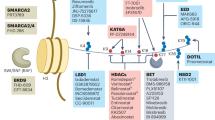
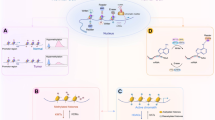
Abstract
Rapid advances in the field of epigenetics have facilitated the development of novel therapeutics targeting epigenetic mechanisms that are hijacked by cancer cells to support tumour growth and progression. Several epigenetic agents have been approved by the FDA for the treatment of cancer; however, the efficacy of these drugs is dependent on the underlying biology and drivers of the disease, with inherent differences between solid tumours and haematological malignancies. The efficacy of epigenetic drugs as single agents remains limited across most cancer types, which has spurred the clinical development of combination therapies, with the hope of attaining synergistic activity and/or overcoming treatment resistance. In this Review we discuss clinical advances that have been achieved with the use of epigenetic agents in combination with chemotherapies, immunotherapies or other targeted agents, including epigenetic–epigenetic combinations, as well as limitations and challenges associated with these combinatorial strategies. So far, the success of combination therapies targeting epigenetic mechanisms has generally been confined to haematological malignancies, with limited efficacy observed in patients with solid tumours. Nevertheless, this Review captures the field of epigenetic combination therapies across the spectra of haematology and oncology, highlighting opportunities for precision therapy to effectively harness the potential of epigenetic agents and produce meaningful improvements in clinical outcomes.
Key points
-
The activity of epigenetic drugs is dependent on underlying tumour biology, with distinct differences across solid tumours and haematological malignancies.
-
Current epigenetic drugs have meaningful clinical efficacy as single agents in certain haematological malignancies, particularly acute myeloid leukaemia, peripheral T cell lymphoma or cutaneous T cell lymphoma, but generally limited efficacy in solid tumour settings.
-
Epigenetic agents have been combined with chemotherapies, immunotherapies and targeted therapies, including other epigenetic drugs, in an effort to target parallel pathways of tumorigenesis, enhance cancer cell death and overcome mechanisms of resistance.
-
Epigenetic combination therapies have had limited success in solid tumours, the reasons for which are poorly defined, but this pattern might signal that solid tumours have greater complexity and are not highly dependent on altered epigenetic pathways.
-
Haematological malignancies including peripheral T cell lymphoma, particularly subtypes with a T follicular helper phenotype, and acute myeloid leukaemia also seem to be uniquely sensitive to epigenetic combination therapies, underscoring that successful outcomes are possible if cancers that are highly dependent on aberrant epigenetic pathways can be identified and selectively targeted.
-
Altogether, the experience with epigenetic combination therapies so far highlights the importance of precision medicine and pinpointing specific epigenetic drivers of disease to translate promising preclinical results into clinically meaningful change for patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Waddington, C. H. The epigenotype. Endeavour 1, 18–20 (1942).
Dupont C., Armant D. R. & Brenner C. A. Epigenetics: definition, mechanisms and clinical perspective. Semin. Reprod. Med. 27, 351–357 (2009).
Huang, H., Weng, H. & Chen, J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell 37, 270–288 (2020).
Tarakhovsky, A. Tools and landscapes of epigenetics. Nat. Immunol. 11, 565–568 (2010).
Ahuja, N., Easwaran, H. & Baylin, S. B. Harnessing the potential of epigenetic therapy to target solid tumors. J. Clin. Invest. 124, 56–63 (2014).
Plass, C. et al. Mutations in regulators of the epigenome and their connections to global chromatin patterns in cancer. Nat. Rev. Genet. 14, 765–780 (2013).
Bates, S. E. Epigenetic therapies for cancer. N. Engl. J. Med. 383, 650–663 (2020).
Davalos, V. & Esteller, M. Cancer epigenetics in clinical practice. CA Cancer J. Clin. 73, 376–424 (2023).
Mellinghoff et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma. N. Engl. J. Med. 389, 589–601 (2023).
US Food and Drug Administration. FDA approves vorasidenib for grade 2 astrocytoma or oligodendroglioma susceptible to IDH1 or IDH2 mutation. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-vorasidenib-grade-2-astrocytoma-or-oligodendroglioma-susceptible-idh1-or-idh2-mutation (FDA, 2024).
Mabe, N. W., Perry, J. A., Malone, C. F. & Stegmaier, K. Pharmacological targeting of the cancer epigenome. Nat. Cancer 5, 844–865 (2024).
Amengual, J. E. Can we use epigenetics to prime chemoresistant lymphomas? Hematology 2020, 85–94 (2020).
DiNardo, C. D. et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 383, 617–629 (2020).
US Food and Drug Administration. FDA grants regular approval to venetoclax in combination for untreated acute myeloid leukemia. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-venetoclax-combination-untreated-acute-myeloid-leukemia (FDA, 2024).
Bruzzese, F. et al. Synergistic antitumor effect between vorinostat and topotecan in small cell lung cancer cells is mediated by generation of reactive oxygen species and DNA damage-induced apoptosis. Mol. Cancer Ther. 8, 3075–3087 (2009).
Li, J. et al. Epigenetic targeting drugs potentiate chemotherapeutic effects in solid tumor therapy. Sci. Rep. 7, 4035 (2017).
