2024).
Menkin, S. et al. Insights into soft short circuit-based degradation of lithium metal batteries. Faraday Discuss. 248, 277–297 (2024).
Deng, W. et al. Quantification of reversible and irreversible lithium in practical lithium-metal batteries. Nat. Energy 7, 1031–1041 (2022). This work reports the method for quantifying CELi in Ah-level LMBs.
Wood, K. N. et al. Dendrites and pits: untangling the complex behavior of lithium metal anodes through operando video microscopy. ACS Cent. Sci. 2, 790–801 (2016).
Chen, K. H. et al. Dead lithium: mass transport effects on voltage, capacity, and failure of lithium metal anodes. J. Mater. Chem. A 5, 11671–11681 (2017). This work highlights the presence and impact of the dead Li layer on the degradation pathways of LMBs.
Lu, D. et al. Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv. Energy Mater. 5, 1400993 (2015).
Xiang, Y. X. et al. Quantitatively analyzing the failure processes of rechargeable Li metal batteries. Sci. Adv. 7, eabj3423 (2021).
Zeng, L. et al. Voltage decay of Li-rich layered oxides: mechanism, modification strategies, and perspectives. Adv. Funct. Mater. 33, 2213260 (2023).
House, R. A. et al. First-cycle voltage hysteresis in Li-rich 3d cathodes associated with molecular O2 trapped in the bulk. Nat. Energy 5, 777–785 (2020).
Xu, B., Fell, C. R., Chi, M. & Meng, Y. S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: a joint experimental and theoretical study. Energy Environ. Sci. 4, 2223–2233 (2011).
Yan, P. et al. Injection of oxygen vacancies in the bulk lattice of layered cathodes. Nat. Nanotechnol. 14, 602–608 (2019).
Li, X. et al. Dependence of initial capacity irreversibility on oxygen framework chemistry in Li-rich layered cathode oxides. Energy Environ. Mater. 7, e12722 (2024).
Liu, T. et al. Origin of structural degradation in Li-rich layered oxide cathode. Nature 606, 305–312 (2022). This work highlights the role of lattice strain or displacement in driving voltage decay and oxygen loss in LLOs.
Croy, J. R., Balasubramanian, M., Gallagher, K. G. & Burrell, A. K. Review of the US Department of Energy’s “deep dive” effort to understand voltage fade in Li- and Mn-rich cathodes. Acc. Chem. Res. 48, 2813–2821 (2015).
Eum, D. et al. Voltage decay and redox asymmetry mitigation by reversible cation migration in lithium-rich layered oxide electrodes. Nat. Mater. 19, 419–427 (2020).
Wang, E. et al. Al/Ti synergistic doping enhanced cycle stability of Li-rich layered oxides. Adv. Funct. Mater. 32, 2201744 (2022).
Qing, R. P. et al. Enhancing the kinetics of Li-rich cathode materials through the pinning effects of gradient surface Na+ doping. Adv. Energy Mater. 6, 1501914 (2016).
Li, Q. et al. K+-doped Li1.2Mn0.54Co0.13Ni0.13O2: a novel cathode material with an enhanced cycling stability for lithium-ion batteries. ACS Appl. Mater. Interfaces 6, 10330–10341 (2014).
Wang, M., Chen, L., Liu, M., Chen, Y. & Gu, Y. Enhanced electrochemical performance of La-doped Li-rich layered cathode material. J. Alloys Compd. 848, 156620 (2020).
Gao, Y., Wang, X., Ma, J., Wang, Z. & Chen, L. Selecting substituent elements for Li-rich Mn-based cathode materials by density functional theory (DFT) calculations. Chem. Mater. 27, 3456–3461 (2015).
Wang, M. et al. Enhanced electrochemical performances of cerium-doped Li-Rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode materials. J. Alloys Compd. 861, 158000 (2021).
Peng, Z. et al. Enhanced electrochemical performance of layered Li-rich cathode materials for lithium ion batteries via aluminum and boron dual-doping. Ceram. Int. 45, 4184–4192 (2019).
Seaby, T., Lin, T.-E., Hu, Y.-X., Yuan, Q.-H. & Wang, L.-Z. An analysis of F-doping in Li-rich cathodes. Rare Met. 41, 1771–1796 (2022).
