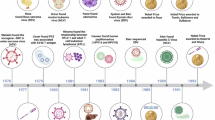
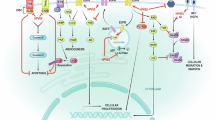
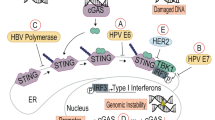
Abstract
A diverse range of viruses have well-established roles as the primary driver of oncogenesis in various haematological malignancies and solid tumours. Indeed, estimates suggest that approximately 1.5 million patients annually are diagnosed with virus-related cancers. The predominant human oncoviruses include Epstein–Barr virus (EBV), Kaposi sarcoma-associated herpesvirus (KSHV), hepatitis B and C viruses (HBV and HCV), human papillomavirus (HPV), human T-lymphotropic virus type 1 (HTLV1), and Merkel cell polyomavirus (MCPyV). In addition, although not inherently oncogenic, human immunodeficiency virus (HIV) is associated with immunosuppression that contributes to the development of AIDS-defining cancers (specifically, Kaposi sarcoma, aggressive B cell non-Hodgkin lymphoma and cervical cancer). Given that an adaptive T cell-mediated immune response is crucial for the control of viral infections, increasing research is being focused on evaluating virus-specific T cell therapies for the treatment of virus-associated cancers. In this Review, we briefly outline the roles of viruses in the pathogenesis of these malignancies before describing progress to date in the field of virus-specific T cell therapy and evaluating the potential utility of these therapies to treat or possibly even prevent virus-related malignancies.
Key points
-
Viruses can contribute to the development of cancer via inflammation, disruption of the cell cycle by viral oncoproteins, direct integration into the genome, and genomic instability and are often required for the proliferation of malignant cells.
-
Virus-specific T cell (VST) therapies have demonstrated a favourable safety profile and can be manufactured from autologous, allogeneic donor and third-party sources.
-
Although historically costly and time-consuming to manufacture, novel rapid manufacturing techniques promise to reduce the costs of and increase accessibility to VST therapies.
-
VSTs targeting Epstein–Barr virus (EBV) have the most substantial evidence of efficacy, with few adverse events, leading to the first regulatory approval of VSTs for use in oncology practice.
-
Adoptive cell therapy has not yet achieved success in the treatment of all virus-associated cancers owing to multiple barriers, including the immunosuppressive tumour microenvironment, tumour heterogeneity and viral immune evasion mechanisms.
-
Combinatorial treatment strategies might expand the clinical utility of VST therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Plummer, M. et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob. Health 4, e609-16 (2016).
Volesky-Avellaneda, K. D. et al. Cancers attributable to infections in the US in 2017: a meta-analysis. JAMA Oncol. 9, 1678–1687 (2023).
Farrell, P. J. Epstein-Barr virus and cancer. Annu. Rev. Pathol. 14, 29–53 (2019).
Chowdhary, S. et al. Recent updates on viral oncogenesis: available preventive and therapeutic entities. Mol. Pharm. 20, 3698–3740 (2023).
Krump, N. A. & You, J. Molecular mechanisms of viral oncogenesis in humans. Nat. Rev. Microbiol. 16, 684–698 (2018).
Skolnik, J. M. & Morrow, M. P. Vaccines for HPV-associated diseases. Mol. Aspects Med. 94, 101224 (2023).
Balfour, H. H. Jr., Schmeling, D. O. & Grimm-Geris, J. M. The promise of a prophylactic Epstein-Barr virus vaccine. Pediatr. Res. 87, 345–352 (2020).
Cohen, J. I. Vaccine development for Epstein-Barr virus. Adv. Exp. Med. Biol. 1045, 477–493 (2018).
Hont, A. B. et al. The generation and application of antigen-specific T cell therapies for cancer and viral-associated disease. Mol. Ther. 30, 2130–2152 (2022).
Toner, K. & Bollard, C. M. EBV+ lymphoproliferative diseases: opportunities for leveraging EBV as a therapeutic target. Blood 139, 983–994 (2022).