Thurn, K. T., Thomas, S., Raha, P., Qureshi, I. & Munster, P. N. Histone deacetylase regulation of ATM-mediated DNA damage signaling. Mol. Cancer Ther. 12, 2078–2087 (2013).
Chen, C.-S. et al. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce DNA double-strand breaks by targeting Ku70 acetylation. Cancer Res. 67, 5318–5327 (2007).
Liu, K., Cang, S., Ma, Y. & Chiao, J. W. Synergistic effect of paclitaxel and epigenetic agent phenethyl isothiocyanate on growth inhibition, cell cycle arrest and apoptosis in breast cancer cells. Cancer Cell Int. 13, 1–8 (2013).
Qin, T. et al. Effect of cytarabine and decitabine in combination in human leukemic cell lines. Clin. Cancer Res. 13, 4225–4232 (2007).
Hollenbach, P. W. et al. A comparison of azacitidine and decitabine activities in acute myeloid leukemia cell lines. PLoS ONE 5, e9001 (2010).
Kurimoto, M. et al. Pretreatment of leukemic cells with low-dose decitabine markedly enhances the cytotoxicity of gemtuzumab ozogamicin. Leukemia 27, 233–235 (2013).
Smonskey, M. et al. EZH2 inhibition re-sensitizes multidrug resistant B-cell lymphomas to etoposide mediated apoptosis. Oncoscience 3, 21–30 (2016).
Candelaria, M. et al. A phase II study of epigenetic therapy with hydralazine and magnesium valproate to overcome chemotherapy resistance in refractory solid tumors. Ann. Oncol. 18, 1529–1538 (2007).
Zhang, Y.-W. et al. Integrated analysis of DNA methylation and mRNA expression profiling reveals candidate genes associated with cisplatin resistance in non-small cell lung cancer. Epigenetics 9, 896–909 (2014).
Pan, M. R. et al. The histone methyltransferase G9a as a therapeutic target to override gemcitabine resistance in pancreatic cancer. Oncotarget 7, 61136–61151 (2016).
Gao, J. et al. Methyltransferase-like 3 inhibition limits intrahepatic cholangiocarcinoma metabolic reprogramming and potentiates the efficacy of chemotherapy. Oncogene 42, 2507–2520 (2023).
Plumb, J. A., Strathdee, G., Sludden, J., Kaye, S. B. & Brown, R. Reversal of drug resistance in human tumor xenografts by 2′-deoxy-5-azacytidine-induced demethylation of the hMLH1 gene promoter. Cancer Res. 60, 6039–6044 (2000).
Kaminskas, E., Farrell, A. T., Wang, Y.-C., Sridhara, R. & Pazdur, R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza™) for injectable suspension. Oncologist 10, 176–182 (2005).
US Food and Drug Administration. FDA approves onureg (azacitidine tablets) for acute myeloid leukemia. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-onureg-azacitidine-tablets-acute-myeloid-leukemia (FDA, 2024).
Kim, N. et al. FDA approval summary: decitabine and cedazuridine tablets for myelodysplastic syndromes. Clin. Cancer Res. 28, 3411–3416 (2022).
Pfeifer, G. P., Kadam, S. & Jin, S.-G. 5-Hydroxymethylcytosine and its potential roles in development and cancer. Epigenetics Chromatin 6, 1–9 (2013).
Tulstrup, M. et al. TET2 mutations are associated with hypermethylation at key regulatory enhancers in normal and malignant hematopoiesis. Nat. Commun. 12, 6061 (2021).
Issa, J.-P. J., Kantarjian, H. M. & Kirkpatrick, P. Azacitidine. Nat. Rev. Drug Discov. 4, 275–277 (2005).
Baylin, S. B. & Jones, P. A. A decade of exploring the cancer epigenome — biological and translational implications. Nat. Rev. Cancer 11, 726–734 (2011).
Tsai, H.-C. et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell 21, 430–446 (2012).
Scheller, M. et al. Hotspot DNMT3A mutations in clonal hematopoiesis and acute myeloid leukemia sensitize cells to azacytidine via viral mimicry response. Nat. Cancer 2, 527–544 (2021).
Valdez, B. C. et al. Combination of a hypomethylating agent and inhibitors of PARP and HDAC traps PARP1 and DNMT1 to chromatin, acetylates DNA repair proteins, down-regulates NuRD and induces apoptosis in human leukemia and lymphoma cells. Oncotarget 9, 3908–3921 (2018).
Roulois, D. et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 162, 961–973 (2015).
Thépot, S. et al. Azacitidine in untreated acute myeloid leukemia: a report on 149 patients. Am. J. Hematol. 89, 410–416 (2014).
Kaminskas, E. et al. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin. Cancer Res. 11, 3604–3608 (2005).
Kuendgen, A. et al. Efficacy of azacitidine is independent of molecular and clinical characteristics — an analysis of 128 patients with myelodysplastic syndromes or acute myeloid leukemia and a review of the literature. Oncotarget 9, 27882–27894 (2018).
Dombret, H. et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126, 291–299 (2015).
Zavras, P. D., Shastri, A., Goldfinger, M., Verma, A. K. & Saunthararajah, Y. Clinical trials assessing hypomethylating agents combined with other therapies: causes for failure and potential solutions. Clin. Cancer Res. 27, 6653–6661 (2021).