Yan, H., Li, B., Yu, Z., Chu, W. & Xia, D. First-principles study: tuning the redox behavior of lithium-rich layered oxides by chlorine doping. J. Phys. Chem. C 121, 7155–7163 (2017).
Nayak, P. K. et al. Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries. Adv. Energy Mater. 6, 1502398 (2016).
Dahiya, P., Ghanty, C., Sahoo, K., Basu, S. & Majumder, S. Suppression of voltage decay and improvement in electrochemical performance by zirconium doping in Li-rich cathode materials for Li-ion batteries. J. Electrochem. Soc. 165, A3114 (2018).
Feng, Z. et al. Adjusting oxygen redox reaction and structural stability of Li- and Mn-rich cathodes by Zr-Ti dual-doping. ACS Appl. Mater. Interfaces 14, 5308–5317 (2022).
Yang, P. et al. A gradient doping strategy toward superior electrochemical performance for Li-rich Mn-based cathode materials. Small 19, 2207797 (2023).
Lu, C. et al. Enhanced electrochemical performance of Li-rich Li1.2Mn0.52Co0.08Ni0.2O2 cathode materials for Li-ion batteries by vanadium doping. Electrochim. Acta 209, 448–455 (2016).
Meng, J. et al. Modulating crystal and interfacial properties by W-gradient doping for highly stable and long life Li-rich layered cathodes. Adv. Funct. Mater. 32, 2113013 (2022).
Yang, J. et al. Encouraging voltage stability upon long cycling of Li-rich Mn-based cathode materials by Ta–Mo dual doping. ACS Appl. Mater. Interfaces 13, 25981–25992 (2021).
Eum, D. et al. Electrochemomechanical failure in layered oxide cathodes caused by rotational stacking faults. Nat. Mater. 23, 1093–1099 (2024).
Zheng, J. & Archer, L. A. Crystallographically textured electrodes for rechargeable batteries: symmetry, fabrication, and characterization. Chem. Rev. 122, 14440–14470 (2022).
Celeste, A. et al. On the elusive crystallography of lithium-rich layered oxides: novel structural models. Small Methods 8, 2301466 (2024).
Yin, C. et al. Structural insights into composition design of Li-rich layered cathode materials for high-energy rechargeable battery. Mater. Today 51, 15–26 (2021).
Zeng, L. et al. Quenching-induced lattice modifications endowing Li-rich layered cathodes with ultralow voltage decay and long life. Energy Environ. Sci. 18, 284–299 (2025).
Csernica, P. M. et al. Persistent and partially mobile oxygen vacancies in Li-rich layered oxides. Nat. Energy 6, 642–652 (2021).
Wandt, J., Freiberg, A. T., Ogrodnik, A. & Gasteiger, H. A. Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries. Mater. Today 21, 825–833 (2018).
Marie, J.-J. et al. Trapped O2 and the origin of voltage fade in layered Li-rich cathodes. Nat. Mater. 23, 818–825 (2024).
Lee, S., Su, L., Mesnier, A., Cui, Z. & Manthiram, A. Cracking vs. surface reactivity in high-nickel cathodes for lithium-ion batteries. Joule 7, 2430–2444 (2023).
Zhu, Z. et al. Gradient Li-rich oxide cathode particles immunized against oxygen release by a molten salt treatment. Nat. Energy 4, 1049–1058 (2019).
Qiu, B. et al. Gas–solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries. Nat. Commun. 7, 12108 (2016). This work reports a method for constructing surface oxygen vacancy on LLOs for practical application.
Li, A. et al. Enhancing cycling stability in Li-rich layered oxides by atomic layer deposition of LiNbO3 nanolayers. Solid State Ion 417, 116727 (2024).
Jung, R., Metzger, M., Maglia, F., Stinner, C. & Gasteiger, H. A. Chemical versus electrochemical electrolyte oxidation on NMC111, NMC622, NMC811, LNMO, and conductive carbon. J. Phys. Chem. Lett. 8, 4820–4825 (2017).
Li, Y. et al. A novel 3D Li/Li9Al4/Li-Mg alloy anode for superior lithium metal batteries. Adv. Funct. Mater. 33, 2213905 (2023).
