Abstract
Lung cancer remains the leading cause of cancer-related mortality globally, with many patients diagnosed with advanced-stage disease. Treatment in this setting relies on systemic therapies, including chemotherapy, targeted therapy and immunotherapy. Immune-checkpoint inhibitors (ICIs), which promote or restore antitumour immunity by inhibiting immunosuppressive signalling pathways, are currently the most widely used immunotherapies in these patients. However, immune-related adverse events (irAEs) or disease progression often necessitate discontinuation of these agents, leaving many patients with limited subsequent treatment options. In this scenario, ICI rechallenge has emerged as a potential strategy. Despite this potential, evidence for ICI rechallenge after either disease progression or irAEs in patients with non-small-cell lung cancer is limited and evidence for those with small cell lung cancer seems to be non-existent. In this Review, we provide a comprehensive overview of the available data on ICI rechallenge in the context of both disease progression and irAEs, including a summary of current guidance on clinical management and detailed discussions of safety and efficacy. We also highlight important unanswered questions in an attempt to guide future research in this area.
Key points
-
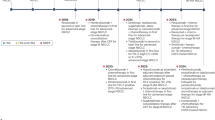
Rechallenge with an immune-checkpoint inhibitor (ICI) in patients with non-small-cell lung cancer (NSCLC) can be considered to be either retreatment or resensitization based on whether other treatments were administered between initial ICI administration and rechallenge.
-
The success of ICI rechallenge is often dependent on the initial reasons for disease progression: drug resistance (primary or secondary resistance) and discontinuation-related progression (probable resistance-related discontinuation progression and complete discontinuation progression).
-
ICI rechallenge is encouraged after discontinuation progression but should be pursued with caution after resistance progression.
-
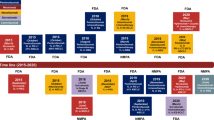
Several guidelines (American Society of Clinical Oncology, Chinese Society of Clinical Oncology, National Comprehensive Cancer Network, Society for Immunotherapy of Cancer) provide recommendations on ICI rechallenge after discontinuation owing to immune-related adverse events, although recommendations differ between guidelines.
-
Performance status, PD-L1 expression, treatment interval and initial duration of treatment are all predictive of effective ICI rechallenge.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Bhopal, A., Peake, M. D., Gilligan, D. & Cosford, P. Lung cancer in never-smokers: a hidden disease. J. R. Soc. Med. 112, 269–271 (2019).
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F. & Heist, R. S. Lung cancer. Lancet 398, 535–554 (2021).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 6, 38 (2020).
Hoos, A. Development of immuno-oncology drugs — from CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 15, 235–247 (2016).
Zugazagoitia, J., Osma, H., Baena, J., Ucero, A. C. & Paz-Ares, L. Facts and hopes on cancer immunotherapy for small cell lung cancer. Clin. Cancer Res. 30, 2872–2883 (2024).
Doroshow, D. B. et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin. Cancer Res. 25, 4592–4602 (2019).
Garon, E. B. et al. Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J. Clin. Oncol. 37, 2518–2527 (2019).
Topalian, S. L. et al. Five-year survival and correlates among patients with advanced melanoma, renal cell carcinoma, or non-small cell lung cancer treated with nivolumab. JAMA Oncol. 5, 1411–1420 (2019).
Bagchi, S., Yuan, R. & Engleman, E. G. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249 (2021).
Michot, J. M. et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer 54, 139–148 (2016).
Insa, A. et al. Which treatment after first line therapy in NSCLC patients without genetic alterations in the era of immunotherapy? Crit. Rev. Oncol. Hematol. 169, 103538 (2022).
Kaira, K. et al. Pooled analysis of the reports of erlotinib after failure of gefitinib for non-small cell lung cancer. Lung Cancer 68, 99–104 (2010).
Kobayashi, K. et al. Successful retreatment using pembrolizumab for non-small-cell lung cancer after severe immune-related hepatitis: a case report. Clin. Lung Cancer 21, e30–e32 (2020).
van der Westhuizen, A. et al. Repurposing azacitidine and carboplatin to prime immune checkpoint blockade-resistant melanoma for anti-PD-L1 rechallenge. Cancer Res. Commun. 2, 814–826 (2022).
Cao, J., Ding, X., Ji, J., Zhang, L. & Luo, C. Efficacy and safety of immune checkpoint inhibitors rechallenge in advanced solid tumors: a systematic review and meta-analysis. Front. Oncol. 14, 1475502 (2024).
