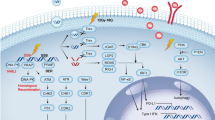
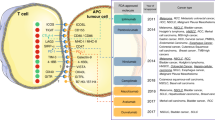
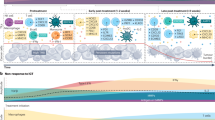
Abstract
Radiotherapy has an established role in the clinical treatment of patients with a variety of cancers owing to the ability to preferentially kill malignant cells mostly while sparing their non-malignant counterparts. Results from phase I–II trials also suggest that radiotherapy can have therapeutically relevant immunostimulatory effects, especially when combined with immune-checkpoint inhibitors. Over the past two decades, evidence has emerged showing that intestinal microbial communities have a major influence on the immunological tonus of patients with cancer and can influence sensitivity to various immunotherapies, including immune-checkpoint inhibitors and chimeric antigen receptor T cells. Here, we critically discuss the effects of such microbial ecosystems on radiotherapy-induced toxicities and tumour-targeting immune responses, with a focus on the clinical potential of these relationships for predictive and therapeutic clinical applications.
Key points
-
Both the intestinal and intratumoural microbiota have emerged as regulators of innate and adaptive antitumour immunity during disease progression and in response to radiotherapy.
-
The composition of microbial ecosystems populating mucosae and their interactions with epithelial membranes influence the severity of radiotherapy-induced mucositis.
-
Intestinal irradiation has metabolic and immunological effects with systemic outreach that can either suppress or promote cancer immunosurveillance, depending on the irradiated organ, radiotherapy dose and target volume as well as the microbiota composition at baseline.
-
Carefully designed microbiota-centred interventions provide promising methods of promoting radiotherapy-induced antitumour immunity while alleviating treatment-associated toxicities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bates, J. E., Sanders, T., Arnone, A., Elmore, S. N. C. & Royce, T. J. Geographic density of linear accelerators and receipt of radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 111, e351–e352 (2021).
Williams, G. R., Manjunath, S. H., Butala, A. A. & Jones, J. A. Palliative radiotherapy for advanced cancers: indications and outcomes. Surg. Oncol. Clin. N. Am. 30, 563–580 (2021).
Gerard, J. P., Romestaing, P. & Chapet, O. Radiotherapy alone in the curative treatment of rectal carcinoma. Lancet Oncol. 4, 158–166 (2003).
De Ruysscher, D. et al. Radiotherapy toxicity. Nat. Rev. Dis. Prim. 5, 13 (2019).
Bucci, M. K., Bevan, A. & Roach, M. 3rd Advances in radiation therapy: conventional to 3D, to IMRT, to 4D, and beyond. CA Cancer J. Clin. 55, 117–134 (2005).
Lo, S. S. et al. Stereotactic body radiation therapy: a novel treatment modality. Nat. Rev. Clin. Oncol. 7, 44–54 (2010).
Thariat, J. et al. Image-guided radiation therapy for muscle-invasive bladder cancer. Nat. Rev. Urol. 9, 23–29 (2011).
Lu, Z. et al. Deciphering the biological effects of radiotherapy in cancer cells. Biomolecules 12, 1167 (2022).
Groelly, F. J., Fawkes, M., Dagg, R. A., Blackford, A. N. & Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 23, 78–94 (2023).
Pilié, P. G., Tang, C., Mills, G. B. & Yap, T. A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 16, 81–104 (2019).
Petroni, G., Buqué, A., Coussens, L. M. & Galluzzi, L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat. Rev. Drug Discov. 21, 440–462 (2022).
Hahn, W. C. et al. An expanded universe of cancer targets. Cell 184, 1142–1155 (2021).
Galluzzi, L., Aryankalayil, M. J., Coleman, C. N. & Formenti, S. C. Emerging evidence for adapting radiotherapy to immunotherapy. Nat. Rev. Clin. Oncol. 20, 543–557 (2023).
Kroemer, G., Chan, T. A., Eggermont, A. M. M. & Galluzzi, L. Immunosurveillance in clinical cancer management. CA Cancer J. Clin. 74, 187–202 (2024).
Galassi, C., Chan, T. A., Vitale, I. & Galluzzi, L. The hallmarks of cancer immune evasion. Cancer Cell 42, 1825–1863 (2024).
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Lhuillier, C. et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Invest. 131, e138740 (2021).
Yamazaki, T. et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 21, 1160–1171 (2020).
Marchi, S., Guilbaud, E., Tait, S. W. G., Yamazaki, T. & Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 23, 159–173 (2023).
DeSelm, C. et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol. Ther. 26, 2542–2552 (2018).
Sugita, M. et al. Radiation therapy improves CAR T cell activity in acute lymphoblastic leukemia. Cell Death Dis. 14, 305 (2023).
Rodriguez-Ruiz, M. E., Vitale, I., Harrington, K. J., Melero, I. & Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 21, 120–134 (2020).
Pointer, K. B., Pitroda, S. P. & Weichselbaum, R. R. Radiotherapy and immunotherapy: open questions and future strategies. Trends Cancer 8, 9–20 (2022).
