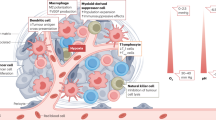
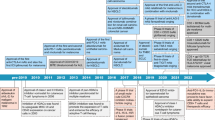
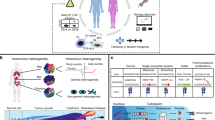
Abstract
Immune-checkpoint inhibitors and chimeric antigen receptor (CAR) T cells are revolutionizing oncology and haematology practice. With these and other immunotherapies, however, systemic biodistribution raises safety issues, potentially requiring the use of suboptimal doses or even precluding their clinical development. Delivering or attracting immune cells or immunomodulatory factors directly to the tumour and/or draining lymph nodes might overcome these problems. Hence, intratumoural delivery and tumour tissue-targeted compounds are attractive options to increase the in situ bioavailability and, thus, the efficacy of immunotherapies. In mouse models, intratumoural administration of immunostimulatory monoclonal antibodies, pattern recognition receptor agonists, genetically engineered viruses, bacteria, cytokines or immune cells can exert powerful effects not only against the injected tumours but also often against uninjected lesions (abscopal or anenestic effects). Alternatively, or additionally, biotechnology strategies are being used to achieve higher functional concentrations of immune mediators in tumour tissues, either by targeting locally overexpressed moieties or engineering ‘unmaskable’ agents to be activated by elements enriched within tumour tissues. Clinical trials evaluating these strategies are ongoing, but their development faces issues relating to the administration methodology, pharmacokinetic parameters, pharmacodynamic end points, and immunobiological and clinical response assessments. Herein, we discuss these approaches in the context of their historical development and describe the current landscape of intratumoural or tumour tissue-targeted immunotherapies.
Key points
-
Repeated intratumoural injections with agents designed to enhance antitumour immune responses constitutes a feasible strategy to reduce the risk of systemic toxicities and achieve higher local bioactive drug concentrations.
-
Spearheaded by the oncolytic virus talimogene laheparepvec, the first intratumoural immunotherapy approved by the FDA and EMA, and supported by a strong preclinical rationale, many intratumoural immunotherapies are now being developed in clinical trials.
-
These immunotherapies include microorganisms (viruses or bacteria) and synthetic compounds mimicking infectious agents (such as pattern recognition receptor agonists), as well as immunomodulatory monoclonal antibodies, cytokines and chimeric proteins.
-
Higher locoregional concentrations of immunotherapy agents can also be achieved through molecular engineering, for example, to target them towards moieties that are enriched in the tumour microenvironment.
-
Increased specificity in tumour targeting can also be attained through the development of prodrug forms of immunotherapies that become functional only after entering tumour tissue (pro-immunodrugs).
-
Procedural, pharmaceutical, regulatory and analytical challenges require multidisciplinary expert consensus and systematic research to maximize the potential of these modes of administration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Guedan, S., Ruella, M. & June, C. H. Emerging cellular therapies for cancer. Annu. Rev. Immunol. 37, 145–171 (2019).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Cuesta, A. M., Sainz-Pastor, N., Bonet, J., Oliva, B. & Alvarez-Vallina, L. Multivalent antibodies: when design surpasses evolution. Trends Biotechnol. 28, 355–362 (2010).
Melero, I., Rouzaut, A., Motz, G. T. & Coukos, G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 4, 522–526 (2014).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Leonard, J. P. et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90, 2541–2548 (1997).
Ascierto, P. A. et al. Overall survival at 5 years of follow-up in a phase III trial comparing ipilimumab 10 mg/kg with 3 mg/kg in patients with advanced melanoma. J. Immunother. Cancer 8, e000391 (2020).
Wang, Y. L., Peng, H. H., Su, S. Y. & Lin, C. T. Combined immunotherapy (OK-432, IL-2) with chemotherapy decrease the recurrence rate in advanced ovarian cancer. Reprod. Sci. 26, 244–249 (2019).
Marabelle, A., Tselikas, L., de Baere, T. & Houot, R. Intratumoral immunotherapy: using the tumor as the remedy. Ann. Oncol. 28, xii33–xii43 (2017).
Margolin, K. et al. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin. Cancer Res. 24, 5552–5561 (2018).
Barlesi, F. et al. Phase Ib study of selicrelumab (CD40 agonist) in combination with atezolizumab (anti-PD-L1) in patients with advanced solid tumors [abstract 291]. J. Immunother. Cancer 8 (Suppl. 3), A178 (2020).
Chandrasekaran, S. & King, M. R. Microenvironment of tumor-draining lymph nodes: opportunities for liposome-based targeted therapy. Int. J. Mol. Sci. 15, 20209–20239 (2014).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Vormehr, M., Tureci, O. & Sahin, U. Harnessing tumor mutations for truly individualized cancer vaccines. Annu. Rev. Med. 70, 395–407 (2019).
Linette, G. P. et al. Immunological ignorance is an enabling feature of the oligo-clonal T cell response to melanoma neoantigens. Proc. Natl Acad. Sci. USA 116, 23662–23670 (2019).
Harrington, K., Freeman, D. J., Kelly, B., Harper, J. & Soria, J. C. Optimizing oncolytic virotherapy in cancer treatment. Nat. Rev. Drug Discov. 18, 689–706 (2019).
Coley, W. B. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Clin. Orthop. Relat. Res. 262, 3–11 (1991).
Nauts, H. C., Swift, W. E. & Coley, B. L. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res. 6, 205–216 (1946).
Tsung, K. & Norton, J. A. Lessons from Coley’s toxin. Surg. Oncol. 15, 25–28 (2006).