Lan, S. & Lu, S. P2.03a-058 is there a place for pemetrexed rechallenge in advanced lung adenocarcinoma?: topic: clinical trials. J. Thorac. Oncol. 12, S924–S925 (2017).
Metro, G. et al. Successful response to osimertinib rechallenge after intervening chemotherapy in an EGFR T790M-positive lung cancer patient. Clin. Drug Investig. 38, 983–987 (2018).
Ceresoli, G. L. et al. Retreatment with pemetrexed-based chemotherapy in patients with malignant pleural mesothelioma. Lung Cancer 72, 73–77 (2011).
Song, Y. et al. Efficacy and safety of gefitinib as third-line treatment in NSCLC patients with activating EGFR mutations treated with first-line gefitinib followed by second-line chemotherapy: a single-arm, prospective, multicenter phase ii study (RE-CHALLENGE, CTONG1304). Am. J. Clin. Oncol. 42, 432–439 (2019).
Li, M. S. et al. P2.09-24 risk of recurrent interstitial lung disease (ILD) from EGFR TKI rechallenge after osimertinib induced ILD. J. Thorac. Oncol. 18, S342 (2023).
Zaremba, A. et al. The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur. J. Cancer 155, 268–280 (2021).
Gebhardt, C. et al. The concepts of rechallenge and retreatment in melanoma: a proposal for consensus definitions. Eur. J. Cancer 138, 68–76 (2020).
Kitagawa, S., Hakozaki, T., Kitadai, R. & Hosomi, Y. Switching administration of anti-PD-1 and anti-PD-L1 antibodies as immune checkpoint inhibitor rechallenge in individuals with advanced non-small cell lung cancer: case series and literature review. Thorac. Cancer 11, 1927–1933 (2020).
Mouri, A. et al. Clinical difference between discontinuation and retreatment with nivolumab after immune-related adverse events in patients with lung cancer. Cancer Chemother. Pharmacol. 84, 873–880 (2019).
Yang, J. et al. Efficacy, prognosis and safety analysis of anti-PD-1/PD-L1 inhibitor rechallenge in advanced lung cancer patients: a cohort study. Transl. Lung Cancer Res. 11, 1038–1050 (2022).
Santini, F. C. et al. Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol. Res. 6, 1093–1099 (2018).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Kluger, H. M. et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC immunotherapy resistance taskforce. J. Immunother. Cancer 8, e000398 (2020).
Kluger, H. et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for resistance to combinations of immune checkpoint inhibitors. J. Immunother. Cancer 11, e005921 (2023).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575, 299–309 (2019).
Fares, C. M., Van Allen, E. M., Drake, C. G., Allison, J. P. & Hu-Lieskovan, S. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am. Soc. Clin. Oncol. Educ. Book 39, 147–164 (2019).
Whiteside, T. L., Demaria, S., Rodriguez-Ruiz, M. E., Zarour, H. M. & Melero, I. Emerging opportunities and challenges in cancer immunotherapy. Clin. Cancer Res. 22, 1845–1855 (2016).
Shergold, A. L., Millar, R. & Nibbs, R. J. B. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol. Res. 145, 104258 (2019).
Weichselbaum, R. R., Liang, H., Deng, L. & Fu, Y.-X. Radiotherapy and immunotherapy: a beneficial liaison? Nat. Rev. Clin. Oncol. 14, 365–379 (2017).
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Melanoma: cutaneous, version 2.2025. NCCN https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf (2025).
Amaral, T. et al. Cutaneous melanoma: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 36, 10–30 (2025).
Sondak, V. K. et al. Systemic therapy for melanoma: ASCO guideline update Q and A. JCO Oncol. Pract. 20, 173–177 (2024).
Seth, R. et al. Systemic therapy for melanoma: ASCO guideline update. J. Clin. Oncol. 41, 4794–4820 (2023).
Rathmell, W. K. et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J. Clin. Oncol. 40, 2957–2995 (2022).
Powles, T. et al. Renal cell carcinoma: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 35, 692–706 (2024).
Rini, B. I. et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J. Immunother. Cancer 7, 354 (2019).
Govindan, R. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of lung cancer and mesothelioma. J. Immunother. Cancer 10, e003956 (2022).
Hendriks, L. E. et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34, 358–376 (2023).