Turchan, W. T., Pitroda, S. P. & Weichselbaum, R. R. Radiotherapy and immunotherapy combinations in the treatment of patients with metastatic disease: current status and future focus. Clin. Cancer Res. 27, 5188–5194 (2021).
Altorki, N. K. et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 22, 824–835 (2021).
Antonia, S. J. et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 377, 1919–1929 (2017).
Fizazi, K. et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur. Urol. 78, 822–830 (2020).
Lin, Z. Y. et al. Neoadjuvant short-course radiotherapy followed by camrelizumab and chemotherapy in locally advanced rectal cancer (UNION): early outcomes of a multicenter randomized phase III trial. Ann. Oncol. 35, 882–891 (2024).
Grossman, S. A. et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 17, 5473–5480 (2011).
Cramer, J. D., Burtness, B., Le, Q. T. & Ferris, R. L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 16, 669–683 (2019).
de Kermenguy, F. et al. Radio-induced lymphopenia in the era of anti-cancer immunotherapy. Int. Rev. Cell Mol. Biol. 378, 1–30 (2023).
Yovino, S., Kleinberg, L., Grossman, S. A., Narayanan, M. & Ford, E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 31, 140–144 (2013).
Nakamura, N., Kusunoki, Y. & Akiyama, M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat. Res. 123, 224–227 (1990).
de Kermenguy, F. et al. LymphoDose: a lymphocyte dose estimation framework-application to brain radiotherapy. Phys. Med. Biol. 69, https://doi.org/10.1088/1361-6560/ad3c8d (2024).
Ghosh, S. et al. Radiation-induced circulating myeloid-derived suppressor cells induce systemic lymphopenia after chemoradiotherapy in patients with glioblastoma. Sci. Transl. Med. 15, eabn6758 (2023).
Zitvogel, L., Fidelle, M. & Kroemer, G. Long-distance microbial mechanisms impacting cancer immunosurveillance. Immunity 57, 2013–2029 (2024).
Routy, B. et al. The gut microbiota influences anticancer immunosurveillance and general health. Nat. Rev. Clin. Oncol. 15, 382–396 (2018).
Price, J. M., Prabhakaran, A. & West, C. M. L. Predicting tumour radiosensitivity to deliver precision radiotherapy. Nat. Rev. Clin. Oncol. 20, 83–98 (2023).
Zitvogel, L., Ayyoub, M., Routy, B. & Kroemer, G. Microbiome and anticancer immunosurveillance. Cell 165, 276–287 (2016).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Viaud, S. et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342, 971–976 (2013).
Roberti, M. P. et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat. Med. 26, 919–931 (2020).
Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020).
Derosa, L. et al. Custom scoring based on ecological topology of gut microbiota associated with cancer immunotherapy outcome. Cell 187, 3373–3389 (2024).
Derosa, L. et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 29, 1437–1444 (2018).
Eng, L. et al. Impact of antibiotic exposure before immune checkpoint inhibitor treatment on overall survival in older adults with cancer: a population-based study. J. Clin. Oncol. 41, 3122–3134 (2023).
Pinato, D. J. et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 5, 1774–1778 (2019).
Smith, M. et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 28, 713–723 (2022).
Stein-Thoeringer, C. K. et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 29, 906–916 (2023).
Derosa, L. et al. Microbiota-centered interventions: the next breakthrough in immuno-oncology? Cancer Discov. 11, 2396–2412 (2021).
Elkrief, A. et al. Antibiotics are associated with worse outcomes in lung cancer patients treated with chemotherapy and immunotherapy. NPJ Precis. Oncol. 8, 143 (2024).
Lurienne, L. et al. NSCLC immunotherapy efficacy and antibiotic use: a systematic review and meta-analysis. J. Thorac. Oncol. 15, 1147–1159 (2020).
Tsikala-Vafea, M., Belani, N., Vieira, K., Khan, H. & Farmakiotis, D. Use of antibiotics is associated with worse clinical outcomes in patients with cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Int. J. Infect. Dis. 106, 142–154 (2021).
Yu, Y. et al. Effects of antibiotic use on outcomes in cancer patients treated using immune checkpoint inhibitors: a systematic review and meta-analysis. J. Immunother. 44, 76–85 (2021).
Zhou, J. et al. The impact of antibiotic use on clinical features and survival outcomes of cancer patients treated with immune checkpoint inhibitors. Front. Immunol. 13, 968729 (2022).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Fidelle, M. et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 380, eabo2296 (2023).
Gacesa, R. et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 604, 732–739 (2022).
Yonekura, S. et al. Cancer induces a stress ileopathy depending on beta-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 12, 1128–1151 (2022).
Zitvogel, L., Ma, Y., Raoult, D., Kroemer, G. & Gajewski, T. F. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 359, 1366–1370 (2018).
Daillere, R. et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016).
Thomas, A. M. et al. Gut oncomicrobiome signatures (GOMS) as next-generation biomarkers for cancer immunotherapy. Nat. Rev. Clin. Oncol. 20, 583–603 (2023).
