Abstract
Human papillomavirus (HPV)-positive (HPV+) oropharyngeal squamous cell carcinoma (OPSCC) has one of the most rapidly increasing incidences of any cancer in high-income countries. The most recent (8th) edition of the UICC/AJCC staging system separates HPV+ OPSCC from its HPV-negative (HPV−) counterpart to account for the improved prognosis seen in the former. Indeed, owing to its improved prognosis and greater prevalence in younger individuals, numerous ongoing trials are examining the potential for treatment de-intensification as a means to improve quality of life while maintaining acceptable survival outcomes. In addition, owing to the distinct biology of HPV+ OPSCCs, targeted therapies and immunotherapies have become an area of particular interest. Importantly, OPSCC is often detected at an advanced stage owing to a lack of symptoms in the early stages; therefore, a need exists to identify and validate possible diagnostic biomarkers to aid in earlier detection. In this Review, we provide a summary of the epidemiology, molecular biology and clinical management of HPV+ OPSCC in an effort to highlight important advances in the field. Ultimately, a need exists for improved understanding of the molecular basis and clinical course of this disease to guide efforts towards early detection and precision care, and to improve patient outcomes.
Key points
-
The incidence of human papillomavirus-associated oropharyngeal cancer (HPV+ OPSCC) is expected to continue to rise over the coming decades until the benefits of gender-neutral prophylactic HPV vaccination begin to become manifest.
-
The incidence of HPV+ OPSCC appears to be highest in high-income countries, although more epidemiological data are needed from low- and middle-income countries, in which HPV vaccination coverage remains low.
-
The substantially better prognosis of patients with HPV+ OPSCC compared to those with HPV– OPSCC has been recognized in the American Joint Committee on Cancer TNM8 staging guidelines, which recommend stratification by HPV status to improve staging.
-
The molecular biology and genomic features of HPV+ OPSCC are similar to those of other HPV-associated malignancies, with HPV oncogenes (E6 and E7) acting as key drivers of pathogenesis.
-
Treatment de-intensification is being pursued in clinical trials, although identifying the ~15% of patients with HPV+ OPSCC who have recurrent disease, and who therefore require more intensive treatment, remains a key challenge.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chaturvedi, A. K. et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 29, 4294–4301 (2011).
Gillison, M. L., Chaturvedi, A. K., Anderson, W. F. & Fakhry, C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 33, 3235–3242 (2015).
Senkomago, V. et al. Human papillomavirus-attributable cancers — United States, 2012–2016. MMWR Morb. Mortal. Wkly Rep. 68, 724–728 (2019).
Schache, A. G. et al. HPV-related oropharynx cancer in the United Kingdom: an evolution in the understanding of disease etiology. Cancer Res. 76, 6598–6606 (2016).
Lei, J. et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 383, 1340–1348 (2020).
Craig, S. G. et al. Recommendations for determining HPV status in patients with oropharyngeal cancers under TNM8 guidelines: a two-tier approach. Br. J. Cancer 120, 827–833 (2019).
Gillison, M. L. et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl Cancer Inst. 100, 407–420 (2008).
Lechner, M., Jones, O. S., Breeze, C. E. & Gilson, R. Gender-neutral HPV vaccination in the UK, rising male oropharyngeal cancer rates, and lack of HPV awareness. Lancet Infect. Dis. 19, 131–132 (2019).
Faraji, F. et al. The prevalence of human papillomavirus in oropharyngeal cancer is increasing regardless of sex or race, and the influence of sex and race on survival is modified by human papillomavirus tumor status. Cancer 125, 761–769 (2019).
Argirion, I. et al. Increasing prevalence of HPV in oropharyngeal carcinoma suggests adaptation of p16 screening in Southeast Asia. J. Clin. Virol. 132, 104637 (2020).
Hwang, T. Z., Hsiao, J. R., Tsai, C. R. & Chang, J. S. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995-2009. Int. J. Cancer 137, 395–408 (2015).
Wittekindt, C. et al. Increasing incidence rates of oropharyngeal squamous cell carcinoma in Germany and significance of disease burden attributed to human papillomavirus. Cancer Prev. Res. 12, 375–382 (2019).
Zamani, M. et al. The current epidemic of HPV-associated oropharyngeal cancer: an 18-year Danish population-based study with 2,169 patients. Eur. J. Cancer 134, 52–59 (2020).
Del Mistro, A. et al. Age-independent increasing prevalence of human papillomavirus-driven oropharyngeal carcinomas in North-East Italy. Sci. Rep. 10, 1–10 (2020).
Morbini, P. et al. The evolving landscape of human papillomavirus-related oropharyngeal squamous cell carcinoma at a single institution in northern Italy. Acta Otorhinolaryngol. Ital. 39, 9–17 (2019).
Haeggblom, L. et al. Changes in incidence and prevalence of human papillomavirus in tonsillar and base of tongue cancer during 2000-2016 in the Stockholm region and Sweden. Head. Neck 41, 1583–1590 (2019).
Donà, M. G. et al. Evolving profile of HPV-driven oropharyngeal squamous cell carcinoma in a national cancer institute in Italy: a 10-year retrospective study. Microorganisms 8, 1–12 (2020).
Girardi, F. M., Wagner, V. P., Martins, M. D., Abentroth, A. L. & Hauth, L. A. Prevalence of p16 expression in oropharyngeal squamous cell carcinoma in southern Brazil. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 130, 681–691 (2020).
Rietbergen, M. M. et al. Epidemiologic associations of HPV-positive oropharyngeal cancer and (pre)cancerous cervical lesions. Int. J. Cancer 143, 283–288 (2018).
Carlander, A. F. et al. A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses 13, 1326 (2021).
Chen, S. Y. et al. The association of smoking and outcomes in HPV-positive oropharyngeal cancer: a systematic review. Am. J. Otolaryngol. 41, 102592 (2020).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010).