Gao, P. et al. Optimization of magnesium-doped lithium metal anode for high performance lithium metal batteries through modeling and experiment. Angew. Chem. Int. Ed. 60, 16506–16513 (2021).
Lu, Y. et al. The carrier transition from Li atoms to Li vacancies in solid-state lithium alloy anodes. Sci. Adv. 7, eabi5520 (2021).
Wang, X. et al. Glassy Li metal anode for high-performance rechargeable Li batteries. Nat. Mater. 19, 1339–1345 (2020).
Pei, A., Zheng, G., Shi, F., Li, Y. & Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 17, 1132–1139 (2017).
Yan, K. et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 1–8 (2016).
Deng, W., Zhou, X., Fang, Q. & Liu, Z. Microscale lithium metal stored inside cellular graphene scaffold toward advanced metallic lithium anodes. Adv. Energy Mater. 8, 1703152 (2018).
Chazalviel, J.-N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 42, 7355 (1990).
Bai, P., Li, J., Brushett, F. R. & Bazant, M. Z. Transition of lithium growth mechanisms in liquid electrolytes. Energy Environ. Sci. 9, 3221–3229 (2016).
Wang, S. H. et al. Stable Li metal anodes via regulating lithium plating/stripping in vertically aligned microchannels. Adv. Mater. 29, 1703729 (2017).
Alexander, G. V., Shi, C., O’Neill, J. & Wachsman, E. D. Extreme lithium-metal cycling enabled by a mixed ion- and electron-conducting garnet three-dimensional architecture. Nat. Mater. 22, 1136–1143 (2023).
Hu, A. et al. An artificial hybrid interphase for an ultrahigh-rate and practical lithium metal anode. Energy Environ. Sci. 14, 4115–4124 (2021).
Liu, Y. et al. Electro-chemo-mechanical modeling of artificial solid electrolyte interphase to enable uniform electrodeposition of lithium metal anodes. Adv. Energy Mater. 12, 2103589 (2022).
Han, Z. et al. A protective layer for lithium metal anode: why and how. Small Methods 5, 2001035 (2021).
Santhosha, A., Medenbach, L., Buchheim, J. R. & Adelhelm, P. The indium−lithium electrode in solid-state lithium-ion batteries: phase formation, redox potentials, and interface stability. Batt. Supercaps 4, 1654–1654 (2021).
Sayavong, P. et al. Dissolution of the solid electrolyte interphase and its effects on lithium metal anode cyclability. J. Am. Chem. Soc. 145, 12342–12350 (2023).
Lin, D. et al. Fast galvanic lithium corrosion involving a Kirkendall-type mechanism. Nat. Chem. 11, 382–389 (2019).
Wang, H. et al. Application-driven design of non-aqueous electrolyte solutions through quantification of interfacial reactions in lithium metal batteries. Nat. Nanotechnol. 20, 1034–1042 (2025).
Rynearson, L. et al. Speciation of transition metal dissolution in electrolyte from common cathode materials. Angew. Chem. Int. Ed. 136, e202317109 (2024).
Sim, R., Su, L., Dolocan, A. & Manthiram, A. Delineating the impact of transition-metal crossover on solid-electrolyte interphase formation with ion mass spectrometry. Adv. Mater. 36, 2311573 (2024).
Xu, H. et al. Impacts of dissolved Ni2+ on the solid electrolyte interphase on a graphite anode. Angew. Chem. Int. Ed. 134, e202202894 (2022).
Jin, C. et al. Inhibiting and rejuvenating dead lithium in battery materials. Nat. Rev. Chem. 9, 553–568 (2025).
Werres, M. et al. Origin of heterogeneous stripping of lithium in liquid electrolytes. ACS Nano 17, 10218–10228 (2023).
Wang, C. et al. Tension-induced cavitation in Li-metal stripping. Adv. Mater. 35, 2209091 (2023).
Sanchez, A. J. et al. Plan-view operando video microscopy of Li metal anodes: identifying the coupled relationships among nucleation, morphology, and reversibility. ACS Energy Lett. 5, 994–1004 (2020).