Papadopoulou, A., Alvanou, M., Karavalakis, G., Tzannou, I. & Yannaki, E. Pathogen-specific T cells: targeting old enemies and new invaders in transplantation and beyond. Hemasphere 7, e809 (2023).
Martinov, T. & Greenberg, P. D. Targeting driver oncogenes and other public neoantigens using T cell receptor-based cellular therapy. Annu. Rev. Cancer Biol. 7, 331–351 (2023).
McLaughlin, L. P., Gottschalk, S., Rooney, C. M. & Bollard, C. M. EBV-directed T cell therapeutics for EBV-associated lymphomas. Methods Mol. Biol. 1532, 255–265 (2017).
Keam, S. J. Tabelecleucel: first approval. Mol. Diagn. Ther. 27, 425–431 (2023).
Keller, M. D. et al. T-cell receptor sequencing demonstrates persistence of virus-specific T cells after antiviral immunotherapy. Br. J. Haematol. 187, 206–218 (2019).
Galati, L., Chiantore, M. V., Marinaro, M. & Di Bonito, P. Human oncogenic viruses: characteristics and prevention strategies-lessons learned from human papillomaviruses. Viruses 16, 416 (2024).
Cesarman, E. et al. Kaposi sarcoma. Nat. Rev. Dis. Prim. 5, 9 (2019).
Vasudevan, H. N. & Yom, S. S. Nasopharyngeal carcinoma and its association with epstein-barr virus. Hematol. Oncol. Clin. North Am. 35, 963–971 (2021).
Cohen, J. I., Bollard, C. M., Khanna, R. & Pittaluga, S. Current understanding of the role of Epstein-Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leuk. Lymphoma 49, 27–34 (2008).
Latour, S. & Fischer, A. Signaling pathways involved in the T-cell-mediated immunity against Epstein-Barr virus: lessons from genetic diseases. Immunol. Rev. 291, 174–189 (2019).
Schiller, J. T. & Lowy, D. R. An introduction to virus infections and human cancer. Recent. Results Cancer Res. 217, 1–11 (2021).
Qian, Z. et al. HBV integrations reshaping genomic structures promote hepatocellular carcinoma. Gut 73, 1169–1182 (2024).
Jia, Y. et al. HBV DNA polymerase upregulates the transcription of PD-L1 and suppresses T cell activity in hepatocellular carcinoma. J. Transl. Med. 22, 272 (2024).
Dong, W., Wang, H., Li, M., Li, P. & Ji, S. Virus-induced host genomic remodeling dysregulates gene expression, triggering tumorigenesis. Front. Cell Infect. Microbiol. 14, 1359766 (2024).
Zoulim, F., Chen, P. J., Dandri, M., Kennedy, P. & Seeger, C. Hepatitis B virus DNA integration: implications for diagnostics, therapy, and outcome. J. Hepatol. https://doi.org/10.1016/j.jhep.2024.06.037 (2024).
Vallejo-Ruiz, V., Gutiérrez-Xicotencatl, L., Medina-Contreras, O. & Lizano, M. Molecular aspects of cervical cancer: a pathogenesis update. Front. Oncol. 14, 1356581 (2024).
Li, W. et al. The characteristics of HPV integration in cervical intraepithelial cells. J. Cancer 10, 2783–2787 (2019).
Tian, R. et al. Risk stratification of cervical lesions using capture sequencing and machine learning method based on HPV and human integrated genomic profiles. Carcinogenesis 40, 1220–1228 (2019).
Wang, L. et al. Epstein-Barr virus episome physically interacts with active regions of the host genome in lymphoblastoid cells. J. Virol. 94, e01390-20 (2020).
Alcami, A. & Koszinowski, U. H. Viral mechanisms of immune evasion. Trends Microbiol. 8, 410–418 (2000).
Sabourirad, S., Dimitriadis, E. & Mantamadiotis, T. Viruses exploit growth factor mechanisms to achieve augmented pathogenicity and promote tumorigenesis. Arch. Microbiol. 206, 193 (2024).
Zhao, B. Epstein-barr virus B cell growth transformation: the nuclear events. Viruses 15, 832 (2023).