Hoption Cann, S. A., van Netten, J. P. & van Netten, C. Dr William Coley and tumour regression: a place in history or in the future. Postgrad. Med. J. 79, 672–680 (2003).
Morales, A., Eidinger, D. & Bruce, A. W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 116, 180–183 (1976).
Mori, K., Lamm, D. L. & Crawford, E. D. A trial of bacillus Calmette-Guérin versus adriamycin in superficial bladder cancer: a South-West Oncology Group Study. Urol. Int. 41, 254–259 (1986).
Mostafid, A. H., Palou Redorta, J., Sylvester, R. & Witjes, J. A. Therapeutic options in high-risk non-muscle-invasive bladder cancer during the current worldwide shortage of bacille Calmette-Guérin. Eur. Urol. 67, 359–360 (2015).
van Puffelen, J. H. et al. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 17, 513–525 (2020).
Yang, J. et al. Insights into local tumor microenvironment immune factors associated with regression of cutaneous melanoma metastases by Mycobacterium bovis bacille Calmette-Guérin. Front. Oncol. 7, 61 (2017).
Svejda, J., Mechl, Z., Sopkova, B. & Foukal, T. Histologic changes in the human skin melanoma after intratumorous treatment with BCG. Neoplasma 26, 215–221 (1979).
Agarwala, S. S., Neuberg, D., Park, Y. & Kirkwood, J. M. Mature results of a phase III randomized trial of bacillus Calmette-Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I-III melanoma (E1673): a trial of the Eastern Oncology Group. Cancer 100, 1692–1698 (2004).
Fisher, R. I. et al. Adjuvant immunotherapy or chemotherapy for malignant melanoma. Preliminary report of the National Cancer Institute randomized clinical trial. Surg. Clin. North Am. 61, 1267–1277 (1981).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353 (2015).
Matzinger, P. An innate sense of danger. Ann. NY Acad. Sci. 961, 341–342 (2002).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Kandimalla, E. R. et al. Immunomodulatory oligonucleotides containing a cytosine-phosphate-2′-deoxy-7-deazaguanosine motif as potent toll-like receptor 9 agonists. Proc. Natl Acad. Sci. USA 102, 6925–6930 (2005).
Wooldridge, J. E. & Weiner, G. J. CpG DNA and cancer immunotherapy: orchestrating the antitumor immune response. Curr. Opin. Oncol. 15, 440–445 (2003).
Krieg, A. M. Development of TLR9 agonists for cancer therapy. J. Clin. Invest. 117, 1184–1194 (2007).
Manegold, C. et al. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J. Clin. Oncol. 26, 3979–3986 (2008).
Hirsh, V. et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J. Clin. Oncol. 29, 2667–2674 (2011).
Belani, C. P. et al. Phase 2 trial of erlotinib with or without PF-3512676 (CPG 7909, a Toll-like receptor 9 agonist) in patients with advanced recurrent EGFR-positive non-small cell lung cancer. Cancer Biol. Ther. 14, 557–563 (2013).
Brody, J. D. et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J. Clin. Oncol. 28, 4324–4332 (2010).
Kim, Y. H. et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 119, 355–363 (2012).
Frank, M. J. et al. In situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 8, 1258–1269 (2018).
Ribas, A. et al. SD-101 in combination with pembrolizumab in advanced melanoma: results of a phase Ib, multicenter study. Cancer Discov. 8, 1250–1257 (2018).
Li, J. et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J. Immunol. 179, 2493–2500 (2007).
Haymaker, C. et al. Final results from ILLUMINATE-204, a phase I/II trial of intratumoral tilsotolimod in combination with ipilimumab in PD-1 inhibitor refractory advanced melanoma [abstract 1083MO]. Ann. Oncol. 31 (Suppl. 4), S736 (2020).
Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998).
Weighardt, H. et al. Identification of a TLR4- and TRIF-dependent activation program of dendritic cells. Eur. J. Immunol. 34, 558–564 (2004).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Persing, D. H. et al. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10, S32–S37 (2002).
Chicoine, M. R., Won, E. K. & Zahner, M. C. Intratumoral injection of lipopolysaccharide causes regression of subcutaneously implanted mouse glioblastoma multiforme. Neurosurgery 48, 607–614; discussion 614–5 (2001).
Bhatia, S. et al. Intratumoral G100, a TLR4 agonist, induces antitumor immune responses and tumor regression in patients with Merkel cell carcinoma. Clin. Cancer Res. 25, 1185–1195 (2019).
Flowers, C. et al. Intratumoral G100 induces systemic immunity and abscopal tumor regression in patients with follicular lymphoma: results of a phase 1/2 study examining G100 alone and in combination with pembrolizumab [abstract]. Blood 130 (Suppl. 1), 2771 (2017).
Flowers, C. R. et al. Long term follow-up of a phase 2 study examining intratumoral G100 alone and in combination with pembrolizumab in patients with follicular lymphoma [abstract]. Blood 132 (Suppl. 1), 2892 (2018).
Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Nemes, M. M., Tytell, A. A., Lampson, G. P., Field, A. K. & Hilleman, M. R. Inducers of interferon and host resistance. VI. Antiviral efficacy of poly I:C in animal models. Proc. Soc. Exp. Biol. Med. 132, 776–783 (1969).
Schulz, O. et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433, 887–892 (2005).
Degre, M. & Elgjo, K. Methylcholanthrene-induced skin carcinogenesis modified by treatment with polyinosinic:polycytidylic acid (poly I:C). Acta Pathol. Microbiol. Scand. A 79, 687–688 (1971).
Fujimura, T., Nakagawa, S., Ohtani, T., Ito, Y. & Aiba, S. Inhibitory effect of the polyinosinic-polycytidylic acid/cationic liposome on the progression of murine B16F10 melanoma. Eur. J. Immunol. 36, 3371–3380 (2006).