Liu, H. et al. Plating/stripping behavior of actual lithium metal anode. Adv. Energy Mater. 9, 1902254 (2019).
Niu, C. et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy 6, 723–732 (2021).
Liu, X. et al. Advances in multi-scale design and fabrication processes for thick electrodes in lithium-ion batteries. Energy Rev. 3, 100066 (2024).
Kuang, Y., Chen, C., Kirsch, D. & Hu, L. Thick electrode batteries: principles, opportunities, and challenges. Adv. Energy Mater. 9, 1901457 (2019).
Jiao, S. et al. Behavior of lithium metal anodes under various capacity utilization and high current density in lithium metal batteries. Joule 2, 110–124 (2018).
Cai, X. et al. Characterization and quantification of multi-field coupling in lithium-ion batteries under mechanical constraints. J. Energy Chem. 95, 364–379 (2024).
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396 (2005).
Xu, C., Ahmad, Z., Aryanfar, A., Viswanathan, V. & Greer, J. R. Enhanced strength and temperature dependence of mechanical properties of Li at small scales and its implications for Li metal anodes. Proc. Natl Acad. Sci. USA 114, 57–61 (2017).
Zhang, W. et al. Design principles of functional polymer separators for high-energy, metal-based batteries. Small 14, 1703001 (2018).
Seo, J. et al. Recent progress of advanced functional separators in lithium metal batteries. Small 20, 2312132 (2024).
Fang, C. C. et al. Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat. Energy 6, 987–994 (2021).
Liu, D. et al. Controlled large-area lithium deposition to reduce swelling of high-energy lithium metal pouch cells in liquid electrolytes. Nat. Energy 9, 559–569 (2024).
Masias, A., Felten, N., Garcia-Mendez, R., Wolfenstine, J. & Sakamoto, J. Elastic, plastic, and creep mechanical properties of lithium metal. J. Mater. Sci. 54, 2585–2600 (2019). This work characterizes the elastic and plastic mechanical properties and creep behaviour of Li metal.
Huang, Y. et al. Mechanism of lithium plating and stripping in lithium-ion batteries induced by overhang failure defects. Cell Rep. Phys. Sci. 5, 102299 (2024).
Krauskopf, T., Mogwitz, B., Rosenbach, C., Zeier, W. G. & Janek, J. Diffusion limitation of lithium metal and Li–Mg alloy anodes on LLZO type solid electrolytes as a function of temperature and pressure. Adv. Energy Mater. 9, 1902568 (2019).
Lei, Y. et al. Surface modification of Li-rich Mn-based layered oxide cathodes: challenges, materials, methods, and characterization. Adv. Energy Mater. 10, 2002506 (2020).
Wu, J. et al. From fundamental understanding to engineering design of high-performance thick electrodes for scalable energy-storage systems. Adv. Mater. 33, 2101275 (2021).
Park, N.-Y. et al. Degradation mechanism of Ni-rich cathode materials: focusing on particle interior. ACS Energy Lett. 7, 2362–2369 (2022).
Ji, W., Qu, H., Zhang, X., Zheng, D. & Qu, D. Electrode architecture design to promote charge-transport kinetics in high-loading and high-energy lithium-based batteries. Small Methods 5, 2100518 (2021).
Dienemann, L. L., Saigal, A. & Zimmerman, M. A. Creep and anisotropy of free-standing lithium metal foils in an industrial dry room. J. Electrochem. Energy 18, 040908 (2021).
Zhang, K. et al. Unveiling the influence of formation voltage on Li-rich layered oxide cathode. Angew. Chem. Int. Ed. 64, e202515719 (2025).
Zhang, S. et al. The lasting impact of formation cycling on the Li-ion kinetics between SEI and the Li-metal anode and its correlation with efficiency. Sci. Adv. 10, eadj8889 (2024).
Zhou, M. et al. Correlating the potential-holding formation protocol of solid–electrolyte interphases with improving calendar aging on lithium metal anode. ACS Energy Lett. 8, 4702–4710 (2023).
Chang, W. et al. Relating chemo-mechanical hysteresis and formation protocols for anode-free lithium metal batteries. J. Electrochem. Soc. 171, 040506 (2024).