Riely, G. J. et al. Non-small cell lung cancer, version 4.2024, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 22, 249–274 (2024).
Chinese Society of Clinical Oncology (CSCO) Guidelines Working Committee. Guideline on Toxicity Management Associated with Immune Checkpoint Inhibitors (People’s Medical Publishing House, 2021).
Gandara, D. R. et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK study. J. Thorac. Oncol. 13, 1906–1918 (2018).
Stinchcombe, T. E., Miksad, R. A., Gossai, A., Griffith, S. D. & Torres, A. Z. Real-world outcomes for advanced non-small cell lung cancer patients treated with a PD-L1 inhibitor beyond progression. Clin. Lung Cancer 21, 389–394.e3 (2020).
Salous, T. et al. A phase 2 trial of chemotherapy plus pembrolizumab in patients with advanced non-small cell lung cancer previously treated with a PD-1 or PD-L1 inhibitor: big ten cancer research consortium BTCRC-LUN15-029. Cancer 129, 264–271 (2023).
Waterhouse, D. M. et al. Continuous versus 1-year fixed-duration nivolumab in previously treated advanced non-small-cell lung cancer: CheckMate 153. J. Clin. Oncol. 38, 3863–3873 (2020).
Fortman, D. et al. Brief report: phase II clinical trial of atezolizumab in advanced nonsmall cell lung cancer patients previously treated with PD-1-directed therapy. Clin. Lung Cancer 26, 78–81 (2025).
Hakozaki, T., Okuma, Y. & Kashima, J. Re-challenging immune checkpoint inhibitor in a patient with advanced non-small cell lung cancer: a case report. BMC Cancer 18, 302 (2018).
Hirano, S. et al. Drastic response of rechallenge of nivolumab in a patient with NSCLC who progressed on the first nivolumab treatment. J. Thorac. Oncol. 15, e20–e22 (2020).
Watanabe, H. et al. The effect and safety of immune checkpoint inhibitor rechallenge in non-small cell lung cancer. Jpn J. Clin. Oncol. 49, 762–765 (2019).
Herbst, R. S. et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J. Clin. Oncol. 38, 1580–1590 (2020).
Reck, M. et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥50. J. Clin. Oncol. 39, 2339–2349 (2021).
de Castro, G. et al. Five-year outcomes with pembrolizumab versus chemotherapy as first-line therapy in patients with non-small-cell lung cancer and programmed death ligand-1 tumor proportion score ≥ 1% in the KEYNOTE-042 study. J. Clin. Oncol. 41, 1986–1991 (2023).
Nomura, S. et al. A randomized phase III study comparing continuation and discontinuation of PD-1 pathway inhibitors for patients with advanced non-small-cell lung cancer (JCOG1701, SAVE study). Jpn J. Clin. Oncol. 50, 821–825 (2020).
Schoenfeld, A. J. et al. Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Ann. Oncol. 32, 1597–1607 (2021).
Herbst, R. S. et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N. Engl. J. Med. 383, 1328–1339 (2020).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 375, 1823–1833 (2016).
Akamatsu, H. et al. Nivolumab retreatment in non-small cell lung cancer patients who responded to prior immune checkpoint inhibitors and had ICI-free intervals (WJOG9616L). Clin. Cancer Res. 28, OF1–OF7 (2022).
Xu, C. et al. Comparative safety of immune checkpoint inhibitors in cancer: systematic review and network meta-analysis. BMJ 363, k4226 (2018).
Yan, Y.-D. et al. A network comparison on safety profiling of immune checkpoint inhibitors in advanced lung cancer. Front. Immunol. 12, 760737 (2021).
Allouchery, M. et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J. Immunother. Cancer 8, e001622 (2020).
Nishino, M., Giobbie-Hurder, A., Hatabu, H., Ramaiya, N. H. & Hodi, F. S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2, 1607–1616 (2016).
Arnaud-Coffin, P. et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int. J. Cancer 145, 639–648 (2019).
Wang, D. Y. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728 (2018).
Thompson, J. A. et al. NCCN guidelines® insights: management of immunotherapy-related toxicities, version 2.2024. J. Natl Compr. Cancer Netw. 22, 582–592 (2024).
Schneider, B. J. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 39, 4073–4126 (2021).
Puzanov, I. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J. Immunother. Cancer 5, 95 (2017).
Brahmer, J. R. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 9, e002435 (2021).