Gooi, Z., Chan, J. Y. K. & Fakhry, C. The epidemiology of the human papillomavirus related to oropharyngeal head and neck cancer. Laryngoscope 126, 894–900 (2016).
D’Souza, G. et al. Sex differences in risk factors and natural history of oral human papillomavirus infection. J. Infect. Dis. 213, 1893–1896 (2016).
de Martel, C., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 141, 664–670 (2017).
Blumberg, J., Monjane, L., Prasad, M., Carrilho, C. & Judson, B. L. Investigation of the presence of HPV related oropharyngeal and oral tongue squamous cell carcinoma in Mozambique. Cancer Epidemiol. 39, 1000–1005 (2015).
Rettig, E. M. et al. Oral human papillomavirus infection and head and neck squamous cell carcinoma in rural northwest Cameroon. OTO Open 3, 2473974X18818415 (2019).
Ndiaye, C. et al. The role of human papillomavirus in head and neck cancer in Senegal. Infect. Agent. Cancer 8, 14 (2013).
Kofi, B. et al. Infrequent detection of human papillomavirus infection in head and neck cancers in the Central African Republic: a retrospective study. Infect. Agent Cancer 14, 9 (2019).
Oga, E. A. et al. Paucity of HPV-related head and neck cancers (HNC) in Nigeria. PLoS ONE 11, e0152828 (2016).
Chaturvedi, A. K. & Zumsteg, Z. S. A snapshot of the evolving epidemiology of oropharynx. cancers Cancer 124, 2893–2896 (2018).
Tota, J. E. et al. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J. Clin. Oncol. 37, 1538–1546 (2019).
Kreimer, A. R. et al. Summary from an international cancer seminar focused on human papillomavirus (HPV)-positive oropharynx cancer, convened by scientists at IARC and NCI. Oral. Oncol. 108, 104736 (2020).
Mariz BALA, K. L. P. et al. Global prevalence of human papillomavirus-driven oropharyngeal squamous cell carcinoma following the ASCO guidelines: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 156, 103116 (2020).
Windon, M. J. et al. Increasing prevalence of human papillomavirus–positive oropharyngeal cancers among older adults. Cancer 124, 2993–2999 (2018).
Rettig, E. M., Fakhry, C., Khararjian, A. & Westra, W. H. Age profile of patients with oropharyngeal squamous cell carcinoma. JAMA Otolaryngol. 144, 538–539 (2018).
Zumsteg, Z. S. et al. Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol. 2, 1617–1623 (2016).
Mahal, B. A. et al. Incidence and demographic burden of HPV-associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol. Biomark. Prev. 28, 1660–1667 (2019).
Ramer, I. et al. Racial disparities in incidence of human papillomavirus-associated oropharyngeal cancer in an urban population. Cancer Epidemiol. 44, 91–95 (2016).
Liederbach, E. et al. The national landscape of human papillomavirus-associated oropharynx squamous cell carcinoma. Int. J. Cancer 140, 504–512 (2017).
Falcaro, M. et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet 398, 2084–2092 (2021).
Masterson, L. & Lechner, M. HPV vaccination in boys — will the UK join the fight? Nat. Rev. Clin. Oncol. 13, 721–722 (2016).
HPV Vaccination Uptake. Australia National Control Indicators. Published 2019. https://ncci.canceraustralia.gov.au/prevention/hpv-vaccination-uptake/hpv-vaccination-uptake (2021).
Walker, T. Y. et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2019. MMWR Morb. Mortal. Wkly Rep. 69, 1109–1116 (2020).
Public Health England. Human papillomavirus (HPV) vaccination coverage in adolescent females and males in England: academic year 2019 to 2020. Heal. Prot. Rep. 14, 1–15 (2020).
Radisic, G., Chapman, J., Flight, I. & Wilson, C. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: a systematic review. Prev. Med. 95, 26–37 (2017).
Sonawane, K. et al. Parental intent to initiate and complete the human papillomavirus vaccine series in the USA: a nationwide, cross-sectional survey. Lancet Public Heal. 5, e484–e492 (2020).
Gottvall, M., Stenhammar, C. & Grandahl, M. Parents’ views of including young boys in the Swedish national school-based HPV vaccination programme: a qualitative study. BMJ Open 7, 11–13 (2017).
Thompson, E. L. et al. Awareness and knowledge of HPV and HPV vaccination among adults ages 27–45 years. Vaccine 38, 3143–3148 (2020).
Waller, J. et al. Decision-making about HPV vaccination in parents of boys and girls: a population-based survey in England and Wales. Vaccine 38, 1040–1047 (2020).
Sherman, S. M., Cohen, C. R., Denison, H. J., Bromhead, C. & Patel, H. A survey of knowledge, attitudes and awareness of the human papillomavirus among healthcare professionals across the UK. Eur. J. Public Health 30, 10–16 (2020).
Lechner, M. et al. A cross-sectional survey of awareness of human papillomavirus-associated oropharyngeal cancers among general practitioners in the UK. BMJ Open 8, 1–6 (2018).
Katz, J. The impact of HPV vaccination on the prevalence of oropharyngeal cancer (OPC) in a hospital-based population: a cross-sectional study of patient’s registry. J. Oral. Pathol. Med. 50, 47–51 (2021).
Herrero, R. et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS ONE 8, e68329 (2013).
Chaturvedi, A. K. et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J. Clin. Oncol. 36, 262–267 (2018).
Hirth, J. M., Chang, M., Resto, V. A., Guo, F. & Berenson, A. B. Prevalence of oral human papillomavirus by vaccination status among young adults (18–30 years old). Vaccine 35, 3446–3451 (2017).
Zhang, Y., Fakhry, C. & D’Souza, G. Projected association of human papillomavirus vaccination with oropharynx cancer incidence in the US, 2020–2045. JAMA Oncol. 7, e212907 (2021).