Liu, Z. et al. Revealing the degradation pathways of layered Li-rich oxide cathodes. Nat. Nanotechnol. 19, 1821–1830 (2024).
Li, L. et al. Self-heating-induced healing of lithium dendrites. Science 359, 1513–1516 (2018).
Li, Q., Tan, S., Li, L., Lu, Y. & He, Y. Understanding the molecular mechanism of pulse current charging for stable lithium-metal batteries. Sci. Adv. 3, e1701246 (2017).
Zhang, Y. et al. Unveiling the impacts of charge/discharge rate on the cycling performance of Li-metal batteries. ACS Energy Lett. 10, 872–880 (2025).
Kim, S. et al. Calendar life of lithium metal batteries: accelerated aging and failure analysis. Energy Storage Mater. 65, 103147 (2024).
Wood, S. M. et al. Predicting calendar aging in lithium metal secondary batteries: the impacts of solid electrolyte interphase composition and stability. Adv. Energy Mater. 8, 1801427 (2018).
Boyle, D. T. et al. Corrosion of lithium metal anodes during calendar ageing and its microscopic origins. Nat. Energy 6, 487–494 (2021).
Li, N. et al. Understanding and quantifying capacity loss in storage aging of Ah-level Li metal pouch cells. InfoMat 5, e12402 (2023).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 3, 373–386 (2018).
Luo, K. et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 8, 684–691 (2016).
Seo, D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Eum, D. et al. Coupling structural evolution and oxygen-redox electrochemistry in layered transition metal oxides. Nat. Mater. 21, 664–672 (2022).
Qiu, B. et al. Metastability and reversibility of anionic redox-based cathode for high-energy rechargeable batteries. Cell Rep. Phys. Sci. 1, 100028 (2020).
Kang, S., Lee, S., Lee, H. & Kang, Y.-M. Manipulating disorder within cathodes of alkali-ion batteries. Nat. Rev. Chem. 8, 587–604 (2024).
Zhang, M. et al. High pressure effect on structural and electrochemical properties of anionic redox-based lithium transition metal oxides. Matter 4, 164–181 (2021).
Zuo, W. et al. Li-rich cathodes for rechargeable Li-based batteries: reaction mechanisms and advanced characterization techniques. Energy Environ. Sci. 13, 4450–4497 (2020).
Jeong, H.-T. & Kim, W. J. Deformation mechanism maps of pure lithium: their application in determining stack pressure for all-solid-state lithium-ion batteries. ACS Energy Lett. 9, 3237–3251 (2024).
Chang, W., Xu, T. & Steingart, D. Chemo-mechanical effects of stack pressure and temperature on anode-free lithium metal batteries. J. Electrochem. Soc. 169, 090530 (2022).
Kasnatscheew, J. et al. Determining oxidative stability of battery electrolytes: validity of common electrochemical stability window (ESW) data and alternative strategies. Phys. Chem. Chem. Phys. 19, 16078–16086 (2017).
Zhou, M. Y. et al. Quantifying the apparent electron transfer number of electrolyte decomposition reactions in anode-free batteries. Joule 6, 2122–2137 (2022).
Gu, Y. et al. Resolving nanostructure and chemistry of solid-electrolyte interphase on lithium anodes by depth-sensitive plasmon-enhanced Raman spectroscopy. Nat. Commun. 14, 3536 (2023).
Wang, J. et al. Visualizing and regulating dynamic evolution of interfacial electrolyte configuration during de-solvation process on lithium-metal anode. Angew. Chem. Int. Ed. 63, e202400254 (2024).
Glasbeek, M. & Zhang, H. Femtosecond studies of solvation and intramolecular configurational dynamics of fluorophores in liquid solution. Chem. Rev. 104, 1929–1954 (2004).
Litman, Y., Chiang, K.-Y., Seki, T., Nagata, Y. & Bonn, M. Surface stratification determines the interfacial water structure of simple electrolyte solutions. Nat. Chem. 16, 644–650 (2024).
Yao, N., Chen, X., Fu, Z.-H. & Zhang, Q. Applying classical, ab initio, and machine-learning molecular dynamics simulations to the liquid electrolyte for rechargeable batteries. Chem. Rev. 122, 10970–11021 (2022).