Naidoo, J. et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology. J. Immunother. Cancer 11, e006398 (2023).
Delaunay, M. et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. 50, 1700050 (2017).
Ma, K. et al. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front. Pharmacol. 9, 1430 (2018).
Ricciuti, B. et al. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J. Clin. Oncol. 37, 1927–1934 (2019).
Delanoy, N. et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol. 6, e48–e57 (2019).
US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (2017).
Dolladille, C. et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 6, 865–871 (2020).
Moslehi, J. J., Salem, J.-E., Sosman, J. A., Lebrun-Vignes, B. & Johnson, D. B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 391, 933 (2018).
Escudier, M. et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation 136, 2085–2087 (2017).
Ganatra, S. & Neilan, T. G. Immune checkpoint inhibitor-associated myocarditis. Oncologist 23, 879–886 (2018).
Thompson, J. A. et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 20, 387–405 (2022).
Collins, M. et al. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann. Oncol. 28, 2860–2865 (2017).
Wei, S. C. et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell 170, 1120–1133.e17 (2017).
Barnes, M. J. et al. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 6, 324–334 (2013).
Hellmann, M. D. et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 381, 2020–2031 (2019).
Ready, N. et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J. Clin. Oncol. 37, 992–1000 (2019).
Hellmann, M. D. et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 18, 31–41 (2017).
Rizvi, N. A. et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 6, 661–674 (2020).
Brahmer, J. R. et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J. Clin. Oncol. 41, 1200–1212 (2023).
Spänkuch, I. et al. Severe hepatitis under combined immunotherapy: resolution under corticosteroids plus anti-thymocyte immunoglobulins. Eur. J. Cancer 81, 203–205 (2017).
Kataoka, S. et al. Re-administration of nivolumab after immune checkpoint inhibitor-induced cholangitis: the first reported case. Clin. J. Gastroenterol. 15, 467–474 (2022).
Ribas, A. et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 31, 616–622 (2013).
Eggermont, A. M. M. et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 16, 522–530 (2015).
Weber, J. S. et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 16, 375–384 (2015).
Herbst, R. S. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387, 1540–1550 (2016).
Zhao, Q. et al. Safety and efficacy of the rechallenge of immune checkpoint inhibitors after immune-related adverse events in patients with cancer: a systemic review and meta-analysis. Front. Immunol. 12, 730320 (2021).
Haanen, J. et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: review of the literature and suggested prophylactic strategy. J. Immunother. Cancer 8, e000604 (2020).
Nagao, K. et al. Pancreatic injury in patients treated with immune checkpoint inhibitors: a retrospective multicenterstudy. J. Gastroenterol. 59, 424–433 (2024).
ElSayed, N. A. et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care 46, S19–S40 (2023).
Stamatouli, A. M. et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 67, 1471–1480 (2018).
Barroso-Sousa, R. et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 4, 173–182 (2018).
Byun, D. J., Wolchok, J. D., Rosenberg, L. M. & Girotra, M. Cancer immunotherapy — immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 13, 195–207 (2017).
Clotman, K., Janssens, K., Specenier, P., Weets, I. & De Block, C. E. M. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J. Clin. Endocrinol. Metab. 103, 3144–3154 (2018).
Aleksova, J., Lau, P. K. H., Soldatos, G. & McArthur, G. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma. BMJ Case Rep. 2016, bcr2016217454 (2016).
Bhanderi, H. et al. Autoimmune diabetes from pembrolizumab: a case report and review of literature. World J. Clin. Oncol. 14, 535–543 (2023).
Ross, D. S. et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid 26, 1343–1421 (2016).
Illouz, F., Briet, C. & Rodien, P. Immune checkpoint inhibitor-related thyroid dysfunction. Ann. Endocrinol. 84, 346–350 (2023).
Wright, J. J., Powers, A. C. & Johnson, D. B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol. 17, 389–399 (2021).
Mosaferi, T. et al. Optimal thyroid hormone replacement dose in immune checkpoint inhibitor-associated hypothyroidism is distinct from Hashimoto’s thyroiditis. Thyroid 32, 496–504 (2022).
Idrees, T., Palmer, S., Maciel, R. M. B. & Bianco, A. C. Liothyronine and desiccated thyroid extract in the treatment of hypothyroidism. Thyroid 30, 1399–1413 (2020).
Shakir, M. K. M. et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J. Clin. Endocrinol. Metab. 106, e4400–e4413 (2021).