Lechner, M., Breeze, C. E., O’Mahony, J. F. & Masterson, L. Early detection of HPV-associated oropharyngeal cancer. Lancet 393, 2123 (2019).
Kreimer, A. R. et al. Timing of HPV16-E6 antibody seroconversion before OPSCC: findings from the HPVC3 consortium. Ann. Oncol. 30, 1335–1343 (2019).
Kreimer, A. R., Clifford, G. M., Boyle, P. & Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systemic review. Cancer Epidemiol. Biomark. Prev. 14, 467–475 (2005).
Egawa, N., Egawa, K., Griffin, H. & Doorbar, J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 7, 3863–3890 (2015).
Doorbar, J. et al. The biology and life-cycle of human papillomaviruses. Vaccine 30, F55–F70 (2012).
Graham, S. V. Keratinocyte differentiation-dependent human papillomavirus gene regulation. Viruses 9, 245 (2017).
Parfenov, M. et al. Characterization of HPV and host genome interactions in primary head and neck cancers. Proc. Natl Acad. Sci. USA 111, 15544–15549 (2014).
Vinokurova, S. et al. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 68, 307–313 (2008).
Ramqvist, T. et al. Studies on human papillomavirus (HPV) 16 E2, E5 and E7 mRNA in HPV-positive tonsillar and base of tongue cancer in relation to clinical outcome and immunological parameters. Oral. Oncol. 51, 1126–1131 (2015).
Koneva, L. A. et al. HPV integration in HNSCC correlates with survival outcomes, immune response signatures, and candidate drivers. Mol. Cancer Res. 16, 90–102 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Mesri, E. A., Feitelson, M. A. & Munger, K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe 15, 266–282 (2014).
Huibregtse, J. M., Scheffner, M. & Howley, P. M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 10, 4129–4135 (1991).
Scheffner, M., Werness, B. A., Huibregtse, J. M., Levine, A. J. & Howley, P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63, 1129–1136 (1990).
Scheffner, M., Huibregtse, J. M., Vierstra, R. D. & Howley, P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75, 495–505 (1993).
Huh, K. et al. Human papillomavirus type 16 E7 oncoprotein associates with the Cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J. Virol. 81, 9737–9747 (2007).
Dyson, N., Howley, P. M., Munger, K. & Harlow, E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243, 934–938 (1986).
Münger, K., Phelps, W. C., Bubb, V., Howley, P. M. & Schlegel, R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J. Virol. 63, 4417–4421 (1989).
Božinović, K. et al. Genome-wide miRNA profiling reinforces the importance of miR-9 in human papillomavirus associated oral and oropharyngeal head and neck cancer. Sci. Rep. 9, 2306 (2019).
Boscolo-Rizzo, P., Furlan, C., Lupato, V., Polesel, J. & Fratta, E. Novel insights into epigenetic drivers of oropharyngeal squamous cell carcinoma: role of HPV and lifestyle factors. Clin. Epigenetics 9, 124 (2017).
Barr, J. A. et al. Long non-coding RNA FAM83H-AS1 is regulated by human papillomavirus 16 E6 independently of p53 in cervical cancer cells. Sci. Rep. 9, 3662 (2019).
Lechner, M. et al. Identification and functional validation of HPV-mediated hypermethylation in head and neck squamous cell carcinoma. Genome Med. 5, 15 (2013).
Burgers, W. A. et al. Viral oncoproteins target the DNA methyltransferases. Oncogene 26, 1650–1655 (2007).
Chalertpet, K., Pakdeechaidan, W., Patel, V., Mutirangura, A. & Yanatatsaneejit, P. Human papillomavirus type 16 E7 oncoprotein mediates CCNA1 promoter methylation. Cancer Sci. 106, 1333–1340 (2015).
Cicchini, L. et al. Suppression of antitumor immune responses by human papillomavirus through epigenetic downregulation of CXCL14. mBio 7, e00270-16 (2016).
Cicchini, L. et al. High-risk human papillomavirus E7 alters host DNA methylome and represses HLA-E expression in human keratinocytes. Sci. Rep. 7, 3633 (2017).
Munger, K. & Jones, D. L. Human papillomavirus carcinogenesis: an identity crisis in the retinoblastoma tumor suppressor pathway. J. Virol. 89, 4708–4711 (2015).
Magaldi, T. G. et al. Primary human cervical carcinoma cells require human papillomavirus E6 and E7 expression for ongoing proliferation. Virology 422, 114–124 (2012).
Weinstein, I. B. Addiction to oncogenes–the Achilles heal of cancer. Science 297, 63–64 (2002).
McLaughlin-Drubin, M. E., Crum, C. P. & Münger, K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc. Natl Acad. Sci. USA 108, 2130–2135 (2011).
McLaughlin-Drubin, M. E., Park, D. & Munger, K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc. Natl Acad. Sci. USA 110, 16175–16180 (2013).
Spring, L. M. et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet 395, 817–827 (2020).
Soto, D. R., Barton, C., Munger, K. & McLaughlin-Drubin, M. E. KDM6A addiction of cervical carcinoma cell lines is triggered by E7 and mediated by p21CIP1 suppression of replication stress. PLoS Pathog. 13, 1–25 (2017).
Ganti, K. et al. The human papillomavirus E6 PDZ binding motif: from life cycle to malignancy. Viruses 7, 3530–3551 (2015).
Mittal, S. & Banks, L. Molecular mechanisms underlying human papillomavirus E6 and E7 oncoprotein-induced cell transformation. Mutat. Res. 772, 23–35 (2017).
Roman, A. & Munger, K. The papillomavirus E7 proteins. Virology 445, 138–168 (2013).
Moody, C. A. & Laimins, L. A. Human papillomavirus oncoproteins: pathways to transformation. Nat. Rev. Cancer 10, 550–560 (2010).