Salazar, A. M., Erlich, R. B., Mark, A., Bhardwaj, N. & Herberman, R. B. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol. Res. 2, 720–724 (2014).
Rodriguez-Ruiz, M. E. et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 29, 1312–1319 (2018).
Caskey, M. et al. Synthetic double-stranded RNA induces innate immune responses similar to a live viral vaccine in humans. J. Exp. Med. 208, 2357–2366 (2011).
Okada, H. et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J. Clin. Oncol. 29, 330–336 (2011).
Aznar, M. A. et al. Immunotherapeutic effects of intratumoral nanoplexed poly I:C. J. Immunother. Cancer 7, 116 (2019).
Tormo, D. et al. Targeted activation of innate immunity for therapeutic induction of autophagy and apoptosis in melanoma cells. Cancer Cell 16, 103–114 (2009).
Kalbasi, A. et al. Uncoupling interferon signaling and antigen presentation to overcome immunotherapy resistance due to JAK1 loss in melanoma. Sci. Transl Med. 12, eabb0152 (2020).
Marquez-Rodas, I. et al. Intratumoral nanoplexed poly I:C BO-112 in combination with systemic anti-PD-1 for patients with anti-PD-1-refractory tumors. Sci. Transl Med. 12, eabb0391 (2020).
Barral, P. M. et al. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol. Ther. 124, 219–234 (2009).
Diebold, S. S. et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424, 324–328 (2003).
Ishizuka, J. J. et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature 565, 43–48 (2019).
Liu, H. et al. Tumor-derived IFN triggers chronic pathway agonism and sensitivity to ADAR loss. Nat. Med. 25, 95–102 (2019).
Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Kariko, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Hemmi, H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200 (2002).
Sidky, Y. A. et al. Inhibition of murine tumor growth by an interferon-inducing imidazoquinolinamine. Cancer Res. 52, 3528–3533 (1992).
Syed, T. A., Ahmadpour, O. A., Ahmad, S. A. & Ahmad, S. H. Management of female genital warts with an analog of imiquimod 2% in cream: a randomized, double-blind, placebo-controlled study. J. Dermatol. 25, 429–433 (1998).
Stary, G. et al. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J. Exp. Med. 204, 1441–1451 (2007).
van Seters, M. et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N. Engl. J. Med. 358, 1465–1473 (2008).
Adams, S. et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin. Cancer Res. 18, 6748–6757 (2012).
Rook, A. H. et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood 126, 1452–1461 (2015).
Chen, Q., Sun, L. & Chen, Z. J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 17, 1142–1149 (2016).
Yang, Y. G., Lindahl, T. & Barnes, D. E. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131, 873–886 (2007).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Woo, S. R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 41, 830–842 (2014).
Vanpouille-Box, C. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618 (2017).
Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 11, 1018–1030 (2015).
Lara, P. N. Jr. et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J. Clin. Oncol. 29, 2965–2971 (2011).
Harrington, K. J. et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas [abstract LBA15]. Ann. Oncol. 29 (Suppl. 8), viii712 (2018).
Meric-Bernstam, F. et al. Phase Ib study of MIW815 (ADU-S100) in combination with spartalizumab (PDR001) in patients (pts) with advanced/metastatic solid tumors or lymphomas [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 2507 (2019).
Flood, B. A., Higgs, E. F., Li, S., Luke, J. J. & Gajewski, T. F. STING pathway agonism as a cancer therapeutic. Immunol. Rev. 290, 24–38 (2019).
Giguere, C. M. et al. Treatment of lymphangiomas with OK-432 (Picibanil) sclerotherapy: a prospective multi-institutional trial. Arch. Otolaryngol. Head. Neck Surg. 128, 1137–1144 (2002).
Aluffi Valletti, P. et al. A single-center experience in the management of head and neck lymphangiomas. Oral. Maxillofac. Surg. 24, 109–115 (2020).
Kasahara, K. et al. Randomized phase II trial of OK-432 in patients with malignant pleural effusion due to non-small cell lung cancer. Anticancer Res. 26, 1495–1499 (2006).
Yamaguchi, Y. et al. Locoregional immunotherapy of malignant effusion from colorectal cancer using the streptococcal preparation OK-432 plus interleukin-2: induction of autologous tumor-reactive CD4+ Th1 killer lymphocytes. Br. J. Cancer 89, 1876–1884 (2003).
Hirayama, M. et al. Overcoming regulatory T-cell suppression by a lyophilized preparation of Streptococcus pyogenes. Eur. J. Immunol. 43, 989–1000 (2013).
Andtbacka, R. H. I. et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J. Immunother. Cancer 7, 145 (2019).
Andtbacka, R. H. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 33, 2780–2788 (2015).
Hamid, O. et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 30, 582–588 (2019).
Andtbacka, R. H. et al. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase III clinical trial. Ann. Surg. Oncol. 23, 4169–4177 (2016).
Dillman, R. O. An update on GM-CSF and its potential role in melanoma management. Melanoma Manag. 7, MMT49 (2020).
Puzanov, I. et al. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 34, 2619–2626 (2016).
Ribas, A. et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119.e10 (2017).
Middleton, M. et al. An open-label, multicenter, phase 1/2 clinical trial of Rp1, an enhanced potency oncolytic Hsv, combined with nivolumab: updated results from the skin cancer cohorts [abstract 422]. J. Immunother. Cancer 8 (Suppl. 3), A257 (2020).
Heo, J. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 19, 329–336 (2013).
Kim, M. K. et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci. Transl Med. 5, 185ra63 (2013).