Moro, H. et al. Epigenetic priming sensitizes gastric cancer cells to irinotecan and cisplatin by restoring multiple pathways. Gastric Cancer 23, 105–115 (2020).
Halpern, A. B. et al. Mitoxantrone, etoposide and cytarabine following epigenetic priming with decitabine in adults with relapsed/refractory acute myeloid leukemia or other high-grade myeloid neoplasms: a phase 1/2 study. Leukemia 31, 2560–2567 (2017).
Zhou, X. et al. Epigenetic priming with decitabine followed by low dose idarubicin and cytarabine in acute myeloid leukemia evolving from myelodysplastic syndromes and higher-risk myelodysplastic syndromes: a prospective multicenter single-arm trial. Hematol. Oncol. 38, 531–540 (2020).
Kadia, T. M. et al. Cladribine and low-dose cytarabine alternating with decitabine as front-line therapy for elderly patients with acute myeloid leukaemia: a phase 2 single-arm trial. Lancet Haematol. 5, e411–e421 (2018).
Im, A. et al. Phase 2 study of epigenetic priming with decitabine followed by cytarabine for acute myeloid leukemia in older patients. Am. J. Hematol. 99, 380–386 (2024).
Garcia-Manero, G. et al. A randomized phase III study of standard versus high-dose cytarabine with or without vorinostat for AML. Leukemia 38, 58–66 (2024).
Daver, N., Schlenk, R. F., Russell, N. H. & Levis, M. J. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33, 299–312 (2019).
Stone, R. M. et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 377, 454–464 (2017).
Cortes, J. E. et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 20, 984–997 (2019).
Perl, A. E. et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N. Engl. J. Med. 381, 1728–1740 (2019).
Stein, E. M. et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130, 722–731 (2017).
DiNardo, C. D. et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N. Engl. J. Med. 378, 2386–2398 (2018).
Hamann, P. R. et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody−calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 13, 47–58 (2002).
Ma, H., O’Connor, O. A. & Marchi, E. New directions in treating peripheral T-cell lymphomas (PTCL): leveraging epigenetic modifiers alone and in combination. Expert Rev. Hematol. 12, 137–146 (2019).
Dobay, M. P. et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. Haematologica 102, e148 (2017).
Ghione, P. et al. T follicular helper phenotype predicts response to histone deacetylase inhibitors in relapsed/refractory peripheral T-cell lymphoma. Blood Adv. 4, 4640–4647 (2020).
Amengual, J. E. et al. A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood 131, 397–407 (2018).
Camus, V. et al. Romidepsin plus cyclophosphamide, doxorubicin, vincristine, and prednisone versus cyclophosphamide, doxorubicin, vincristine, and prednisone in patients with previously untreated peripheral T-cell lymphoma: final analysis of the Ro-CHOP trial. J. Clin. Oncol. 42, 1612–1618 (2024).
Piekarz, R. L. et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood 117, 5827–5834 (2011).
O’Connor, O. A. et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J. Clin. Oncol. 29, 1182–1189 (2011).
Coiffier, B. et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J. Hematol. Oncol. 7, 11 (2014).
Shimony, S. et al. Romidepsin treatment for relapsed or refractory peripheral and cutaneous T‐cell lymphoma: real‐life data from a national multicenter observational study. Hematol. Oncol. 37, 569–577 (2019).
Whittaker, S. J. et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J. Clin. Oncol. 28, 4485–4491 (2010).
Piekarz, R. L. et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J. Clin. Oncol. 27, 5410–5417 (2009).
Bachy, E. et al. Romidepsin plus CHOP versus CHOP in patients with previously untreated peripheral T-cell lymphoma: results of the Ro-CHOP phase III study (conducted by LYSA). J. Clin. Oncol. 40, 242–251 (2022).
Mellgard, G. S., Fojo, T. & Bates, S. E. Lessons from withdrawn accelerated approvals in oncology. Nat. Cancer 5, 211–215 (2024).
Chiappella, A. et al. Romidepsin–CHOEP followed by high-dose chemotherapy and stem-cell transplantation in untreated peripheral T-cell lymphoma: results of the PTCL13 phase Ib/II study. Leukemia 37, 433–440 (2023).
Janikova, A. et al. First-line therapy for T cell lymphomas: a retrospective population-based analysis of 906 T cell lymphoma patients. Ann. Hematol. 98, 1961–1972 (2019).
Johnston, P. B. et al. Belinostat in combination with standard cyclophosphamide, doxorubicin, vincristine and prednisone as first-line treatment for patients with newly diagnosed peripheral T-cell lymphoma. Exp. Hematol. Oncol. 10, 15 (2021).
Ryu Tiger Y. K. et al. Phase II study of the novel antifolate agent pralatrexate in combination with the histone deacetylase inhibitor romidepsin for the treatment of patients with mature T-cell lymphoma. Leuk. Lymphoma. https://doi.org/10.1080/10428194.2024.2329996 (2024).
Ruan, J. et al. Multicenter phase 2 study of oral azacitidine (CC-486) plus CHOP as initial treatment for PTCL. Blood 141, 2194–2205 (2023).
National Comprehensive Cancer Network. T-cell lymphomas (version 2.2024) https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf (NCCN, 2024).
Grant, C. et al. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev. Anticancer Ther. 10, 997–1008 (2010).
O’Connor, O. A. et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J. Clin. Oncol. 33, 2492–2499 (2015).