Medhat, A., Arzumanyan, A. & Feitelson, M. A. Hepatitis B x antigen (HBx) is an important therapeutic target in the pathogenesis of hepatocellular carcinoma. Oncotarget 12, 2421–2433 (2021).
Mittal, S. & Banks, L. Molecular mechanisms underlying human papillomavirus E6 and E7 oncoprotein-induced cell transformation. Mutat. Res. Rev. Mutat. Res. 772, 23–35 (2017).
Mueller, S. N. & Rouse, B. T. in Clinical Immunology 3rd edn (eds Robert. R. R. et al.) 421–431 (Mosby, 2008).
Latour, S. & Winter, S. Inherited immunodeficiencies with high predisposition to Epstein-Barr virus-driven lymphoproliferative diseases. Front. Immunol. 9, 1103 (2018).
Cohen, J. I. Primary immunodeficiencies associated with EBV disease. Curr. Top. Microbiol. Immunol. 390, 241–265 (2015).
Sacco, K. A., Notarangelo, L. D. & Delmonte, O. M. When to suspect inborn errors of immunity in Epstein-Barr virus-related lymphoproliferative disorders. Clin. Microbiol. Infect. 29, 457–462 (2023).
Cohen, J. I. Epstein-Barr virus infection. N. Engl. J. Med. 343, 481–492 (2000).
Aggarwal, S., Agarwal, P. & Singh, A. K. Human papilloma virus vaccines: a comprehensive narrative review. Cancer Treat. Res. Commun. 37, 100780 (2023).
Davies, S. I. et al. Robust production of Merkel cell polyomavirus oncogene specific t cells from healthy donors for adoptive transfer. Front. Immunol. 11, 592721 (2020).
Chapuis, A. G. et al. Regression of metastatic Merkel cell carcinoma following transfer of polyomavirus-specific T cells and therapies capable of re-inducing HLA class-I. Cancer Immunol. Res. 2, 27–36 (2014).
Mohanty, S. & Harhaj, E. W. Mechanisms of innate immune sensing of HTLV-1 and viral immune evasion. Pathogens 12, 735 (2023).
Nagarsheth, N. B. et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 27, 419–425 (2021).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Kahan, S. M., Wherry, E. J. & Zajac, A. J. T cell exhaustion during persistent viral infections. Virology 479-480, 180–193 (2015).
Bollard, C. M. et al. Tumor-specific T-cells engineered to overcome tumor immune evasion induce clinical responses in patients with relapsed Hodgkin lymphoma. J. Clin. Oncol. 36, 1128–1139 (2018).
Bell, M. & Gottschalk, S. Engineered cytokine signaling to improve CAR T cell effector function. Front. Immunol. 12, 684642 (2021).
Rooney, C. M. et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 345, 9–13 (1995).
Harris, K. M., Davila, B. J., Bollard, C. M. & Keller, M. D. Virus-specific T cells: current and future use in primary immunodeficiency disorders. J. Allergy Clin. Immunol. Pract. 7, 809–818 (2019).
Gustafson, M. P. et al. Emerging frontiers in immuno- and gene therapy for cancer. Cytotherapy 25, 20–32 (2023).
Qian, C. et al. Viral-specific T-cell transfer from HSCT donor for the treatment of viral infections or diseases after HSCT. Bone Marrow Transpl. 53, 114–122 (2018).
Wehler, T. C. et al. Rapid identification and sorting of viable virus-reactive CD4+ and CD8+ T cells based on antigen-triggered CD137 expression. J. Immunol. Methods 339, 23–37 (2008).
Frentsch, M. et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 11, 1118–1124 (2005).
Thomas, S. et al. Framework engineering to produce dominant T cell receptors with enhanced antigen-specific function. Nat. Commun. 10, 4451 (2019).
Rosenberg, S. A. et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 319, 1676–1680 (1988).
Kazemi, M. H. et al. Tumor-infiltrating lymphocytes for treatment of solid tumors: it takes two to tango? Front. Immunol. 13, 1018962 (2022).
Stevanovic, S. et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 33, 1543–1550 (2015).
Xie, N. et al. Neoantigens: promising targets for cancer therapy. Signal. Transduct. Target. Ther. 8, 9 (2023).