Transgene. Transgene provides an update after the interim futility analysis of the PHOCUS study of Pexa-Vec in liver cancer. Transgene https://www.transgene.fr/en/news/#pressreleases (2019).
Zamarin, D. et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl Med. 6, 226ra32 (2014).
Curti, B. D. et al. Activity of a novel immunotherapy combination of intralesional Coxsackievirus A21 and systemic ipilimumab in advanced melanoma patients previously treated with anti-PD1 blockade therapy [abstract]. J. Clin. Oncol. 35 (Suppl. 15), 3014 (2017).
Lang, F. F. et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 36, 1419–1427 (2018).
Rosa, K. Japanese approval sought for oncolytic virus teserpaturev for malignanat glioma. OncLive https://www.onclive.com/view/japanese-approval-sought-for-oncolytic-virus-teserpaturev-for-malignant-glioma (2021).
Nakao, S. et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade. Sci. Transl Med. 12, eaax7992 (2020).
Kepp, O., Marabelle, A., Zitvogel, L. & Kroemer, G. Oncolysis without viruses – inducing systemic anticancer immune responses with local therapies. Nat. Rev. Clin. Oncol. 17, 49–64 (2020).
Swift, L., Zhang, C., Trippett, T. & Narendran, A. Potent in vitro and xenograft antitumor activity of a novel agent, PV-10, against relapsed and refractory neuroblastoma. Onco Targets Ther. 12, 1293–1307 (2019).
Toomey, P. et al. Intralesional injection of rose bengal induces a systemic tumor-specific immune response in murine models of melanoma and breast cancer. PLoS ONE 8, e68561 (2013).
Read, T. A. et al. Intralesional PV-10 for the treatment of in-transit melanoma metastases – Results of a prospective, non-randomized, single center study. J. Surg. Oncol. 117, 579–587 (2018).
Foote, M. et al. Results of a phase II, open-label, non-comparative study of intralesional PV-10 followed by radiotherapy for the treatment of in-transit or metastatic melanoma. J. Surg. Oncol. 115, 891–897 (2017).
Liu, H. et al. T cell mediated immunity after combination therapy with intralesional PV-10 and blockade of the PD-1/PD-L1 pathway in a murine melanoma model. PLoS ONE 13, e0196033 (2018).
De Ridder, T. R. et al. Randomized controlled clinical study evaluating the efficacy and safety of intratumoral treatment of canine mast cell tumors with tigilanol tiglate (EBC-46). J. Vet. Intern. Med. 35, 415–429 (2020).
Panizza, B. J. et al. Phase I dose-escalation study to determine the safety, tolerability, preliminary efficacy and pharmacokinetics of an intratumoral injection of tigilanol tiglate (EBC-46). EBioMedicine 50, 433–441 (2019).
Boyle, G. M. et al. Intra-lesional injection of the novel PKC activator EBC-46 rapidly ablates tumors in mouse models. PLoS ONE 9, e108887 (2014).
Zanin-Zhorov, A., Dustin, M. L. & Blazar, B. R. PKC-theta function at the immunological synapse: prospects for therapeutic targeting. Trends Immunol. 32, 358–363 (2011).
Anel, A. et al. Protein kinase C-θ (PKC-θ) in natural killer cell function and anti-tumor immunity. Front. Immunol. 3, 187 (2012).
Zanin-Zhorov, A. et al. Protein kinase C-θ mediates negative feedback on regulatory T cell function. Science 328, 372–376 (2010).
Kong, K. F. et al. Protein kinase C-η controls CTLA-4-mediated regulatory T cell function. Nat. Immunol. 15, 465–472 (2014).
Tornesello, A. L., Borrelli, A., Buonaguro, L., Buonaguro, F. M. & Tornesello, M. L. Antimicrobial peptides as anticancer agents: functional properties and biological activities. Molecules 25, 2850 (2020).
Zhou, H. et al. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 7, e2134 (2016).
Zhou, H. et al. The oncolytic peptide LTX-315 kills cancer cells through Bax/Bak-regulated mitochondrial membrane permeabilization. Oncotarget 6, 26599–26614 (2015).
Baurain, J. F. et al. A phase I study of the oncolytic peptide LTX-315 generates de novo T-cell responses and clinical benefit in patients with advanced sarcoma [abstract]. Cancer Res. 79 (Suppl. 13), CT010 (2019).
Dubrot, J. et al. Intratumoral injection of interferon-α and systemic delivery of agonist anti-CD137 monoclonal antibodies synergize for immunotherapy. Int. J. Cancer 128, 105–118 (2011).
Vom Berg, J. et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J. Exp. Med. 210, 2803–2811 (2013).
Negrier, S. et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N. Engl. J. Med. 338, 1272–1278 (1998).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Wrangle, J. M. et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol. 19, 694–704 (2018).
Diab, A. et al. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov. 10, 1158–1173 (2020).
Lotze, M. T. et al. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA 256, 3117–3124 (1986).
Jackaman, C. et al. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J. Immunol. 171, 5051–5063 (2003).
Gutwald, J. G., Groth, W. & Mahrle, G. Peritumoral injections of interleukin 2 induce tumour regression in metastatic malignant melanoma. Br. J. Dermatol. 130, 541–542 (1994).
Weide, B. et al. Survival after intratumoral interleukin-2 treatment of 72 melanoma patients and response upon the first chemotherapy during follow-up. Cancer Immunol. Immunother. 60, 487–493 (2011).
Charych, D. H. et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin. Cancer Res. 22, 680–690 (2016).
Bentebibel, S. E. et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 9, 711–721 (2019).
Mauldin, I. S. et al. Intratumoral interferon-gamma increases chemokine production but fails to increase T cell infiltration of human melanoma metastases. Cancer Immunol. Immunother. 65, 1189–1199 (2016).