Appleton, K. et al. Phase I and pharmacodynamic trial of the DNA methyltransferase inhibitor decitabine and carboplatin in solid tumors. J. Clin. Oncol. 25, 4603–4609 (2007).
Chang, X. et al. Identification of hypermethylated genes associated with cisplatin resistance in human cancers. Cancer Res. 70, 2870–2879 (2010).
Fu, S. et al. Phase Ib–IIa study to reverse platinum resistance by the use of a hypomethylating agent azacitidine in platinum-resistant or refractory epithelial ovarian cancer. Cancer 117, 1661–1669 (2011).
Glasspool, R. et al. A randomised, phase II trial of the DNA-hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in combination with carboplatin vs carboplatin alone in patients with recurrent, partially platinum-sensitive ovarian cancer. Br. J. Cancer 110, 1923–1929 (2014).
Teodoridis, J. M. et al. CpG island methylation of DNA damage response genes in advanced ovarian cancer. Cancer Res. 65, 8961–8967 (2005).
Kwon, N.-H., Kim, J.-S., Lee, J.-Y., Oh, M.-J. & Choi, D.-C. DNA methylation and the expression of IL-4 and IFN-γ promoter genes in patients with bronchial asthma. J. Clin. Immunol. 28, 139–146 (2008).
Singal, R. et al. Phase I/II study of azacitidine, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel-based therapy. Clin. Genitourin. Cancer 13, 22–31 (2015).
Fan H. et al. Low-dose decitabine-based chemoimmunotherapy for patients with refractory advanced solid tumors: a phase I/II report. J. Immunol. Res. 2014, 371087 (2014).
Sharma, A. et al. Hypomethylating agents synergize with irinotecan to improve response to chemotherapy in colorectal cancer cells. PLoS ONE 12, e0176139 (2017).
Lee, V. et al. A phase II study of guadecitabine combined with irinotecan vs regorafenib or TAS-102 in irinotecan-refractory metastatic colorectal cancer patients. Int. J. Cancer 154, 1794–1801 (2024).
Mak, M. P. et al. Valproic acid combined with cisplatin-based chemoradiation in locally advanced head and neck squamous cell carcinoma patients and associated biomarkers. Ecancermedicalscience 14, 1155 (2020).
Chan, E. et al. Phase I/II study of mocetinostat in combination with gemcitabine for patients with advanced pancreatic cancer and other advanced solid tumors. Cancer Chemother. Pharm. 81, 355–364 (2018).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Duruisseaux, M. et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: a multicentre, retrospective analysis. Lancet Respir. Med. 6, 771–781 (2018).
Jung, H. et al. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat. Commun. 10, 4278 (2019).
Luoto, S. et al. Computational characterization of suppressive immune microenvironments in glioblastoma. Cancer Res. 78, 5574–5585 (2018).
Berghoff, A. S. et al. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro Oncol. 19, 1460–1468 (2017).
Waitkus, M. S., Diplas, B. H. & Yan, H. Biological role and therapeutic potential of IDH mutations in cancer. Cancer Cell 34, 186–195 (2018).
Ghoneim, H. E. et al. De novo epigenetic programs inhibit PD-1 blockade-mediated T cell rejuvenation. Cell 170, 142–157.e19 (2017).
Chiappinelli, K. B. et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).
Wang, L. et al. Decitabine enhances lymphocyte migration and function and synergizes with CTLA-4 blockade in a murine ovarian cancer model. Cancer Immunol. Res. 3, 1030–1041 (2015).
Durek, P. et al. Epigenomic profiling of human CD4+ T cells supports a linear differentiation model and highlights molecular regulators of memory development. Immunity 45, 1148–1161 (2016).
Garaud, S. et al. FOXP1 is a regulator of quiescence in healthy human CD4+ T cells and is constitutively repressed in T cells from patients with lymphoproliferative disorders. Eur. J. Immunol. 47, 168–179 (2017).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015).
Yoshihama, S. et al. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc. Natl Acad. Sci. USA 113, 5999–6004 (2016).
Zhou, L., Mudianto, T., Ma, X., Riley, R. & Uppaluri, R. Targeting EZH2 enhances antigen presentation, antitumor immunity, and circumvents anti-PD-1 resistance in head and neck cancer. Clin. Cancer Res. 26, 290–300 (2020).
Magner, W. J. et al. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J. Immunol. 165, 7017–7024 (2000).
Krishnadas, D. K., Bao, L., Bai, F., Chencheri, S. C. & Lucas, K. Decitabine facilitates immune recognition of sarcoma cells by upregulating CT antigens, MHC molecules, and ICAM-1. Tumor Biol. 35, 5753–5762 (2014).
Nie, J. et al. Addition of low-dose decitabine to anti-PD-1 antibody camrelizumab in relapsed/refractory classical Hodgkin lymphoma. J. Clin. Oncol. 37, 1479–1489 (2019).
Luo, N. et al. DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat. Commun. 9, 248 (2018).
Manning, J. et al. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen‐processing machinery components in a murine model for human papilloma virus 16‐associated tumours. Immunology 123, 218–227 (2008).
Nie, Y. et al. DNA hypermethylation is a mechanism for loss of expression of the HLA class I genes in human esophageal squamous cell carcinomas. Carcinogenesis 22, 1615–1623 (2001).