Seiwert, T. Y. et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin. Cancer Res. 21, 632–641 (2015).
The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517, 576–582 (2015).
Gillison, M. L. et al. Human papillomavirus and the landscape of secondary genetic alterations in oral cancers. Genome Res. 29, 1–17 (2019).
Dogan, S. et al. Identification of prognostic molecular biomarkers in 157 HPV-positive and HPV-negative squamous cell carcinomas of the oropharynx. Int. J. Cancer 145, 3152–3162 (2019).
Lechner, M. et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV− tumors. Genome Med. 5, 49 (2013).
Hayes, D. N., Van Waes, C. & Seiwert, T. Y. Genetic landscape of human papillomavirus-associated head and neck cancer and comparison to tobacco-related tumors. J. Clin. Oncol. 33, 3227–3234 (2015).
Henderson, S., Chakravarthy, A., Su, X., Boshoff, C. & Fenton, T. R. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep. 7, 1833–1841 (2014).
Zhu, B. et al. Mutations in the HPV16 genome induced by APOBEC3 are associated with viral clearance. Nat. Commun. 11, 1–12 (2020).
Faden, D. L. et al. APOBEC mutagenesis is concordant between tumor and viral genomes in HPV-positive head and neck squamous cell carcinoma. Viruses 13, 1666 (2021).
Smith, N. J. & Fenton, T. R. The APOBEC3 genes and their role in cancer: insights from human papillomavirus. J. Mol. Endocrinol. 62, R269–R287 (2019).
Fenton, T. R. Accumulation of host cell genetic errors following high-risk HPV infection. Curr. Opin. Virol. 51, 1–8 (2021).
Warren, C. J., Westrich, J. A., Van Doorslaer, K. & Pyeon, D. Roles of APOBEC3A and APOBEC3B in human papillomavirus infection and disease progression. Viruses 9, 1–20 (2017).
Lui, V. W. Y. et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 3, 761–769 (2013).
Nichols, A. C. et al. High frequency of activating PIK3CA mutations in human papillomavirus- positive oropharyngeal cancer. JAMA Otolaryngol. 139, 617–622 (2013).
Hanna, G. J. et al. Improved outcomes in PI3K-pathway-altered metastatic HPV oropharyngeal cancer. JCI Insight 3, e122799 (2018).
Beaty, B. T. et al. PIK3CA mutation in HPV-associated OPSCC patients receiving deintensified chemoradiation. J. Natl Cancer Inst. 112, 855–858 (2019).
Hedberg, M. L. et al. Use of nonsteroidal anti-inflammatory drugs predicts improved patient survival for PIK3CA-altered head and neck cancer. J. Exp. Med. 216, 419–427 (2019).
Cai, Y., Yousef, A., Grandis, J. R. & Johnson, D. E. NSAID therapy for PIK3CA-altered colorectal, breast, and head and neck cancer. Adv. Biol. Regul. 75, 100653 (2020).
Paleari, L. et al. PIK3CA mutation, aspirin use after diagnosis and survival of colorectal cancer. a systematic review and meta-analysis of epidemiological studies. Clin. Oncol. 28, 317–326 (2016).
Nyman, P. E., Buehler, D. & Lambert, P. F. Loss of function of canonical Notch signaling drives head and neck carcinogenesis. Clin. Cancer Res. 24, 6308–6318 (2018).
Kranjec, C. et al. Modulation of basal cell fate during productive and transforming HPV-16 infection is mediated by progressive E6-driven depletion of Notch. J. Pathol. 242, 448–462 (2017).
Beglin, M., Melar-New, M. & Laimins, L. Human papillomaviruses and the interferon response. J. Interf. Cytokine Res. 29, 629–635 (2009).
Dhawan, A. et al. Role of gene signatures combined with pathology in classification of oropharynx head and neck cancer. Sci. Rep. 10, 10226 (2020).
She, Y. et al. Immune-related gene signature for predicting the prognosis of head and neck squamous cell carcinoma. Cancer Cell Int. 20, 22 (2020).
Chan, T. A. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol. 30, 44–56 (2019).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Steinbach, A. & Riemer, A. B. Immune evasion mechanisms of human papillomavirus: an update. Int. J. Cancer 142, 224–229 (2018).
Ashrafi, G. H., Haghshenas, M. R., Marchetti, B., O’Brien, P. M. & Campo, M. S. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int. J. Cancer 113, 276–283 (2005).
Ashrafi, G. H., Haghshenas, M., Marchetti, B. & Campo, M. S. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int. J. Cancer 119, 2105–2112 (2006).
Campo, M. S. et al. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 407, 137–142 (2010).
Georgopoulos, N. T., Proffitt, J. L. & Blair, G. E. Transcriptional regulation of the major histocompatibility complex (MHC) class I heavy chain, TAP1 and LMP2 genes by the human papillomavirus (HPV) type 6b, 16 and 18 E7 oncoproteins. Oncogene 19, 4930–4935 (2000).
Li, H., Ou, X., Xiong, J. & Wang, T. HPV16E7 mediates HADC chromatin repression and downregulation of MHC class I genes in HPV16 tumorigenic cells through interaction with an MHC class I promoter. Biochem. Biophys. Res. Commun. 349, 1315–1321 (2006).
Bottley, G. et al. High-risk human papillomavirus E7 expression reduces cell-surface MHC class I molecules and increases susceptibility to natural killer cells. Oncogene 27, 1794–1799 (2008).
Heusinkveld, M. et al. Systemic and local human papillomavirus 16-specific T-cell immunity in patients with head and neck cancer. Int. J. Cancer 131, 74–85 (2012).
Welters, M. J. P. et al. Intratumoral HPV16-specific T cells constitute a type I-oriented tumor microenvironment to improve survival in HPV16-driven oropharyngeal cancer. Clin. Cancer Res. 24, 634–647 (2018).