Grob, J. J. et al. Large keratoacanthomas treated with intralesional interferon alfa-2a. J. Am. Acad. Dermatol. 29, 237–241 (1993).
Thestrup-Pedersen, K., Jacobsen, I. E. & Frentz, G. Intralesional interferon-alpha 2b treatment of basal cell carcinoma. Acta Derm. Venereol. 70, 512–514 (1990).
McDonald, R. R. & Georgouras, K. Treatment of basal cell carcinoma with intralesional interferon alpha: a case report and literature review. Australas. J. Dermatol. 33, 81–86 (1992).
Hanlon, A., Kim, J. & Leffell, D. J. Intralesional interferon alfa-2b for refractory, recurrent squamous cell carcinoma of the face. J. Am. Acad. Dermatol. 69, 1070–1072 (2013).
Buechner, S. A. et al. Regression of basal cell carcinoma by intralesional interferon-alpha treatment is mediated by CD95 (Apo-1/Fas)-CD95 ligand-induced suicide. J. Clin. Invest. 100, 2691–2696 (1997).
Spaapen, R. M. et al. Therapeutic activity of high-dose intratumoral IFN-beta requires direct effect on the tumor vasculature. J. Immunol. 193, 4254–4260 (2014).
Santana Carrero, R. M. et al. IL-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses. Proc. Natl Acad. Sci. USA 116, 599–608 (2019).
Ugen, K. E. et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther. 13, 969–974 (2006).
Hu, Q. et al. Discovery of a novel IL-15 based protein with improved developability and efficacy for cancer immunotherapy. Sci. Rep. 8, 7675 (2018).
Bajetta, E. et al. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin. Cancer Res. 4, 75–85 (1998).
Motzer, R. J. et al. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin. Cancer Res. 4, 1183–1191 (1998).
Gollob, J. A. et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-gamma induction is associated with clinical response. Clin. Cancer Res. 6, 1678–1692 (2000).
Atkins, M. B. et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 3, 409–417 (1997).
Algazi, A. et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann. Oncol. 31, 532–540 (2020).
Algazi, A. P. et al. Phase II trial of IL-12 plasmid transfection and PD-1 blockade in immunologically quiescent melanoma. Clin. Cancer Res. 26, 2827–2837 (2020).
Greaney, S. K. et al. Intratumoral plasmid IL12 electroporation therapy in patients with advanced melanoma induces systemic and intratumoral T-cell responses. Cancer Immunol. Res. 8, 246–254 (2020).
Bhatia, S. et al. Intratumoral delivery of plasmid IL12 via electroporation leads to regression of injected and noninjected tumors in Merkel cell carcinoma. Clin. Cancer Res. 26, 598–607 (2020).
Hewitt, S. L. et al. Intratumoral interleukin-12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin. Cancer Res. 26, 6284–6298 (2020).
Hamid, O. et al. Preliminary safety, antitumor activity and pharmacodynamics results of HIT-IT MEDI1191 (mRNA IL-12) in patients with advanced solid tumours and superficial lesions. Ann. Oncol. 32, S9–S13 (2021).
Li, Y. Z. et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 1, 882– 893 (2020).
Kanca, H. et al. Intratumoral recombinant human interferon alpha-2a and vincristine combination therapy in canine transmissible venereal tumour. Vet. Med. Sci. 4, 364–372 (2018).
Vaquero, J. & Martinez, R. Intratumoral immunotherapy with interferon-alpha and interleukin-2 in glioblastoma. Neuroreport 3, 981–983 (1992).
Momin, N. et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci. Transl Med. 11, eaaw2614 (2019).
Wagenaar, T. R. et al. Combinatorial treatment with intratumoral cytokine mRNAs results in high frequency of tumor rejection and development of anti-tumor immunity across a range of preclinical cancer models [abstract]. Cancer Res. 78 (Suppl. 13), LB130 (2018).
Hewitt, S. L. et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl Med. 11, eaat9143 (2019).
Jimeno, A. et al. A phase 1/2, open-label, multicenter, dose escalation and efficacy study of mRNA-2416, a lipid nanoparticle encapsulated mRNA encoding human OX40L, for intratumoral injection alone or in combination with durvalumab for patients with advanced malignancies [abstract]. Cancer Res. 80 (Suppl. 16), CT032 (2020).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Herbst, R. S. et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors [abstract]. J. Clin. Oncol. 31 (Suppl. 15), 3000 (2013).
Francis, D. M. et al. Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci. Transl Med. 12, eaay3575 (2020).
Ascierto, P. A. et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 18, 611–622 (2017).
Sharma, P. et al. Nivolumab alone and with ipilimumab in previously treated metastatic urothelial carcinoma: Checkmate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg expansion cohort results. J. Clin. Oncol. 37, 1608–1616 (2019).
Marabelle, A., Kohrt, H. & Levy, R. Intratumoral anti-CTLA-4 therapy: enhancing efficacy while avoiding toxicity. Clin. Cancer Res. 19, 5261–5263 (2013).
van Hooren, L. et al. Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer. Eur. J. Immunol. 47, 385–393 (2017).
Fransen, M. F., van der Sluis, T. C., Ossendorp, F., Arens, R. & Melief, C. J. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin. Cancer Res. 19, 5381–5389 (2013).
Sandin, L. C. et al. Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo. Oncoimmunology 3, e27614 (2014).
Simmons, A. D. et al. Local secretion of anti-CTLA-4 enhances the therapeutic efficacy of a cancer immunotherapy with reduced evidence of systemic autoimmunity. Cancer Immunol. Immunother. 57, 1263–1270 (2008).