Wang, L.-X. et al. Low dose decitabine treatment induces CD80 expression in cancer cells and stimulates tumor specific cytotoxic T lymphocyte responses. PLoS ONE 8, e62924 (2013).
Brocks, D. et al. DNMT and HDAC inhibitors induce cryptic transcription start sites encoded in long terminal repeats. Nat. Genet. 49, 1052–1060 (2017).
Coral, S. et al. 5-Aza-2′-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: immunotherapeutic implications. Clin. Cancer Res. 8, 2690–2695 (2002).
Dubovsky, J. A. et al. Treatment of chronic lymphocytic leukemia with a hypomethylating agent induces expression of NXF2, an immunogenic cancer testis antigen. Clin. Cancer Res. 15, 3406–3415 (2009).
Hogg, S. J., Beavis, P. A., Dawson, M. A. & Johnstone, R. W. Targeting the epigenetic regulation of antitumour immunity. Nat. Rev. Drug Discov. 19, 776–800 (2020).
Ritter, C. et al. Epigenetic priming restores the HLA class-I antigen processing machinery expression in Merkel cell carcinoma. Sci. Rep. 7, 2290 (2017).
Booth, L., Roberts, J. L., Poklepovic, A., Kirkwood, J. & Dent, P. HDAC inhibitors enhance the immunotherapy response of melanoma cells. Oncotarget 8, 83155–83170 (2017).
Yang, W. et al. A selective HDAC8 inhibitor potentiates antitumor immunity and efficacy of immune checkpoint blockade in hepatocellular carcinoma. Sci. Transl. Med. 13, eaaz6804 (2021).
Burke, B. et al. Inhibition of histone deacetylase (HDAC) enhances checkpoint blockade efficacy by rendering bladder cancer cells visible for T cell-mediated destruction. Front. Oncol. 10, 699 (2020).
Green, M. R. et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116, 3268–3277 (2010).
Ansell, S. M. Hodgkin lymphoma: 2018 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 93, 704–715 (2018).
Chen, R. et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 134, 1144–1153 (2019).
Younes, A. et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 17, 1283–1294 (2016).
Liu, Z. et al. Epigenetic reprogramming of Runx3 reinforces CD8+ T-cell function and improves the clinical response to immunotherapy. Mol. Cancer 22, 84 (2023).
Sermer, D. J. et al. Interim efficacy analysis of a phase II study demonstrates promising activity of the combination of pembrolizumab (PEM) and entinostat (ENT) in relapsed and refractory (R/R) Hodgkin lymphoma (HL). Blood 138, 2447 (2021).
Herrera, A. F. et al. Nivolumab(N)-avd improves progression-free survival compared to brentuximab vedotin(BV)-AVD in advanced stage (AS) classic hodgkin lymphoma (HL): results of SWOG S1826. Hematol. Oncol. 41, 33–35 (2023).
Hawkes, E. A. et al. Avelumab in combination regimens for relapsed/refractory DLBCL: results from the phase Ib JAVELIN DLBCL study. Target. Oncol. 16, 761–771 (2021).
Ansell, S. M. et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J. Clin. Oncol. 37, 481–489 (2019).
Lesokhin, A. M. et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J. Clin. Oncol. 34, 2698–2704 (2016).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Jacobson C. A. et al. Real-world outcomes with CAR T-cell therapies in large B-cell lymphoma: a systematic review and meta-analysis. Transplant. Cell. Ther. 30, 77.e1–77.e15 (2024).
Shah, N. N. et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with B-ALL. J. Clin. Oncol. 39, 1650–1659 (2021).
Sheykhhasan, M. et al. CAR T therapies in multiple myeloma: unleashing the future. Cancer Gene Ther. 31, 667–686 (2024).
Peng, L., Sferruzza, G., Yang, L., Zhou, L. & Chen, S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell. Mol. Immunol. 21, 1089–1108 (2024).
Baker, D. J., Arany, Z., Baur, J. A., Epstein, J. A. & June, C. H. CAR T therapy beyond cancer: the evolution of a living drug. Nature 619, 707–715 (2023).
Garcia-Prieto, C. A. et al. Epigenetic profiling and response to CD19 chimeric antigen receptor T-cell therapy in B-cell malignancies. J. Natl Cancer Inst. 114, 436–445 (2021).
Salz, L. et al. Culture expansion of CAR T cells results in aberrant DNA methylation that is associated with adverse clinical outcome. Leukemia 37, 1868–1878 (2023).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372, eaba1786 (2021).
Isshiki, Y. et al. EZH2 inhibitors enhance CART cell quality, efficacy, in vivo homing, tumor cell binding and killing of fully syngeneic primary B cell lymphomas, as well as reprogramming lymphoma cells to a highly immunogenic and T cell adherent phenotype. Blood 142, 432 (2023).
Porazzi, P. et al. Inhibition of EZH2 improves CART19 immunotherapy by reprogramming lymphoma tumor cells and enhancing T-cell functionality. Blood 142, 1018 (2023).
Cao, J. & Yan, Q. Cancer epigenetics, tumor immunity, and immunotherapy. Trends Cancer 6, 580–592 (2020).
Mamdani, H., Matosevic, S., Khalid, A. B., Durm, G. & Jalal, S. I. Immunotherapy in lung cancer: current landscape and future directions. Front. Immunol. 13, 823618 (2022).