Santegoets, S. J. et al. The anatomical location shapes the immune infiltrate in tumors of same etiology and affects survival. Clin. Cancer Res. 25, 240–252 (2019).
Santegoets, S. J. et al. CD163+ cytokine-producing cDC2 stimulate intratumoral type 1 T cell responses in HPV16-induced oropharyngeal cancer. J. Immunother. Cancer 8, e001053 (2020).
Hoffmann, T. K. et al. T cells specific for HPV16 E7 epitopes in patients with squamous cell carcinoma of the oropharynx. Int. J. Cancer 118, 1984–1991 (2006).
Masterson, L. et al. CD8+ T cell response to human papillomavirus 16 E7 is able to predict survival outcome in oropharyngeal cancer. Eur. J. Cancer 67, 141–151 (2016).
Laban, S. & Hoffmann, T. K. Human papillomavirus immunity in oropharyngeal cancer: time to change the game? Clin. Cancer Res. 24, 505–507 (2018).
Balermpas, P. et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: a multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 138, 171–181 (2016).
Ward, M. J. et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 110, 489–500 (2014).
Mandal, R. et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 1, e89829 (2016).
Chakravarthy, A. et al. Human papillomavirus drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely restricted to the oropharynx. J. Clin. Oncol. 34, 4132–4141 (2016).
Li, H. et al. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol. 144, 519–525 (2018).
Hladíková, K. et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8+ T cells. J. Immunother. Cancer 7, 261 (2019).
Wood, O. et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 7, 56781–56797 (2016).
Ou, D. et al. Influence of tumor-associated macrophages and HLA class I expression according to HPV status in head and neck cancer patients receiving chemo/bioradiotherapy. Radiother. Oncol. 130, 89–96 (2019).
Welters, M. J. P., Santegoets, S. J. & van der Burg, S. H. The tumor microenvironment and immunotherapy of oropharyngeal squamous cell carcinoma. Front. Oncol. 10, 545385 (2020).
Hong, A. M. et al. Significant association of PD-L1 expression with human papillomavirus positivity and its prognostic impact in oropharyngeal cancer. Oral. Oncol. 92, 33–39 (2019).
Cao, S. et al. Dynamic host immune response in virus-associated cancers. Commun. Biol. 2, 109 (2019).
McIlwain, W. R., Sood, A. J., Nguyen, S. A. & Day, T. A. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol. 140, 441–447 (2014).
Khalid, M. B. et al. Initial presentation of human papillomavirus-related head and neck cancer: a retrospective review. Laryngoscope 129, 877–882 (2019).
Tham, T., Ahn, S., Frank, D., Kraus, D. & Costantino, P. Anatomical subsite modifies survival in oropharyngeal squamous cell carcinoma: National Cancer Database study. Head. Neck 42, 434–445 (2020).
Golusinski, P. et al. Evidence for the approach to the diagnostic evaluation of squamous cell carcinoma occult primary tumors of the head and neck. Oral. Oncol. 88, 145–152 (2019).
Zhang, M. Q., El-Mofty, S. K. & Dávila, R. M. Detection of human papillomavirus-related squamous cell carcinoma cytologically and by in situ hybridization in fine-needle aspiration biopsies of cervical metastasis: a tool for identifying the site of an occult head and neck primary. Cancer 114, 118–123 (2008).
Begum, S., Gillison, M. L., Nicol, T. L. & Westra, W. H. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin. Cancer Res. 13, 1186–1191 (2007).
Mehanna, H. et al. Oropharyngeal cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 130, S90–S96 (2016).
Gage, K. L., Thomas, K., Jeong, D., Stallworth, D. G. & Arrington, J. A. Multimodal imaging of head and neck squamous cell carcinoma. Cancer Control. 24, 172–179 (2017).
Schache, A. G. et al. Evaluation of human papilloma virus diagnostic testing in oropharyngeal squamous cell carcinoma: sensitivity, specificity, and prognostic discrimination. Clin. Cancer Res. 17, 6262–6271 (2011).
Lewis, J. S. Morphologic diversity in human papillomavirus-related oropharyngeal squamous cell carcinoma: catch me if you can! Mod. Pathol. 30, S44–S53 (2017).
Chernock, R. D., Lewis, J. S., Zhang, Q. & El-Mofty, S. K. Human papillomavirus-positive basaloid squamous cell carcinomas of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Hum. Pathol. 41, 1016–1023 (2010).
Cho, K. J. et al. Basaloid squamous cell carcinoma of the head and neck: subclassification into basal, ductal, and mixed subtypes based on comparison of clinico-pathologic features and expression of p53, cyclin D1, epidermal growth factor receptor, p16, and human papilloma. J. Pathol. Transl. Med. 51, 374–380 (2017).
Mehrad, M. et al. Papillary squamous cell carcinoma of the head and neck: clinicopathologic and molecular features with special reference to human papillomavirus. Am. J. Surg. Pathol. 37, 1349–1356 (2013).
Carpenter, D. H., El-Mofty, S. K. & Lewis, J. S. Undifferentiated carcinoma of the oropharynx: a human papillomavirus-associated tumor with a favorable prognosis. Mod. Pathol. 24, 1306–1312 (2011).
Singhi, A. D., Stelow, E. B., Mills, S. E. & Westra, W. H. Lymphoepithelial-like carcinoma of the oropharynx: a morphologic variant of HPV-related head and neck carcinoma. Am. J. Surg. Pathol. 34, 800–805 (2010).
Jo, V. Y., Mills, S. E., Stoler, M. H. & Stelow, E. B. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am. J. Surg. Pathol. 33, 1720–1724 (2009).
Bryne, M., Koppang, H. S., Lilleng, R. & Kjærheim, Å. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J. Pathol. 166, 375–381 (1992).