Munz, C. Redirecting T cells against epstein-barr virus infection and associated oncogenesis. Cells 9, 1400 (2020).
Bollard, C. M. et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 32, 798–808 (2014).
Chua, D. et al. Adoptive transfer of autologous Epstein-Barr virus-specific cytotoxic T cells for nasopharyngeal carcinoma. Int. J. Cancer 94, 73–80 (2001).
Heslop, H. E., Savoldo, B. & Rooney, C. M. Cellular therapy of Epstein-Barr-virus-associated post-transplant lymphoproliferative disease. Best Pract. Res. Clin. Haematol. 17, 401–413 (2004).
Lucas, K. G., Small, T. N., Heller, G., Dupont, B. & O’Reilly, R. J. The development of cellular immunity to Epstein-Barr virus after allogeneic bone marrow transplantation. Blood 87, 2594–2603 (1996).
Papadopoulos, E. B. et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N. Engl. J. Med. 330, 1185–1191 (1994).
Bollard, C. M. & Heslop, H. E. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 127, 3331–3340 (2016).
Doubrovina, E. et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 119, 2644–2656 (2012).
Heslop, H. E. et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115, 925–935 (2010).
McLaughlin, L. P. et al. EBV/LMP-specific T cells maintain remissions of T- and B-cell EBV lymphomas after allogeneic bone marrow transplantation. Blood 132, 2351–2361 (2018).
Prockop, S. E. & Vatsayan, A. Epstein-Barr virus lymphoproliferative disease after solid organ transplantation. Cytotherapy 19, 1270–1283 (2017).
Rubinstein, J., Toner, K., Gross, T. & Wistinghausen, B. Diagnosis and management of post-transplant lymphoproliferative disease following solid organ transplantation in children, adolescents, and young adults. Best Pract. Res. Clin. Haematol. 36, 101446 (2023).
Wistinghausen, B., Gross, T. G. & Bollard, C. Post-transplant lymphoproliferative disease in pediatric solid organ transplant recipients. Pediatr. Hematol. Oncol. 30, 520–531 (2013).
Haque, T. et al. Reconstitution of EBV-specific T cell immunity in solid organ transplant recipients. J. Immunol. 160, 6204–6209 (1998).
Khanna, R. et al. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc. Natl Acad. Sci. USA 96, 10391–10396 (1999).
Comoli, P. et al. Treatment of EBV-related post-renal transplant lymphoproliferative disease with a tailored regimen including EBV-specific T cells. Am. J. Transpl. 5, 1415–1422 (2005).
Savoldo, B. et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs). Blood 108, 2942–2949 (2006).
Prockop, S. et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J. Clin. Invest. 130, 733–747 (2020).
Barker, J. N. et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood 116, 5045–5049 (2010).
Wistinghausen, B. et al. ANHL1522: durable immunity to EBV post rituximab and third party LMP-specific T-cells: a children’s oncology group study. Blood Adv. 12, 1116–1127 (2024).
Bollard, C. M. et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood 110, 2838–2845 (2007).
Kim, W. S. et al. Autologous EBV-specific T cell treatment results in sustained responses in patients with advanced extranodal NK/T lymphoma: results of a multicenter study. Ann. Hematol. 100, 2529–2539 (2021).
Comoli, P. et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J. Clin. Oncol. 23, 8942–8949 (2005).
Chia, W. K. et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol. Ther. 22, 132–139 (2014).
Secondino, S. et al. Long-lasting responses with chemotherapy followed by T-cell therapy in recurrent or metastatic EBV-related nasopharyngeal carcinoma. Front. Immunol. 14, 1208475 (2023).
Toh, H. C. et al. 652O Randomized phase III VANCE study: gemcitabine and carboplatin (GC) followed by Epstein Barr virus-specific autologous cytotoxic T lymphocytes (EBV-CTL) versus the same chemotherapy as first-line treatment for advanced nasopharyngeal carcinoma (NPC). Ann. Oncol. 33, S840 (2022).
Haque, T. et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood 110, 1123–1131 (2007).