Jonklaas, J. et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 24, 1670–1751 (2014).
Cook, S. et al. Immune-related adverse events and survival among patients with metastatic NSCLC treated with immune checkpoint inhibitors. JAMA Netw. Open 7, e2352302 (2024).
Cukier, P., Santini, F. C., Scaranti, M. & Hoff, A. O. Endocrine side effects of cancer immunotherapy. Endocr. Relat. Cancer 24, T331–T347 (2017).
Chang, L.-S. et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr. Rev. 40, 17–65 (2019).
Meraz-Muñoz, A. et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J. Immunother. Cancer 8, e000467 (2020).
Gupta, S., Cortazar, F. B., Riella, L. V. & Leaf, D. E. Immune checkpoint inhibitor nephrotoxicity: update 2020. Kidney360 1, 130–140 (2020).
Manohar, S. et al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol. Dial. Transplant. 34, 108–117 (2019).
Seethapathy, H. et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin. J. Am. Soc. Nephrol. 14, 1692–1700 (2019).
Cortazar, F. B. et al. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J. Am. Soc. Nephrol. 31, 435–446 (2020).
Manohar, S. & Albright, R. C. Interstitial nephritis in immune checkpoint inhibitor therapy. Kidney Int. 96, 252 (2019).
Knox, A. et al. Immune-related acute kidney injury in Australian non-small cell lung cancer patients: real-world results. Lung Cancer 184, 107325 (2023).
Mroue, A. et al. Exploring the knowledge gap of immune checkpoint inhibitors in chronic renal failure: a systematic review of the literature. Crit. Rev. Oncol. Hematol. 157, 103169 (2021).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group.KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 105, S117–S314 (2024).
Cuzzubbo, S. et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur. J. Cancer 73, 1–8 (2017).
Diamanti, L. et al. Characterization and management of neurological adverse events during immune-checkpoint inhibitors treatment: an Italian multicentric experience. Neurol. Sci. 43, 2031–2041 (2022).
Eldani, C. et al. Safety of immune checkpoint inhibitor rechallenge after severe immune-related adverse events: a retrospective analysis. Front. Oncol. 14, 1403658 (2024).
Duong, S. L., Barbiero, F. J., Nowak, R. J. & Baehring, J. M. Neurotoxicities associated with immune checkpoint inhibitor therapy. J. Neurooncol. 152, 265–277 (2021).
Park, R. B., Jain, S., Han, H. & Park, J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocul. Surf. 20, 115–129 (2021).
Matsuo, T. & Yamasaki, O. Vogt-Koyanagi-Harada disease-like posterior uveitis in the course of nivolumab (anti-PD-1 antibody), interposed by vemurafenib (BRAF inhibitor), for metastatic cutaneous malignant melanoma. Clin. Case Rep. 5, 694–700 (2017).
Zhou, L. & Wei, X. Ocular immune-related adverse events associated with immune checkpoint inhibitors in lung cancer. Front. Immunol. 12, 701951 (2021).
Young, L. et al. Ocular adverse events in PD-1 and PD-L1 inhibitors. J. Immunother. Cancer 9, e002119 (2021).
Suarez-Almazor, M. E., Kim, S. T., Abdel-Wahab, N. & Diab, A. Review: immune-related adverse events with use of checkpoint inhibitors for immunotherapy of cancer. Arthritis Rheumatol. 69, 687–699 (2017).
Cappelli, L. C., Gutierrez, A. K., Bingham, C. O. & Shah, A. A. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res. 69, 1751–1763 (2017).
Naidoo, J. et al. Inflammatory arthritis: a newly recognized adverse event of immune checkpoint blockade. Oncologist 22, 627–630 (2017).
Woodworth, T. et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: the rheumatology common toxicity criteria v.2.0. J. Rheumatol. 34, 1401–1414 (2007).
Hofmann, L. et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 60, 190–209 (2016).
Wang, F. et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J. Hematol. Oncol. 13, 47 (2020).
Qing, S.-K. et al. Expert consensus on the clinical diagnosis and treatment of camrelizumab induced reactive cutaneous capillary endothelial proliferation. Chin. Clin. Oncol. 25, 840–848 (2020).
Chen, X. et al. Reactive capillary hemangiomas: a novel dermatologic toxicity following anti-PD-1 treatment with SHR-1210. Cancer Biol. Med. 16, 173–181 (2019).