Albergotti, W. G. et al. Defining the prevalence and prognostic value of perineural invasion and angiolymphatic invasion in human papillomavirus-positive oropharyngeal carcinoma. JAMA Otolaryngol. 143, 1236–1243 (2017).
Dirven, R. et al. Tumor thickness versus depth of invasion – analysis of the 8th edition American Joint Committee on Cancer Staging for oral cancer. Oral. Oncol. 74, 30–33 (2017).
Zhan, K. Y. et al. Appraisal of the AJCC 8th edition pathologic staging modifications for HPV−positive oropharyngeal cancer, a study of the National Cancer Data Base. Oral. Oncol. 73, 152–159 (2017).
Elicin, O. et al. Comparison of contemporary staging systems for oropharynx cancer in a surgically treated multi-institutional cohort. Head. Neck 41, 1395–1402 (2019).
Bhattasali, O., Thompson, L. D. R., Schumacher, A. J. & Iganej, S. Radiographic nodal prognostic factors in stage I HPV-related oropharyngeal squamous cell carcinoma. Head. Neck 41, 398–402 (2019).
Sinha, P. et al. High metastatic node number, not extracapsular spread or N-classification is a node-related prognosticator in transorally-resected, neck-dissected p16-positive oropharynx cancer. Oral. Oncol. 51, 514–520 (2015).
Bauer, E. et al. Extranodal extension is a strong prognosticator in HPV-positive oropharyngeal squamous cell carcinoma. Laryngoscope 130, 939–945 (2020).
Freitag, J. et al. Extracapsular extension of neck nodes and absence of human papillomavirus 16-DNA are predictors of impaired survival in p16-positive oropharyngeal squamous cell carcinoma. Cancer 126, 1856–1872 (2020).
Tian, S. et al. Prognostic value of radiographically defined extranodal extension in human papillomavirus-associated locally advanced oropharyngeal carcinoma. Head. Neck 41, 3056–3063 (2019).
Meyer, M. F. et al. The relevance of the lymph node ratio as predictor of prognosis is higher in HPV-negative than in HPV-positive oropharyngeal squamous cell carcinoma. Clin. Otolaryngol. 43, 192–198 (2018).
Chai, R. L. et al. Accuracy of computed tomography in the prediction of extracapsular spread of lymph node metastases in squamous cell carcinoma of the head and neck. JAMA Otolaryngol. 139, 1187–1194 (2013).
Aiken, A. H. et al. Accuracy of preoperative imaging in detecting nodal extracapsular spread in oral cavity squamous cell carcinoma. Am. J. Neuroradiol. 36, 1776–1781 (2015).
Carlton, J. A. et al. Computed tomography detection of extracapsular spread of squamous cell carcinoma of the head and neck in metastatic cervical lymph nodes. Neuroradiol. J. 30, 222–229 (2017).
Douglas, C. et al. Accuracy of contrast-enhanced CT and predictive factors for extracapsular spread in unknown primary head and neck squamous cell cancer. Clin. Radiol. 75, 77.e23–77.e28 (2020).
O’Sullivan, B. et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 17, 440–451 (2016).
Cramer, J. D., Hicks, K. E., Rademaker, A. W., Patel, U. A. & Samant, S. Validation of the eighth edition American Joint Committee on Cancer staging system for human papillomavirus-associated oropharyngeal cancer. Head. Neck 40, 457–466 (2018).
Geltzeiler, M. et al. Staging HPV-related oropharyngeal cancer: validation of AJCC-8 in a surgical cohort. Oral. Oncol. 84, 82–87 (2018).
Van Gysen, K. et al. Validation of the 8th edition UICC/AJCC TNM staging system for HPV associated oropharyngeal cancer patients managed with contemporary chemo-radiotherapy. BMC Cancer 19, 674 (2019).
Würdemann, N. et al. Prognostic impact of AJCC/UICC 8th edition new staging rules in oropharyngeal squamous cell carcinoma. Front. Oncol. 7, 129 (2017).
Nauta, I. H. et al. Evaluation of the eighth TNM classification on p16-positive oropharyngeal squamous cell carcinomas in the Netherlands and the importance of additional HPV DNA testing. Ann. Oncol. 29, 1273–1279 (2018).
Fakhry, C. et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: implications for risk-based therapeutic intensity trials. Cancer 125, 2027–2038 (2019).
Haeggblom, L., Ramqvist, T., Tommasino, M. & Dalianis, T. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res. 4, 1–11 (2017).
Wendt, M. et al. Long-term survival and recurrence in oropharyngeal squamous cell carcinoma in relation to subsites, HPV, and p16-status. Cancers (Basel) 13, 2553 (2021).
Ellis, M. et al. Post-treatment head and neck cancer care: national audit and analysis of current practice in the United Kingdom. Clin. Otolaryngol. 46, 284–294 (2021).
Fakhry, C. et al. Association of oral human papillomavirus DNA persistence with cancer progression after primary treatment for oral cavity and oropharyngeal squamous cell carcinoma. JAMA Oncol. 5, 985–992 (2019).
Chera, B. S. et al. Plasma circulating tumor HPV DNA for the surveillance of cancer recurrence in HPV-associated oropharyngeal cancer. J. Clin. Oncol. 38, 1050–1058 (2020).
Holsinger, F. C. & Ferris, R. L. Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: robotics, lasers, and clinical trials. J. Clin. Oncol. 33, 3285–3292 (2015).
Sinha, P., Haughey, B. H., Kallogjeri, D. & Jackson, R. S. Long-term analysis of transorally resected p16 + oropharynx cancer: outcomes and prognostic factors. Laryngoscope 129, 1141–1149 (2019).
Mahmoud, O., Sung, K., Civantos, F. J., Thomas, G. R. & Samuels, M. A. Transoral robotic surgery for oropharyngeal squamous cell carcinoma in the era of human papillomavirus. Head Neck 40, 710–721 (2018).