Rosenthal, R. et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 567, 479–485 (2019).
Seo, S.-K. et al. Histone deacetylase inhibitors sensitize human non-small cell lung cancer cells to ionizing radiation through acetyl p53-mediated c-myc down-regulation. J. Thorac. Oncol. 6, 1313–1319 (2011).
Ansari, J., Shackelford, R. E. & El-Osta, H. Epigenetics in non-small cell lung cancer: from basics to therapeutics. Transl. Lung Cancer Res. 5, 155–171 (2016).
Hellmann, M. D. et al. Entinostat plus pembrolizumab in patients with metastatic NSCLC previously treated with anti-PD-(L)1 therapy. Clin. Cancer Res. 27, 1019–1028 (2021).
Johnson, M. L. et al. Mocetinostat in combination with durvalumab for patients with advanced NSCLC: results from a phase I/II study. Clin. Lung Cancer 24, 218–227 (2023).
Baretti, M. et al. Entinostat in combination with nivolumab for patients with advanced cholangiocarcinoma and pancreatic adenocarcinoma. J. Clin. Oncol. 36, TPS4151–TPS4151 (2018).
Cadoo, K. A. et al. A phase II randomized study of avelumab plus entinostat versus avelumab plus placebo in patients (pts) with advanced epithelial ovarian cancer (EOC). J. Clin. Oncol. 37, 5511–5511 (2019).
Rodriguez, C. P. et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin. Cancer Res. 26, 837–845 (2020).
Sharma, P. et al. Romidepsin (HDACi) plus cisplatin and nivolumab triplet combination in patients with metastatic triple negative breast cancer (mTNBC). J. Clin. Oncol. 39, 1076–1076 (2021).
Zakharia, Y. et al. Durvalumab and guadecitabine in advanced clear cell renal cell carcinoma: results from the phase Ib/II study BTCRC-GU16-043. Nat. Commun. 15, 972 (2024).
Chen S. et al. Epigenetic priming enhances antitumor immunity in platinum-resistant ovarian cancer. J. Clin. Invest. https://doi.org/10.1172/jci158800 (2022).
Lovly, C. M. & Shaw, A. T. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clin. Cancer Res. 20, 2249–2256 (2014).
Lin, Y., Wang, X. & Jin, H. EGFR-TKI resistance in NSCLC patients: mechanisms and strategies. Am. J. Cancer Res. 4, 411–435 (2014).
Yaghmaie, M. & Yeung, C. C. Molecular mechanisms of resistance to tyrosine kinase inhibitors. Curr. Hematol. Malig. Rep. 14, 395–404 (2019).
Beagle, B. R. et al. mTOR kinase inhibitors synergize with histone deacetylase inhibitors to kill B-cell acute lymphoblastic leukemia cells. Oncotarget 6, 2088 (2015).
Dong, L. H. et al. Histone deacetylase inhibitor potentiated the ability of MTOR inhibitor to induce autophagic cell death in Burkitt leukemia/lymphoma. J. Hematol. Oncol. 6, 1–11 (2013).
Rausch, M. et al. Optimized combination of HDACI and TKI efficiently inhibits metabolic activity in renal cell carcinoma and overcomes sunitinib resistance. Cancers 12, 3172 (2020).
Cheng, C., Yun, F., Ullah, S. & Yuan, Q. Discovery of novel cyclin-dependent kinase (CDK) and histone deacetylase (HDAC) dual inhibitors with potent in vitro and in vivo anticancer activity. Eur. J. Med. Chem. 189, 112073 (2020).
Fan, F. et al. A dual PI3K/HDAC inhibitor induces immunogenic ferroptosis to potentiate cancer immune checkpoint therapy. Cancer Res. 81, 6233–6245 (2021).
Cameron, E. E., Bachman, K. E., Myöhänen, S., Herman, J. G. & Baylin, S. B. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 21, 103–107 (1999).
Huang, W. et al. Dual inhibitors of DNMT and HDAC induce viral mimicry to induce antitumour immunity in breast cancer. Cell Death Discov. 10, 143 (2024).
Ricker, E. C., Estrella, B., Pazos, M. A. & Amengual, J. E. Dual targeting of EZH2 and HDAC with tazemetostat and belinostat promotes immunogenicity in GC-DLBCL. Blood 138, 2410 (2021).
Bose, P., Gandhi, V. & Konopleva, M. Pathways and mechanisms of venetoclax resistance. Leuk. Lymphoma 58, 1–17 (2017).
Jin, S. et al. 5-Azacitidine induces NOXA to prime AML cells for venetoclax-mediated apoptosis. Clin. Cancer Res. 26, 3371–3383 (2020).
Bogenberger, J. M. et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk. Lymphoma 56, 226–229 (2015).
DiNardo, C. D. et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133, 7–17 (2019).
Pollyea, D. A. et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations. Clin. Cancer Res. 28, 2753–2761 (2022).
Pratz, K. W. et al. Long-term follow-up of VIALE-A: venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am. J. Hematol. 99, 615–624 (2024).
Chan, S. M. et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 21, 178–184 (2015).
DiNardo, C. D. et al. 10-Day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 7, e724–e736 (2020).
Chabner B. A. & Longo D. L. (eds). Cancer Chemotherapy, Immunotherapy, and Biotherapy (Lippincott Williams & Wilkins, 2024).