Keller, M. D. et al. Secondary bone marrow graft loss after third-party virus-specific T cell infusion: case report of a rare complication. Nat. Commun. 15, 2749 (2024).
Leen, A. M. et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121, 5113–5123 (2013).
Tzannou, I. et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J. Clin. Oncol. 35, 3547–3557 (2017).
Withers, B. et al. Establishment and operation of a third-party virus-specific T cell bank within an allogeneic stem cell transplant program. Biol. Blood Marrow Transpl. 24, 2433–2442 (2018).
Kazi, S. et al. Long-term follow up after third-party viral-specific cytotoxic lymphocytes for immunosuppression- and Epstein-Barr virus-associated lymphoproliferative disease. Haematologica 104, e356–e359 (2019).
Mahadeo, K. M. et al. Tabelecleucel for allogeneic haematopoietic stem-cell or solid organ transplant recipients with Epstein-Barr virus-positive post-transplant lymphoproliferative disease after failure of rituximab or rituximab and chemotherapy (ALLELE): a phase 3, multicentre, open-label trial. Lancet Oncol. 25, 376–387 (2024).
Bonifacius, A. et al. Patient-tailored adoptive immunotherapy with EBV-specific T cells from related and unrelated donors. J. Clin. Invest. 133, e163548 (2023).
Bollard, C. M. et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood 99, 3179–3187 (2002).
Foster, A. E. et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J. Immunother. 31, 500–505 (2008).
Cruz, C. R. et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 122, 2965–2973 (2013).
McKenna, M. et al. Real-world evidence of the safety and survival with CD19 CAR-T cell therapy for relapsed/refractory solid organ transplant-related PTLD. Br. J. Haematol. 202, 248–255 (2023).
Harputluoglu, M. & Carr, B. I. Hepatitis B before and after hepatocellular carcinoma. J. Gastrointest. Cancer 52, 1206–1210 (2021).
Thomas, D. L. Global elimination of chronic hepatitis. N. Engl. J. Med. 380, 2041–2050 (2019).
Shen, C., Jiang, X., Li, M. & Luo, Y. Hepatitis virus and hepatocellular carcinoma: recent advances. Cancers 15, 533 (2023).
Luna-Cuadros, M. A. et al. Risk of hepatocellular carcinoma after hepatitis C virus cure. World J. Gastroenterol. 28, 96–107 (2022).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Tan, A. T. & Bertoletti, A. HBV-HCC treatment with mRNA electroporated HBV-TCR T cells. Immunother. Adv. 2, ltab026 (2022).
Ozer, M., Goksu, S. Y., Akagunduz, B., George, A. & Sahin, I. Adoptive cell therapy in hepatocellular carcinoma: a review of clinical trials. Cancers 15, 1808 (2023).
Bertoletti, A. et al. T cell receptor-therapy in HBV-related hepatocellularcarcinoma. Oncoimmunology 4, e1008354 (2015).
Bertoletti, A. & Tan, A. T. HBV as a target for CAR or TCR-T cell therapy. Curr. Opin. Immunol. 66, 35–41 (2020).
Qasim, W. et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J. Hepatol. 62, 486–491 (2015).
Tan, A. T. et al. Use of expression profiles of HBV-DNA integrated into genomes of hepatocellular carcinoma cells to select T cells for immunotherapy. Gastroenterology 156, 1862–1876.e9 (2019).
Meng, F. et al. Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial. Hepatol. Int. 15, 1402–1412 (2021).
Yang, F. et al. Messenger RNA electroporated hepatitis B virus (HBV) antigen-specific T cell receptor (TCR) redirected T cell therapy is well-tolerated in patients with recurrent HBV-related hepatocellular carcinoma post-liver transplantation: results from a phase I trial. Hepatol. Int. 17, 850–859 (2023).
Bohne, F. et al. T cells redirected against hepatitis B virus surface proteins eliminate infected hepatocytes. Gastroenterology 134, 239–247 (2008).
Malagón, T., Franco, E. L., Tejada, R. & Vaccarella, S. Epidemiology of HPV-associated cancers past, present and future: towards prevention and elimination. Nat. Rev. Clin. Oncol. 21, 522–538 (2024).