Jackson, R. S. et al. Transoral resection of human papillomavirus (HPV)-positive squamous cell carcinoma of the oropharynx: outcomes with and without adjuvant therapy. Ann. Surg. Oncol. 24, 3494–3501 (2017).
Carey, R. M. et al. Increased rate of recurrence and high rate of salvage in patients with human papillomavirus–associated oropharyngeal squamous cell carcinoma with adverse features treated with primary surgery without recommended adjuvant therapy. Head Neck 43, 1128–1141 (2021).
Sethia, R. et al. Quality of life outcomes of transoral robotic surgery with or without adjuvant therapy for oropharyngeal cancer. Laryngoscope 128, 403–411 (2018).
Ma, D. J. et al. Phase II evaluation of aggressive dose de-escalation for adjuvant chemoradiotherapy in human papillomavirus-associated oropharynx squamous cell carcinoma. J. Clin. Oncol. 37, 1909–1918 (2019).
Hargreaves, S., Beasley, M., Hurt, C., Jones, T. M. & Evans, M. Deintensification of adjuvant treatment after transoral surgery in patients with human papillomavirus-positive oropharyngeal cancer: the conception of the PATHOS study and its development. Front Oncol. 9, 936 (2019).
Ferris, R. L. et al. Updated report of a phase II randomized trial of transoral surgical resection followed by low-dose or standard postoperative therapy in resectable p16+ locally advanced oropharynx cancer: a trial of the ECOG-ACRIN cancer research group (E3311). J. Clin. Oncol. 39, 6010 (2021).
Ferris, R. L. et al. Transoral robotic surgical resection followed by randomization to low- or standard-dose IMRT in resectable p16+ locally advanced oropharynx cancer: a trial of the ECOG-ACRIN Cancer Research Group (E3311). J. Clin. Oncol. 38, 6500 (2020).
Ferris, R. L. et al. Phase II randomized trial of transoral surgery and low-dose intensity modulated radiation therapy in resectable p16+ locally advanced oropharynx cancer: an ECOG-ACRIN Cancer Research Group trial (E3311). J. Clin. Oncol. 40, 138–149 (2022).
Chera, B. S. et al. Phase 2 trial of de-intensified chemoradiation therapy for favorable-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 93, 976–985 (2015).
Chera, B. S. et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 124, 2347–2354 (2018).
Pearlstein, K. A. et al. Quality of life for patients with favorable-risk HPV-associated oropharyngeal cancer after de-intensified chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 103, 646–653 (2019).
Seiwert, T. Y. et al. Optima: a phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann. Oncol. 30, 297–302 (2019).
Marur, S. et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx-ECOG-ACRIN cancer research group. J. Clin. Oncol. 35, 490–497 (2017).
Hegde, J. V. et al. Functional outcomes after de-escalated chemoradiation therapy for human papillomavirus-positive oropharyngeal cancer: secondary analysis of a phase 2 trial. Int. J. Radiat. Oncol. Biol. Phys. 100, 647–651 (2018).
Chen, A. M. et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 18, 803–811 (2017).
Yamamoto, Y. et al. Radiotherapy alone as a possible de-intensified treatment for human papillomavirus-related locally advanced oropharyngeal squamous cell carcinoma. Int. J. Clin. Oncol. 24, 640–648 (2019).
Hall, S. F., Griffiths, R. J., O’Sullivan, B. & Liu, F. F. The addition of chemotherapy to radiotherapy did not reduce the rate of distant metastases in low-risk HPV-related oropharyngeal cancer in a real-world setting. Head Neck 41, 2271–2276 (2019).
Yom, S. S. et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG Oncology HN002). J. Clin. Oncol. 39, 956–965 (2021).
Sher, D. J. et al. Radiation therapy for oropharyngeal squamous cell carcinoma: executive summary of an ASTRO evidence-based clinical practice guideline. Pract. Radiat. Oncol. 7, 246–253 (2017).
Howard, J. et al. Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small-volume primary oropharyngeal carcinoma. Cochrane Database Syst. Rev. 12, CD010963 (2016).
Nichols, A. C. et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol. 20, 1349–1359 (2019).
Ferris, R. L. et al. A novel surgeon credentialing and quality assurance process using transoral surgery for oropharyngeal cancer in ECOG-ACRIN Cancer Research Group trial E3311. Oral. Oncol. 110, 104797 (2020).
de Almeida, J. R. et al. Oncologic outcomes after transoral robotic surgery: a multi-institutional study. JAMA Otolaryngol. 141, 1043–1051 (2015).
Mehanna, H. et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 393, 51–60 (2019).
Gillison, M. L. et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393, 40–50 (2019).
Oosthuizen, J. C. & Doody, J. De-intensified treatment in human papillomavirus-positive oropharyngeal cancer. Lancet 393, 5–7 (2019).
Guo, T. et al. Characterization of functionally active gene fusions in human papillomavirus related oropharyngeal squamous cell carcinoma. Int. J. Cancer 139, 373–382 (2016).
Dunn, L. A. et al. Phase I study of induction chemotherapy with afatinib, ribavirin, and weekly carboplatin and paclitaxel for stage IVA/IVB human papillomavirus-associated oropharyngeal squamous cell cancer. Head. Neck 40, 233–241 (2018).
Frazer, I. H. & Chandra, J. Immunotherapy for HPV associated cancer. Papillomavirus Res. 8, 100176 (2019).
Barra, F. et al. Advances in therapeutic vaccines for treating human papillomavirus-related cervical intraepithelial neoplasia. J. Obstet. Gynaecol. Res. 46, 989–1006 (2020).
Smalley Rumfield, C., Pellom, S. T., Morillon, Y. M., Schlom, J. & Jochems, C. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-000612 (2020).