Derissen, E. J., Beijnen, J. H. & Schellens, J. H. Concise drug review: azacitidine and decitabine. Oncologist 18, 619–624 (2013).
Bazinet, A. et al. A phase I/II study of venetoclax in combination with 5-azacytidine in treatment-naïve and relapsed/refractory high-risk myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia (CMML). Blood 138, 535 (2021).
Zeidan, A. M. et al. A phase 1b study of venetoclax and azacitidine combination in patients with relapsed or refractory myelodysplastic syndromes. Am. J. Hematol. 98, 272–281 (2023).
Montesinos, P. et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N. Engl. J. Med. 386, 1519–1531 (2022).
National Comprehensive Cancer Network. Acute Myeloid Leukemia (version 3.2024) https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (NCCN, 2024).
DiNardo, C. D. et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 22, 1597–1608 (2021).
US Food and Drug Administration. IDHIFA (enasidenib). FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209606s000lbl.pdf (FDA, 2017).
Lu, C. et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012).
Figueroa, M. E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010).
Im, A. et al. DNMT3A and IDH mutations in acute myeloid leukemia and other myeloid malignancies: associations with prognosis and potential treatment strategies. Leukemia 28, 1774–1783 (2014).
Green, C. L. et al. The prognostic significance of IDH2 mutations in AML depends on the location of the mutation. Blood 118, 409–412 (2011).
Thol, F. et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood 116, 614–616 (2010).
Boissel, N. et al. Prognostic impact of isocitrate dehydrogenase enzyme isoforms 1 and 2 mutations in acute myeloid leukemia: a study by the Acute Leukemia French Association group. J. Clin. Oncol. 28, 3717–3723 (2010).
Chou, W. et al. The prognostic impact and stability of Isocitrate dehydrogenase 2 mutation in adult patients with acute myeloid leukemia. Leukemia 25, 246–253 (2011).
Patel, J. P. et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl. J. Med. 366, 1079–1089 (2012).
Lachowiez, C. A. et al. Contemporary outcomes in IDH-mutated acute myeloid leukemia: the impact of co-occurring NPM1 mutations and venetoclax-based treatment. Am. J. Hematol. 97, 1443–1452 (2022).
de Botton, S. et al. Olutasidenib (FT-2102) induces durable complete remissions in patients with relapsed or refractory IDH1-mutated AML. Blood Adv. 7, 3117–3127 (2023).
Krivtsov, A. V. & Armstrong, S. A. MLL translocations, histone modifications and leukaemia stem-cell development. Nat. Rev. Cancer 7, 823–833 (2007).
Yokoyama, A. et al. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 123, 207–218 (2005).
Li, B. E., Gan, T., Meyerson, M., Rabbitts, T. H. & Ernst, P. Distinct pathways regulated by menin and by MLL1 in hematopoietic stem cells and developing B cells. Blood 122, 2039–2046 (2013).
Issa, G. C. et al. The menin inhibitor revumenib in KMT2A-rearranged or NPM1-mutant leukaemia. Nature 615, 920–924 (2023).
Erba, H. P. et al. Update on a phase 1/2 first-in-human study of the menin-KMT2A (MLL) inhibitor ziftomenib (KO-539) in patients with relapsed or refractory acute myeloid leukemia. Blood 140, 153–156 (2022).
Jabbour, E. et al. A first-in-human phase 1 study of the menin-KMT2A (MLL1) inhibitor JNJ-75276617 in adult patients with relapsed/refractory acute leukemia harboring KMT2A or NPM1 alterations. Blood 142, 57 (2023).
Daver, N. et al. Phase 1/2 first-in-human study of the menin-MLL inhibitor DSP-5336 in patients with relapsed or refractory acute leukemia. Blood 142, 2911 (2023).
Issa, G. C. et al. Early results of the phase I/II study investigating the all-oral combination of the menin inhibitor revumenib (SNDX-5613) with decitabine/cedazuridine (ASTX727) and venetoclax in acute myeloid leukemia (SAVE). Blood 142, 58 (2023).
Li, Z. et al. PBX3 and MEIS1 cooperate in hematopoietic cells to drive acute myeloid leukemias characterized by a core transcriptome of the MLL-rearranged disease. Cancer Res. 76, 619–629 (2016).
Sun, Y. et al. HOXA9 reprograms the enhancer landscape to promote leukemogenesis. Cancer Cell 34, 643–658.e5 (2018).
Soto-Feliciano, Y. M. et al. A molecular switch between mammalian MLL complexes dictates response to menin–MLL inhibition. Cancer Discov. 13, 146–169 (2023).
Perillo, B., Tramontano, A., Pezone, A. & Migliaccio, A. LSD1: more than demethylation of histone lysine residues. Exp. Mol. Med. 52, 1936–1947 (2020).
Maiques-Diaz, A. et al. Enhancer activation by pharmacologic displacement of LSD1 from GFI1 induces differentiation in acute myeloid leukemia. Cell Rep. 22, 3641–3659 (2018).
Noce, B., Di Bello, E., Fioravanti, R. & Mai, A. LSD1 inhibitors for cancer treatment: focus on multi-target agents and compounds in clinical trials. Front. Pharm. 14, 1120911 (2023).