Li, T. et al. A multicenter, real-world study on effectiveness and safety of first-line modified PD-1 inhibitors with chemotherapy in advanced non-small cell lung cancer (aNSCLC) with drive gene-negative. Cancer Med. 13, e7024 (2024).
Chan, L. et al. Survival and prognosis of individuals receiving programmed cell death 1 inhibitor with and without immunologic cutaneous adverse events. J. Am. Acad. Dermatol. 82, 311–316 (2020).
Zhang, D. et al. Clinical efficacy of camrelizumab combined with first-line chemotherapy in extensive-stage small-cell lung cancer. Heliyon 10, e22913 (2024).
Qu, W., Wang, F., Qin, S., Sun, Y. & Huang, C. Reactive cutaneous capillary endothelial proliferation following camrelizumab monotherapy or combination therapy for multi-cancers: a large-scale pooled analysis of 10 studies in China. Ther. Adv. Med. Oncol. 16, 17588359241242607 (2024).
O’Neal, R. L., Roberts, K., Hao, Z. & Arnold, S. M. Rechallenging with immune checkpoint inhibition after a treatment-limiting immune-related adverse event. J. Clin. Oncol. 38, 3053–3053 (2020).
Simonaggio, A. et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 5, 1310–1317 (2019).
Guo, M., VanderWalde, A. M., Yu, X., Vidal, G. A. & Tian, G. G. Immune checkpoint inhibitor rechallenge safety and efficacy in stage IV non-small cell lung cancer patients after immune-related adverse events. Clin. Lung Cancer 23, 686–693 (2022).
Schadendorf, D. et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J. Clin. Oncol. 35, 3807–3814 (2017).
Cai, Z., Zhan, P., Song, Y., Liu, H. & Lv, T. Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis. Transl. Lung Cancer Res. 11, 1555–1566 (2022).
Fujisaki, T. et al. The prognostic significance of the continuous administration of anti-PD-1 antibody via continuation or rechallenge after the occurrence of immune-related adverse events. Front. Oncol. 11, 704475 (2021).
Plazy, C., Hannani, D. & Gobbini, E. Immune checkpoint inhibitor rechallenge and resumption: a systematic review. Curr. Oncol. Rep. 24, 1095–1106 (2022).
Gobbini, E. et al. Immune checkpoint inhibitors rechallenge efficacy in non-small-cell lung cancer patients. Clin. Lung Cancer 21, e497–e510 (2020).
Katayama, Y. et al. Retrospective efficacy analysis of immune checkpoint inhibitor rechallenge in patients with non-small cell lung cancer. J. Clin. Med. 9, 102 (2019).
Fujita, K. et al. Retreatment with pembrolizumab in advanced non-small cell lung cancer patients previously treated with nivolumab: emerging reports of 12 cases. Cancer Chemother. Pharmacol. 81, 1105–1109 (2018).
Niki, M. et al. Immune checkpoint inhibitor re-challenge in patients with advanced non-small cell lung cancer. Oncotarget 9, 32298–32304 (2018).
Giaj Levra, M. et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: a national data base analysis. Lung Cancer 140, 99–106 (2020).
Acknowledgements
The authors’ work is supported by grants from Noncommunicable Chronic Diseases — National Science and Technology Major Project (Grant Number 2023ZD0501700), the National Natural Science Foundation of China (Grant No. 82373349), Guangzhou Science and Technology Program (Grant Number 2025A03J4506), MOE Changjiang Distinguished Professor Supporting Project (Grant Number KY0120240205), and Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (Grant Number 2017B030314120).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
C.-R.X. has acted as a consultant and/or advisor of Allist Pharmaceuticals, AstraZeneca, Avistone, BeiGene, Bristol–Myers Squibb, Burning Rock Biotech, CStone Pharmaceuticals, Dizal Pharma, Geneplus, MSD, Pfizer, Roche, SciClone, Takeda and Zhengda Tianqing Pharmaceutical Group. Q.Z. has received honoraria from AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, MSD, Pfizer, Roche and Sanofi for consultancy and/or advisory work. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks J. Alexandre, T. Hakozaki and A. Tufman for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, LB., Peng, YL., Chen, J. et al. Rechallenge with immune-checkpoint inhibitors in patients with advanced-stage lung cancer. Nat Rev Clin Oncol 22, 546–565 (2025). https://doi.org/10.1038/s41571-025-01029-7
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01029-7