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Cohen, E. E. W. et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 393, 156–167 (2019).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Xu, Y. et al. Programmed death-1/programmed death-ligand 1-axis blockade in recurrent or metastatic head and neck squamous cell carcinoma stratified by human papillomavirus status: a systematic review and meta-analysis. Front. Immunol. 12, 645170 (2021).
Wang, J. et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci. Rep. 9, 13404 (2019).
Galvis, M. M. et al. Immunotherapy improves efficacy and safety of patients with HPV positive and negative head and neck cancer: a systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 150, 102966 (2020).
Patel, J. J., Levy, D. A., Nguyen, S. A., Knochelmann, H. M. & Day, T. A. Impact of PD-L1 expression and human papillomavirus status in anti-PD1/PDL1 immunotherapy for head and neck squamous cell carcinoma — systematic review and meta-analysis. Head Neck 42, 774–786 (2019).
Wong, D. J. et al. Abstract CT123: IMvoke010: randomized phase III study of atezolizumab as adjuvant monotherapy after definitive therapy of squamous cell carcinoma of the head and neck. Cancer Res. 79, (Suppl.) 13 (2019).
Leidner, R. et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J. Immunother. Cancer 9, e002485 (2021).
Ferris, R. L. et al. Neoadjuvant nivolumab for patients with resectable HPV-positive and HPV-negative squamous cell carcinomas of the head and neck in the CheckMate 358 trial. J. Immunother. Cancer 9, e002568 (2021).
Ferrarotto, R. et al. Impact of neoadjuvant durvalumab with or without tremelimumab on CD8+ tumor lymphocyte density, safety, and efficacy in patients with oropharynx cancer: CIAO trial results. Clin. Cancer Res. 26, 3211–3219 (2020).
von Witzleben, A., Wang, C., Laban, S., Savelyeva, N. & Ottensmeier, C. H. HNSCC: tumour antigens and their targeting by immunotherapy. Cells 9, 2103 (2020).
Massarelli, E. et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 5, 67–73 (2019).
Aggarwal, C. et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin. Cancer Res. 25, 110–124 (2019).
Fakhry, C. et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 32, 3365–3373 (2014).
Harbison, R. A. et al. The mutational landscape of recurrent versus nonrecurrent human papillomavirus-related oropharyngeal cancer. JCI insight 3, e99327 (2018).
Gleber-netto, F. O. et al. Variations in HPV function are associated with survival in squamous cell carcinoma. JCI Insight 4, e124762 (2019).
King, A. and Broggio, J. Cancer Registration Statistics, England https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/cancerregistrationstatisticsengland/final2016 (2016).
Surveillance Research Program SEER Incidence Data, 1975–2018. https://seer.cancer.gov/data/ (National Cancer Institute, 2020).
Lechner, M. & Fenton, T. R. The genomics, epigenomics, and transcriptomics of HPV-associated oropharyngeal cancer — understanding the basis of a rapidly evolving disease. Adv. Genet. 93, 1–56 (2016).
Machczyński, P., Majchrzak, E., Niewinski, P., Marchlewska, J. & Golusiński, W. A review of the 8th edition of the AJCC staging system for oropharyngeal cancer according to HPV status. Eur. Arch. Otorhinolaryngol. 277, 2407–2412 (2020).
Adelstein, D. J. et al. Role of treatment deintensification in the management of p16+ oropharyngeal cancer: ASCO provisional clinical opinion. J. Clin. Oncol. 37, 1578–1589 (2019).
Posner, M. R. et al. Survival (OS) and progression-free survival (PFS) results after induction chemotherapy (IC) followed by de-escalated chemoradiotherapy (RDCRT) for locally advanced (LA) HPV positive oropharynx cancer (HPVOPC). J. Clin. Oncol. 39, 6058 (2021).
Palma, D. A. et al. A randomized trial of radiotherapy vs. trans-oral surgery for treatment de-escalation in HPV-associated oropharyngeal squamous cell carcinoma (ORATOR2). Int. J. Rad. Oncol. Biophys. 111, 1324–1325 (2021).
Miles, B. A. et al. De-escalated adjuvant therapy after transoral robotic surgery for human papillomavirus-related oropharyngeal carcinoma: the Sinai robotic surgery (SIRS) trial. Oncologist 26, 504–513 (2021).
Swisher–McClure, S. et al. A phase 2 trial of Alternative Volumes of Oropharyngeal Irradiation for De-intensification (AVOID): omission of the resected primary tumor bed after transoral robotic surgery for human papilloma virus-related squamous cell carcinoma of the oropharynx. Int. J. Radiat. Oncol. Biol. Phys. 106, 725–732 (2020).
Acknowledgements
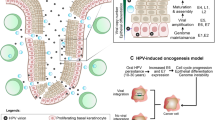
Work on HPV-positive OPSCC in T.R.F’s lab has been supported by the Rosetrees Trust (CM229-CD1) and the Paul Southgate Fund. The authors thank A. Jay (Department of Histopathology, University College London Hospital) for the micrographs shown in Fig. 1.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks T. Dalianis and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lechner, M., Liu, J., Masterson, L. et al. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol 19, 306–327 (2022). https://doi.org/10.1038/s41571-022-00603-7
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41571-022-00603-7
This article is cited by
-
Performance analysis of Leica Biosystems p16 monoclonal antibody in oropharyngeal squamous cell carcinoma
Diagnostic Pathology (2025)
-
Plasma cytokine profiles in oral and oropharyngeal squamous cell carcinoma
BMC Oral Health (2025)
-
Investigating the role of UBASH3B in cancer: structural relevance, physiological functions, and therapeutic possibilities
Journal of Experimental & Clinical Cancer Research (2025)
-
Human papilloma virus (HPV) mediated cancers: an insightful update
Journal of Translational Medicine (2025)
-
Skin microbiome influences the progression of cutaneous squamous cell carcinoma through the immune system
World Journal of Surgical Oncology (2025)