Marabelle, A. et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J. Clin. Invest. 123, 2447–2463 (2013).
Ray, A. et al. A phase I study of intratumoral ipilimumab and interleukin-2 in patients with advanced melanoma. Oncotarget 7, 64390–64399 (2016).
Schwarze, J. K. et al. A phase I clinical trial on intratumoral and intracavitary administration of ipilimumab and nivolumab in patients with recurrent glioblastoma. J. Clin. Oncol. 38, 2534–2534 (2020).
Hamid, O. et al. First in human (FIH) study of an OX40 agonist monoclonal antibody (mAb) PF-04518600 (PF-8600) in adult patients (pts) with select advanced solid tumors: PRELIMINARY safety and pharmacokinetic (PK)/pharmacodynamic results [abstract]. J. Clin. Oncol. 34 (Suppl. 15), 3079 (2016).
Hansen, A. R. et al. A first-in-human phase I dose escalation study of the OX40 agonist MOXR0916 in patients with refractory solid tumors. Cancer Research 76 (Suppl. 14), CT097 (2016).
Bulliard, Y. et al. OX40 engagement depletes intratumoral Tregs via activating FcγRs, leading to antitumor efficacy. Immunol. Cell Biol. 92, 475–480 (2014).
Bulliard, Y. et al. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 210, 1685–1693 (2013).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Sagiv-Barfi, I. et al. Eradication of spontaneous malignancy by local immunotherapy. Sci. Transl Med. 10, eaan4488 (2018).
Vonderheide, R. H. et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 25, 876–883 (2007).
Kwong, B., Gai, S. A., Elkhader, J., Wittrup, K. D. & Irvine, D. J. Localized immunotherapy via liposome-anchored anti-CD137+IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res. 73, 1547–1558 (2013).
Dai, M., Yip, Y. Y., Hellstrom, I. & Hellstrom, K. E. Curing mice with large tumors by locally delivering combinations of immunomodulatory antibodies. Clin. Cancer Res. 21, 1127–1138 (2015).
Sandin, L. C. et al. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunol. Res. 2, 80–90 (2014).
Jackaman, C. & Nelson, D. J. Intratumoral interleukin-2/agonist CD40 antibody drives CD4+-independent resolution of treated-tumors and CD4+-dependent systemic and memory responses. Cancer Immunol. Immunother. 61, 549–560 (2012).
Van De Voort, T. J., Felder, M. A., Yang, R. K., Sondel, P. M. & Rakhmilevich, A. L. Intratumoral delivery of low doses of anti-CD40 mAb combined with monophosphoryl lipid a induces local and systemic antitumor effects in immunocompetent and T cell-deficient mice. J. Immunother. 36, 29–40 (2013).
van Mierlo, G. J. et al. CD40 stimulation leads to effective therapy of CD40− tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc. Natl Acad. Sci. USA 99, 5561–5566 (2002).
Tselikas, L. et al. Pickering emulsions with ethiodized oil and nanoparticles for slow release of intratumoral anti-CTLA4 immune checkpoint antibodies. J. Immunother. Cancer 8, e000579 (2020).
Ishihara, J. et al. Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events. Sci. Transl Med. 9, eaan0401 (2017).
Bol, K. F., Schreibelt, G., Gerritsen, W. R., de Vries, I. J. & Figdor, C. G. Dendritic cell-based immunotherapy: state of the art and beyond. Clin. Cancer Res. 22, 1897–1906 (2016).
Melero, I., Vile, R. G. & Colombo, M. P. Feeding dendritic cells with tumor antigens: self-service buffet or a la carte? Gene Ther. 7, 1167–1170 (2000).
Mazzolini, G. et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J. Clin. Oncol. 23, 999–1010 (2005).
Huarte, E. et al. Intratumoural administration of dendritic cells: hostile environment and help by gene therapy. Expert Opin. Biol. Ther. 5, 7–22 (2005).
Karlsson-Parra, A. et al. Ilixadencel – an allogeneic cell-based anticancer immune primer for intratumoral administration. Pharm. Res. 35, 156 (2018).
Rizell, M. et al. Phase 1 trial with the cell-based immune primer ilixadencel, alone, and combined with sorafenib, in advanced hepatocellular carcinoma. Front. Oncol. 9, 19 (2019).
Frobom, R. et al. Phase I trial evaluating safety and efficacy of intratumorally administered inflammatory allogeneic dendritic cells (ilixadencel) in advanced gastrointestinal stromal tumors. Cancer Immunol. Immunother. 69, 2393–2401 (2020).
Etxeberria, I. et al. Intratumor adoptive transfer of IL-12 mRNA transiently engineered antitumor CD8+ T cells. Cancer Cell 36, 613–629.e7 (2019).
Minute, L. et al. Cellular cytotoxicity is a form of immunogenic cell death. J. Immunother. Cancer 8, e000325 (2020).
Dillman, R. O. et al. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J. Immunother. 32, 914–919 (2009).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016).
Brown, C. E. et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 21, 4062–4072 (2015).
Etxeberria, I. et al. Engineering bionic T cells: signal 1, signal 2, signal 3, reprogramming and the removal of inhibitory mechanisms. Cell Mol. Immunol. 17, 576–586 (2020).
Adusumilli, P. S. et al. Regional delivery of mesothelin-targeted CAR T cells for pleural cancers: safety and preliminary efficacy in combination with anti-PD-1 agent [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 2511 (2019).
Papa, S. et al. A phase I trial of T4 CAR T-cell immunotherapy in head and neck squamous cancer (HNSCC) [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 3046 (2018).