Doran, S. L. et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II study. J. Clin. Oncol. 37, 2759–2768 (2019).
Eskander, R. N. & Tewari, K. S. Immunotherapy: an evolving paradigm in the treatment of advanced cervical cancer. Clin. Ther. 37, 20–38 (2015).
Huang, H. et al. Phase I study of adjuvant immunotherapy with autologous tumor-infiltrating lymphocytes in locally advanced cervical cancer. J. Clin. Invest. 132, e157726 (2022).
Liu, W., MacDonald, M. & You, J. Merkel cell polyomavirus infection and Merkel cell carcinoma. Curr. Opin. Virol. 20, 20–27 (2016).
Veatch, J. et al. Merkel polyoma virus specific T-cell receptor transgenic T-cell therapy in PD-1 inhibitor refractory Merkel cell carcinoma. J. Clin. Oncol. 40, 9549 (2022).
Bangham, C. R. M. HTLV-1 persistence and the oncogenesis of adult T-cell leukemia/lymphoma. Blood 141, 2299–2306 (2023).
Sampaio, G. C. L. et al. Human T cell lymphotropic virus type 1 global prevalence associated with the human development index: systematic review with meta-analysis. AIDS Res. Hum. Retroviruses 39, 145–165 (2023).
Gessain, A. & Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 3, 388 (2012).
Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br. J. Haematol. 79, 428–437 (1991).
Bangham, C. R. & Osame, M. Cellular immune response to HTLV-1. Oncogene 24, 6035–6046 (2005).
Hishizawa, M. et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood 116, 1369–1376 (2010).
Bazarbachi, A. H. et al. Outcome of stem cell transplantation in HTLV-1-associated North American adult T-cell leukemia/lymphoma. Clin. Hematol. Int. 5, 78–91 (2023).
Harashima, N. et al. Graft-versus-Tax response in adult T-cell leukemia patients after hematopoietic stem cell transplantation. Cancer Res. 64, 391–399 (2004).
Harashima, N. et al. Identification of two new HLA-A*1101-restricted tax epitopes recognized by cytotoxic T lymphocytes in an adult T-cell leukemia patient after hematopoietic stem cell transplantation. J. Virol. 79, 10088–10092 (2005).
Suehiro, Y. et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br. J. Haematol. 169, 356–367 (2015).
Kannagi, M. et al. Maintenance of long remission in adult T-cell leukemia by Tax-targeted vaccine: a hope for disease-preventive therapy. Cancer Sci. 110, 849–857 (2019).
Suehiro, Y. Tax-targeted dendritic cell vaccine therapy for long-term remission of adult T-cell leukemia-lymphoma [Japanese]. Rinsho Ketsueki 64, 670–677 (2023).
Takeda, S. et al. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int. J. Cancer 109, 559–567 (2004).
Miura, M. et al. Kinetics of HTLV-1 reactivation from latency quantified by single-molecule RNA FISH and stochastic modelling. PLoS Pathog. 15, e1008164 (2019).
Gaudray, G. et al. The complementary strand of the human T-cell leukemia virus type 1 RNA genome encodes a bZIP transcription factor that down-regulates viral transcription. J. Virol. 76, 12813–12822 (2002).
Chang, Y. et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266, 1865–1869 (1994).
Cesarman, E., Chang, Y., Moore, P. S., Said, J. W. & Knowles, D. M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332, 1186–1191 (1995).
Soulier, J. et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86, 1276–1280 (1995).
Broussard, G. & Damania, B. KSHV: immune modulation and immunotherapy. Front. Immunol. 10, 3084 (2019).
Goncalves, P. H., Uldrick, T. S. & Yarchoan, R. HIV-associated Kaposi sarcoma and related diseases. AIDS 31, 1903–1916 (2017).
Bihl, F. et al. Cellular immune responses and disease control in acute AIDS-associated Kaposi’s sarcoma. AIDS 23, 1918–1922 (2009).
Naimo, E., Zischke, J. & Schulz, T. F. Recent advances in developing treatments of Kaposi’s sarcoma herpesvirus-related diseases. Viruses 13, 1797 (2021).