Stein, E. M. et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood 131, 2661–2669 (2018).
Hideshima, T., Richardson, P. G. & Anderson, K. C. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol. Cancer Ther. 10, 2034–2042 (2011).
Hideshima, T. et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl Acad. Sci. USA 102, 8567–8572 (2005).
San-Miguel, J. F. et al. Phase Ib study of panobinostat and bortezomib in relapsed or relapsed and refractory multiple myeloma. J. Clin. Oncol. 31, 3696–3703 (2013).
Badros, A. et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin. Cancer Res. 15, 5250–5257 (2009).
San-Miguel, J. F. et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 15, 1195–1206 (2014).
Richardson, P. G. et al. Panobinostat plus bortezomib and dexamethasone in previously treated multiple myeloma: outcomes by prior treatment. Blood 127, 713–721 (2016).
San-Miguel, J. F. et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): a randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 3, e506–e515 (2016).
Richardson, P. G. F. et al. Panobinostat for the treatment of relapsed or relapsed/refractory multiple myeloma: pharmacology and clinical outcomes. Expert Rev. Clin. Pharmacol. 9, 35–48 (2016).
Laubach, J. P. et al. Efficacy and safety of oral panobinostat plus subcutaneous bortezomib and oral dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma (PANORAMA 3): an open-label, randomised, phase 2 study. Lancet Oncol. 22, 142–154 (2021).
Pu, J. et al. Exploring the role of histone deacetylase and histone deacetylase inhibitors in the context of multiple myeloma: mechanisms, therapeutic implications, and future perspectives. Exp. Hematol. Oncol. 13, 45 (2024).
Tan, D. et al. Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T-cell lymphoma: an open-label, multicentre phase 2 trial. Lancet Haematol. 2, e326–e333 (2015).
Coiffier, B. et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J. Clin. Oncol. 30, 631–636 (2012).
Falchi, L. et al. Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: a multicenter phase 2 study. Blood 137, 2161–2170 (2021).
Horwitz, S. M. et al. The combination of duvelisib, a PI3K-δ,γ inhibitor, and romidepsin is highly active in relapsed/refractory peripheral T-cell lymphoma with low rates of transaminitis: results of parallel multicenter, phase 1 combination studies with expansion cohorts. Blood 132, 683–683 (2018).
Ruan, J. et al. Multicenter phase 2 study of romidepsin plus lenalidomide for previously untreated peripheral T-cell lymphoma. Blood Adv. 7, 5771–5779 (2023).
Reimer, P. et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J. Clin. Oncol. 27, 106–113 (2009).
Simon, A. et al. Upfront VIP‐reinforced‐ABVD (VIP‐rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS‐LTP95. Br. J. Haematol. 151, 159–166 (2010).
Horwitz, S. et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 393, 229–240 (2019).
Sabnis, G. J., Goloubeva, O. G., Kazi, A. A., Shah, P. & Brodie, A. H. HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7Ca xenografts to aromatase inhibitors through modulation of Her-2. Mol. Cancer Ther. 12, 2804–2816 (2013).
Iwata, H. et al. Efficacy and exploratory biomarker analysis of entinostat plus exemestane in advanced or recurrent breast cancer: phase II randomized controlled trial. Jpn J. Clin. Oncol. 53, 4–15 (2023).
Connolly, R. M. et al. E2112: randomized phase III trial of endocrine therapy plus entinostat or placebo in hormone receptor-positive advanced breast cancer. a trial of the ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 39, 3171–3181 (2021).
Mukohara, T. et al. A phase 1 dose expansion study of a first-in-class KAT6 inhibitor (PF-07248144) in patients with advanced or metastatic ER+ HER2− breast cancer. J. Clin. Oncol. 42, 3006–3006 (2024).
Woessner, R. et al. Abstract LB204: the class Iselective, oral HDAC inhibitor OKI-179 increases tumor regressions when combined with the MEK inhibitor binimetinib inmodels of NRAS melanoma. Cancer Res. 82, LB204–LB204 (2022).
LaFave, L. M. et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat. Med. 21, 1344–1349 (2015).
Zauderer, M. G. et al. EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: a multicentre, open-label, phase 2 study. Lancet Oncol. 23, 758–767 (2022).
Landman, N. et al. Combination of EZH2 and ATM inhibition in BAP1-deficient mesothelioma. Br. J. Cancer 130, 1855–1865 (2024).
Badhai, J. et al. Combined Inhibition of EZH2 and FGFR is synergistic in BAP1-deficient malignant mesothelioma. Cancer Res. Commun. 4, 18–27 (2024).
Yang, J. et al. Epigenetic regulation in the tumor microenvironment: molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 8, 210 (2023).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
A.D.V. has served on advisory boards for Arima Genomics. J.E.A. has served on advisory boards for ADC Therapeutics. B.P. has received research grants from Ono Pharmaceuticals and SciTech. S.E.B. has served on data safety monitoring boards for Acrivon, Amgen, Elmedix, Merck, Pegascy, Pfizer and RenovoRx. S.S.T. declares no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks G. Garcia-Manero and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tolu, S.S., Viny, A.D., Amengual, J.E. et al. Getting the right combination to break the epigenetic code. Nat Rev Clin Oncol 22, 117–133 (2025). https://doi.org/10.1038/s41571-024-00972-1
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41571-024-00972-1