Katz, S. C. et al. HITM-SURE: Hepatic immunotherapy for metastases phase Ib anti-CEA CAR-T study utilizing pressure enabled drug delivery. J. Immunother. Cancer 8, e001097 (2020).
Goebeler, M. E. & Bargou, R. C. T cell-engaging therapies – BiTEs and beyond. Nat. Rev. Clin. Oncol. 17, 418–434 (2020).
Topp, M. S. et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 29, 2493–2498 (2011).
Linke, R., Klein, A. & Seimetz, D. Catumaxomab: clinical development and future directions. mAbs 2, 129–136 (2010).
Heiss, M. M. et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int. J. Cancer 127, 2209–2221 (2010).
Burges, A. et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM × anti-CD3 antibody: a phase I/II study. Clin. Cancer Res. 13, 3899–3905 (2007).
Bacac, M., Klein, C. & Umana, P. CEA TCB: a novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. Oncoimmunology 5, e1203498 (2016).
Damato, B. E., Dukes, J., Goodall, H. & Carvajal, R. D. Tebentafusp: T cell redirection for the treatment of metastatic uveal melanoma. Cancers 11, 971 (2019).
Wong, B., Arron, J. & Choi, Y. T cell receptor signals enhance susceptibility to Fas-mediated apoptosis. J. Exp. Med. 186, 1939–1944 (1997).
Claus, C. et al. Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Sci. Transl Med. 11, eaav5989 (2019).
Lakins, M. A. et al. FS222, a CD137/PD-L1 tetravalent bispecific antibody, exhibits low toxicity and antitumor activity in colorectal cancer models. Clin. Cancer Res. 26, 4154–4167 (2020).
Voeller, J. et al. Combined innate and adaptive immunotherapy overcomes resistance of immunologically cold syngeneic murine neuroblastoma to checkpoint inhibition. J. Immunother. Cancer 7, 344 (2019).
Navid, F. et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J. Clin. Oncol. 32, 1445–1452 (2014).
Federico, S. M. et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin. Cancer Res. 23, 6441–6449 (2017).
Shusterman, S. et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J. Clin. Oncol. 28, 4969–4975 (2010).
Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 (2010).
Johannsen, M. et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur. J. Cancer 46, 2926–2935 (2010).
Danielli, R. et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol. Immunother. 64, 999–1009 (2015).
Heaton, K. M., Ju, G. & Grimm, E. A. Human interleukin 2 analogues that preferentially bind the intermediate-affinity interleukin 2 receptor lead to reduced secondary cytokine secretion: implications for the use of these interleukin 2 analogues in cancer immunotherapy. Cancer Res. 53, 2597–2602 (1993).
Klein, C. et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 6, e1277306 (2017).
Soerensen, M. M. et al. Safety, PK/PD, and anti-tumor activity of RO6874281, an engineered variant of interleukin-2 (IL-2v) targeted to tumor-associated fibroblasts via binding to fibroblast activation protein (FAP). J. Clin. Oncol. 36, e15155 (2018).
Fedele, V. & Melisi, D. Permissive state of EMT: the role of immune cell compartment. Front. Oncol. 10, 587 (2020).
Strauss, J. et al. Phase I evaluation of M7824, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus (HPV)-associated malignancies [abstract]. Cancer Res. 79 (Suppl. 13), CT075 (2019).
Fallon, J. et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget 5, 1869–1884 (2014).
Teijeira, A. et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 52, 856–871.e8 (2020).
Strauss, J. et al. First-in-human phase I trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin. Cancer Res. 25, 99–109 (2019).
Autio, K. A., Boni, V., Humphrey, R. W. & Naing, A. Probody therapeutics: an emerging class of therapies designed to enhance on-target effects with reduced off-tumor toxicity for use in immuno-oncology. Clin. Cancer Res. 26, 984–989 (2020).
Kamata-Sakurai, M. et al. Antibody to CD137 activated by extracellular adenosine triphosphate is tumor selective and broadly effective in vivo without systemic immune activation. Cancer Discov. 11, 158–175 (2021).
Aznar, M. A. et al. Intratumoral delivery of immunotherapy–act locally, think globally. J. Immunol. 198, 31–39 (2017).
Hong, W. X. et al. Intratumoral immunotherapy for early-stage solid tumors. Clin. Cancer Res. 26, 3091–3099 (2020).
Melero, I. P. et al. Repurposing infectious disease vaccines for intratumoral immunotherapy. J. Immunother. Cancer 8, e000443 (2020).
Newman, J. H. et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc. Natl Acad. Sci. USA 117, 1119–1128 (2020).
Shekarian, T. et al. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade. Sci. Transl Med. 11, eaat5025 (2019).
Aznar, M. A. et al. Repurposing the yellow fever vaccine for intratumoral immunotherapy. EMBO Mol. Med. 12, e10375 (2020).
Galluzzi, L., Buque, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Pol, J. et al. Trial Watch: immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology 4, e1008866 (2015).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Halmos, B. et al. A matching-adjusted indirect comparison of pembrolizumab+chemotherapy vs. nivolumab+ipilimumab as first-line therapies in patients with PD-L1 TPS ≥1% metastatic NSCLC. Cancers 12, 3648 (2020).
He, X. et al. Upfront dose-reduced chemotherapy synergizes with immunotherapy to optimize chemoimmunotherapy in squamous cell lung carcinoma. J. Immunother. Cancer 8, e000807 (2020).
Swami, U. et al. Exceptional responses with sequential metronomic temozolomide after pembrolizumab failure in patients with metastatic melanoma. Melanoma Res. 29, 643–647 (2019).
Pfirschke, C. et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 44, 343–354 (2016).
Casares, N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202, 1691–1701 (2005).