Keller, M. D. et al. Antiviral cellular therapy for enhancing T-cell reconstitution before or after hematopoietic stem cell transplantation (ACES): a two-arm, open label phase II interventional trial of pediatric patients with risk factor assessment. Nat. Commun. 15, 3258 (2024).
Gay, C. L. et al. The effects of human immunodeficiency virus type 1 (HIV-1) antigen-expanded specific T-cell therapy and vorinostat on persistent HIV-1 infection in people with HIV on antiretroviral therapy. J. Infect. Dis. 229, 743–752 (2024).
Bekker, L.-G. et al. HIV infection. Nat. Rev. Dis. Prim. 9, 42 (2023).
Grulich, A. E., van Leeuwen, M. T., Falster, M. O. & Vajdic, C. M. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 370, 59–67 (2007).
Yarchoan, R. & Uldrick, T. S. HIV-associated cancers and related diseases. N. Engl. J. Med. 378, 2145 (2018).
Morlat, P. et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS 28, 1181–1191 (2014).
Kim, Y. et al. Trends of cause of death among human immunodeficiency virus patients and the impact of low CD4 counts on diagnosis to death: a retrospective cohort study. J. Korean Med. Sci. 35, e355 (2020).
Walker, R. E. et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood 96, 467–474 (2000).
Mitsuyasu, R. T. et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4ζ gene-modified autologous CD4+ and CD8+ T cells in human immunodeficiency virus-infected subjects. Blood 96, 785–793 (2000).
Van Gulck, E. et al. mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients. AIDS 26, F1–12 (2012).
Allard, S. D. et al. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption. Clin. Immunol. 142, 252–268 (2012).
Sung, J. A. et al. HIV-specific, ex vivo expanded T cell therapy: feasibility, safety, and efficacy in ART-suppressed HIV-infected individuals. Mol. Ther. 26, 2496–2506 (2018).
Hutter, G. et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698 (2009).
Gupta, R. K. et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568, 244–248 (2019).
Jensen, B. O. et al. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 29, 583–587 (2023).
Hsu, J. et al. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186, 1115–1126.e8 (2023).
Dickter, J. K. et al. HIV-1 remission after allogeneic hematopoietic-cell transplantation. N. Engl. J. Med. 390, 669–671 (2024).
Xu, L. et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N. Engl. J. Med. 381, 1240–1247 (2019).
Sáez-Cirión, A. et al. Absence of viral rebound for 18 months without antiretrovirals after allogeneic hematopoietic stem cell transplantation with wild-type CCR5 donor cells to treat a biphenotypic sarcoma. ias2023.org, https://programme.ias2023.org/Abstract/Abstract/?abstractid=5819 (2023).
Ondondo, B. et al. Novel conserved-region T-cell mosaic vaccine with high global HIV-1 coverage is recognized by protective responses in untreated infection. Mol. Ther. 24, 832–842 (2016).
Patel, S. et al. HIV-specific T cells can be generated against non-escaped T cell epitopes with a GMP-compliant manufacturing platform. Mol. Ther. Methods Clin. Dev. 16, 11–20 (2020).
Conarty, J. P. & Wieland, A. The tumor-specific immune landscape in HPV+ head and neck cancer.Viruses 15, 1296 (2023).
Xue, R. et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 612, 141–147 (2022).
Verma, N. K. et al. Obstacles for T-lymphocytes in the tumour microenvironment: therapeutic challenges, advances and opportunities beyond immune checkpoint. EBioMedicine 83, 104216 (2022).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Rossetti, R. et al. Combination of genetically engineered T cells and immune checkpoint blockade for the treatment of cancer. Immunother. Adv. 2, ltac005 (2022).
Siu, L. L. et al. Tabelecleucel in combination with pembrolizumab (Pembro) in platinum-pretreated, recurrent/metastatic Epstein-Barr virus (EBV)-positive nasopharyngeal carcinoma (EBV+NPC). J. Clin. Oncol. 37, TPS6092 (2019).