Zheng, Z. et al. Quantitatively detecting and characterizing metallic lithium in lithium-based batteries. Energy Environ. Sci. 17, 9051–9092 (2024).
Fang, C. C. et al. Quantifying inactive lithium in lithium metal batteries. Nature 572, 511–515 (2019). This work introduces the concept of chemical titration-based quantification for inactive Li in LMB.
Tao, M. M. et al. Quantifying the evolution of inactive Li/lithium hydride and their correlations in rechargeable anode-free Li batteries. Nano Lett. 22, 6775–6781 (2022).
Hobold, G. M., Wang, C., Steinberg, K., Li, Y. & Gallant, B. M. High lithium oxide prevalence in the lithium solid–electrolyte interphase for high coulombic efficiency. Nat. Energy 9, 580–591 (2024).
Fan, X. & Wang, C. High-voltage liquid electrolytes for Li batteries: progress and perspectives. Chem. Soc. Rev. 50, 10486–10566 (2021).
Meng, Y. S., Srinivasan, V. & Xu, K. Designing better electrolytes. Science 378, eabq3750 (2022).
Zhang, H. et al. Electrolyte additives for lithium metal anodes and rechargeable lithium metal batteries: progress and perspectives. Angew. Chem. Int. Ed. 57, 15002–15027 (2018).
Xia, Y. et al. Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries. Nat. Energy 8, 934–945 (2023).
Lu, B. et al. Key parameters in determining the reactivity of lithium metal battery. ACS Energy Lett. 8, 3230–3238 (2023).
Puthusseri, D., Parmananda, M., Mukherjee, P. P. & Pol, V. G. Probing the thermal safety of Li metal batteries. J. Electrochem. Soc. 167, 120513 (2020).
Jiang, F.-N. et al. Thermal safety of dendritic lithium against non-aqueous electrolyte in pouch-type lithium metal batteries. J. Energy Chem. 72, 158–165 (2022).
Zhang, X. et al. Deciphering the thermal failure mechanism of anode-free lithium metal pouch batteries. Adv. Energy Mater. 13, 2203648 (2023).
Xu, X. Q. et al. Dendrite-accelerated thermal runaway mechanisms of lithium metal pouch batteries. SusMat 2, 435–444 (2022).
Cui, X. et al. Safety hazards of lithium metal batteries: from the perspective of lithium dendrites and thermal runaway. Energy Fuels 39, 7665–7690 (2025).
Zhou, Q. et al. A temperature-responsive electrolyte endowing superior safety characteristic of lithium metal batteries. Adv. Energy Mater. 10, 1903441 (2020).
Xie, J. & Lu, Y. C. Designing nonflammable liquid electrolytes for safe Li-ion batteries. Adv. Mater. 37, 2312451 (2025).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (22479158, 52272253, 52472266), the External Cooperation Program of Chinese Academy of Sciences (grant no. 181GJHZ2024126MI), the R&D Project of Jiangsu Province (BKBG2024021), Guangdong Basic and Applied Basic Research Foundation (2024A1515011548), Shenzhen Science and Technology Program (KJZD20240903100707011 and JCYJ20240813155846060), the Natural Science Foundation of Ningbo (2024QL041), and the Youth Innovation Promotion Association of Chinese Academy of Sciences (2022299). Y.S.M. acknowledges the support from the University of Chicago international programme.
Author information
Authors and Affiliations
Contributions
W.D., B.Q., Y.T., Z.L. and Y.S.M. contemplated the topic and structure of this Review. W.D., B.Q. and Y.S.M. contributed to the writing and editing of all sections of the manuscript. All authors contributed to the discussion of content and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Stefan Freunberger, who co-reviewed with Po-Hua Su; Kyu-Young Park; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Molecular Universe of SES AI: https://www.ses.ai
QuantumScape’s FlexFrame: https://www.quantumscape.com/blog/introducing-flexframe/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, W., Qiu, B., Chen, J. et al. Understanding the degradation complexity of ultrahigh-energy lithium metal batteries. Nat Rev Chem (2026). https://doi.org/10.1038/s41570-026-00801-2
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41570-026-00801-2