Oratz, R. et al. Intratumoral cisplatin/adrenaline injectable gel for the treatment of patients with cutaneous and soft tissue metastases of malignant melanoma. Melanoma Res. 13, 59–66 (2003).
Vogl, T. J. et al. CT-guided intratumoural administration of cisplatin/epinephrine gel for treatment of malignant liver tumours. Br. J. Cancer 86, 524–529 (2002).
Khan, F., Anker, C. J., Garrison, G. & Kinsey, C. M. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann. Am. Thorac. Soc. 12, 101–104 (2015).
Mori, V., Roy, G. S., Bates, J. H. T. & Kinsey, C. M. Cisplatin pharmacodynamics following endobronchial ultrasound-guided transbronchial needle injection into lung tumors. Sci. Rep. 9, 6819 (2019).
Hohenforst-Schmidt, W. et al. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des. Devel Ther. 7, 571–583 (2013).
Marabelle, A. et al. Starting the fight in the tumor: expert recommendations for the development of human intratumoral immunotherapy (HIT-IT). Ann. Oncol. 29, 2163–2174 (2018).
Goldmacher, G. V. et al. Response criteria for intratumoral immunotherapy in solid tumors: itRECIST. J. Clin. Oncol. 38, 2667–2676 (2020).
Champiat, S. et al. Intratumoral immunotherapy: from trial design to clinical practice. Clin. Cancer Res. 27, 665–679 (2021).
Acknowledgements
The authors thank the patients, the patients’ families and colleagues who have participated in the authors’ preclinical and clinical research projects on intratumoural immunotherapy and have contributed to the development of the authors’ expertise in that field. The authors thank in particular T. de Baere, L. Tselikas, S. Ammari, S. Farhane and C. Massard for their contribution to the implementation of intratumoural immunotherapy trials at Gustave Roussy. The authors also thank their colleagues at the University of Navarra, especially A. Benito, M. Rodriguez-Ruiz, M.F. Sanmamed, C. de Andrea, J.L. Perez-Gracia, M. Ponz, L. Resano, I. Goicoechea and M. Egaña. The work of M.A. is funded by the Asociación Española contra el Cancer (AECC) Foundation, and the authors acknowledge continued financial support from the Spanish Ministry of Economy and Competitiveness (MINECO SAF2014-52361-R and SAF 2017-83267-C2-1R (AEI/FEDER,UE)), the Cancer Research Institute under the CRI-CLIP, the AECC Foundation under grant GCB15152947MELE, the Joint Translational Call for Proposals 2015 (JTC 2015) TRANSCAN-2 (code TRS715 2016-00000371), and the European Commission within the Horizon 2020 Programme (PROCROP - 718 635122).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
I.M. has received research grants from Alligator, Bioncotech, Bristol Myers Squibb (BMS), Leadartis, Pfizer and Roche; has received speaker’s bureau honoraria from MSD; and is a consultant or advisory board member for Alligator, AstraZeneca, Bayer, Bioncotech, BMS, F-Star, Genmab, Gossamer, Merck Serono, Numab, Pieris and Roche. E.C. is a consultant or advisory board member for AstraZeneca, Beigene, BMS, MSD and Roche. S.C. has received honoraria from Amgen, AstraZeneca, BMS, Janssen, Merck, MSD, Novartis and Roche; is an advisory board member for Amgen and AstraZeneca; has received funding for travel and conference attendance from AstraZeneca, MSD and Roche; and has received research grants from AstraZeneca, BMS, Boehringer Ingelheim, Janssen-Cilag, Merck, Novartis, Onxeo, Pfizer, Roche and Sanofi, and non-financial research support (investigational drugs) from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Medimmune, Merck, NH TherAGuiX, Onxeo, Pfizer and Roche. S.C. has been a principal investigator of academic or industry-sponsored clinical trials of intratumoural immunotherapies for Abbvie, AstraZeneca/Medimmune, BMS, Eisai/H3 Biomedicine, IDERA, Lytix Biopharma, MSD, Nanobiotix and Sanofi/BioNTech. A.M. has been a principal investigator of academic or industry-sponsored clinical trials of intratumoural immunotherapies from AstraZeneca, BMS, Eisai, IDERA, Lytix Biopharma, Merck/MSD, Roche and Transgene; is a member of the Data Safety and Monitoring Board of a trial of a intratumoural TLR3 agonist sponsored by Oncovir (NCT02423863); and has participated in scientific advisory boards or has provided consultancy services on the topic of intratumoural immunotherapies for Amgen, AstraZeneca, Banque Pour l’Investissement, Bayer, Eisai, eTheRNA, Lytix Biopharma, Medincell, MSD, Novartis, Oncosec, Pillar Partners, Rigontec and Sanofi/BioNTech. M.A. declares no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks H. Kaufman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Melero, I., Castanon, E., Alvarez, M. et al. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat Rev Clin Oncol 18, 558–576 (2021). https://doi.org/10.1038/s41571-021-00507-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-021-00507-y
This article is cited by
-
Intratumoural vaccination via checkpoint degradation-coupled antigen presentation
Nature (2026)
-
Treatment of NSCLC after chemoimmunotherapy — are we making headway?
Nature Reviews Clinical Oncology (2025)
-
Integrating antigen capturing nanoparticles and type 1 conventional dendritic cell therapy for in situ cancer immunization
Nature Communications (2025)
-
Orchestrating intratumoral DC-T cell immunity for enhanced tumor control via radiotherapy-activated TLR7/8 prodrugs in mice
Nature Communications (2025)
-
The LINC01315-encoded small protein YAPer-ORF competes with PRP4k to hijack YAP signaling to aberrantly promote cell growth
Cell Death & Differentiation (2025)