Park, J., Thomas, S. & Munster, P. N. Epigenetic modulation with histone deacetylase inhibitors in combination with immunotherapy. Epigenomics 7, 641–652 (2015).
Ghosh, S. K., Perrine, S. P., Williams, R. M. & Faller, D. V. Histone deacetylase inhibitors are potent inducers of gene expression in latent EBV and sensitize lymphoma cells to nucleoside antiviral agents. Blood 119, 1008–1017 (2012).
Lam, S. et al. Broadly-specific cytotoxic T cells targeting multiple HIV antigens are expanded from HIV+ patients: implications for immunotherapy. Mol. Ther. 23, 387–395 (2015).
Lee, P. H., Keller, M. D., Hanley, P. J. & Bollard, C. M. Virus-specific T cell therapies for HIV: lessons learned from hematopoietic stem cell transplantation. Front. Cell Infect. Microbiol. 10, 298 (2020).
Lam, S. & Bollard, C. T-cell therapies for HIV. Immunotherapy 5, 407–414 (2013).
Zhang, J., Guo, Y., Fang, H., Guo, X. & Zhao, L. Oncolytic virus oHSV2 combined with PD-1/PD-L1 inhibitors exert antitumor activity by mediating CD4+ T and CD8+ T cell infiltration in the lymphoma tumor microenvironment. Autoimmunity 56, 2259126 (2023).
Bollard, C. M. et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin’s disease. J. Exp. Med. 200, 1623–1633 (2004).
Gallot, G. et al. T-cell therapy using a bank of EBV-specific cytotoxic T cells: lessons from a phase I/II feasibility and safety study. J. Immunother. 37, 170–179 (2014).
Haque, T. et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet 360, 436–442 (2002).
Vickers, M. A. et al. Establishment and operation of a good manufacturing practice-compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br. J. Haematol. 167, 402–410 (2014).
Pfeiffer, T. et al. Posoleucel, an allogeneic, off-the-shelf multivirus-specific T-cell therapy, for the treatment of refractory viral infections in the post-HCT setting. Clin. Cancer Res. 29, 324–330 (2023).
Louis, C. U. et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood 113, 2442–2450 (2009).
Huang, J. et al. Epstein-Barr virus-specific adoptive immunotherapy for recurrent, metastatic nasopharyngeal carcinoma. Cancer 123, 2642–2650 (2017).
Acknowledgements
The work of the authors is supported by the US National Institutes of Health National Cancer Institute (Award Numbers 5P01 CA148600-09 and 1P01 CA225618-01A1 to C.M.B.); a Leukaemia and Lymphoma Society Translational Research Program award (to C.M.B.); and a Hyundai Hope on Wheels Scholar Grant (to K.T.).
Author information
Authors and Affiliations
Contributions
K.T. and C.D.M. researched data for the article. All authors contributed substantially to discussions of content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.M.B. is on the Board of Directors of Cabaletta Bio and is a scientific co-founder and scientific advisory board member of Catamaran Bio, holds stock in Repertoire Immune Medicines and Neximmune, and serves on the data and safety monitoring board of Swedish Orphan Biovitrum (Sobi). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks B. Eiz-Vesper, who co-reviewed with A. Bonifacius; A. Bertoletti; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toner, K., McCann, C.D. & Bollard, C.M. Applications of cell therapy in the treatment of virus-associated cancers. Nat Rev Clin Oncol 21, 709–724 (2024). https://doi.org/10.1038/s41571-024-00930-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-024-00930-x
This article is cited by
-
In vitro evaluation of bidirectional transcription levels of five types of non-coding control regions of Merkel cell polyomavirus
Virology Journal (2025)
-
ImmunOctoberfest reloaded
Nature Immunology (2025)
-
Viral oncogenesis in cancer: from mechanisms to therapeutics
Signal Transduction and Targeted Therapy (2025)
-
Discovering Tumor Microenvironment Dynamics in HPV-Associated Cancers: Using Organoid-Based Models to Develop Therapeutics
Regenerative Engineering and Translational Medicine (2025)
-
Research trends between acquired immune deficiency syndrome and hematological malignancies: a bibliometric analysis
Discover Oncology (2025)