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
Ciccarese, C. et al. LBA77 Fecal microbiota transplantation (FMT) versus placebo in patients receiving pembrolizumab plus axitinib for metastatic renal cell carcinoma: preliminary results of the randomized phase II TACITO trial. Ann. Oncol. 35, S1264 (2024).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021).
Kim, Y. et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe 32, 1380–1393 (2024).
Routy, B. et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat. Med. 29, 2121–2132 (2023).
Malfertheiner, P. et al. Helicobacter pylori infection. Nat. Rev. Dis. Prim. 9, 19 (2023).
Battaglia, T. W. et al. A pan-cancer analysis of the microbiome in metastatic cancer. Cell 187, 2324–2335 (2024).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806 (2019).
Kalaora, S. et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 592, 138–143 (2021).
Bullman, S. et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017).
Galeano Nino, J. L. et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817 (2022).
LaCourse, K. D., Johnston, C. D. & Bullman, S. The relationship between gastrointestinal cancers and the microbiota. Lancet Gastroenterol. Hepatol. 6, 498–509 (2021).
Parida, S. et al. A procarcinogenic colon microbe promotes breast tumorigenesis and metastatic progression and concomitantly activates notch and β-catenin axes. Cancer Discov. 11, 1138–1157 (2021).
Bertocchi, A. et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 39, 708–724 (2021).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Yu, T. et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170, 548–563 (2017).
Zagato, E. et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat. Microbiol. 5, 511–524 (2020).
Wang, M. et al. Killing tumor-associated bacteria with a liposomal antibiotic generates neoantigens that induce anti-tumor immune responses. Nat. Biotechnol. 42, 1263–1274 (2024).
Choi, Y. et al. Immune checkpoint blockade induces gut microbiota translocation that augments extraintestinal antitumor immunity. Sci. Immunol. 8, eabo2003 (2023).
Gong, T., Liu, L., Jiang, W. & Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 20, 95–112 (2020).
Vanpouille-Box, C., Hoffmann, J. A. & Galluzzi, L. Pharmacological modulation of nucleic acid sensors — therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 18, 845–867 (2019).
Di Luccia, B. et al. TREM2 deficiency reprograms intestinal macrophages and microbiota to enhance anti-PD-1 tumor immunotherapy. Sci. Immunol. 9, eadi5374 (2024).
Galvan-Pena, S., Zhu, Y., Hanna, B. S., Mathis, D. & Benoist, C. A dynamic atlas of immunocyte migration from the gut. Sci. Immunol. 9, eadi0672 (2024).
Wu, Y. et al. Managing strategies of chemotherapy and radiotherapy-induced oral mucositis. Cancer Treat. Rev. 133, 102883 (2025).
Ai, D. et al. Comparison of 3 paclitaxel-based chemoradiotherapy regimens for patients with locally advanced esophageal squamous cell cancer: a randomized clinical trial. JAMA Netw. Open 5, e220120 (2022).
Gebre-Medhin, M. et al. ARTSCAN III: a randomized phase III study comparing chemoradiotherapy with cisplatin versus cetuximab in patients with locoregionally advanced head and neck squamous cell cancer. J. Clin. Oncol. 39, 38–47 (2021).
Miao, J. et al. Adjuvant capecitabine following concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a randomized clinical trial. JAMA Oncol. 8, 1776–1785 (2022).
Tao, Y. et al. Improved outcome by adding concurrent chemotherapy to cetuximab and radiotherapy for locally advanced head and neck carcinomas: results of the GORTEC 2007-01 phase III randomized trial. J. Clin. Oncol. 7, JCO2017762518 (2018).
Bentzen, S. M. et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int. J. Radiat. Oncol. Biol. Phys. 76, S3–S9 (2010).
Hou, J. et al. Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother. Oncol. 129, 44–51 (2018).
Hulpusch, C. et al. Association of skin microbiome dynamics with radiodermatitis in patients with breast cancer. JAMA Oncol. 10, 516–521 (2024).
Iacovacci, J. et al. Intestinal microbiota composition is predictive of radiotherapy-induced acute gastrointestinal toxicity in prostate cancer patients. EBioMedicine 106, 105246 (2024).
Mitra, A. et al. Microbial diversity and composition is associated with patient-reported toxicity during chemoradiation therapy for cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 107, 163–171 (2020).
Reis Ferreira, M. et al. Microbiota- and radiotherapy-induced gastrointestinal side-effects (MARS) study: a large pilot study of the microbiome in acute and late-radiation enteropathy. Clin. Cancer Res. 25, 6487–6500 (2019).
Zhu, X. X. et al. The potential effect of oral microbiota in the prediction of mucositis during radiotherapy for nasopharyngeal carcinoma. EBioMedicine 18, 23–31 (2017).
Danckaert, W. et al. Microbiome and metabolome dynamics during radiotherapy for prostate cancer. Radiother. Oncol. 189, 109950 (2023).
Kalkeri, R. et al. Changes in the gut microbiome community of nonhuman primates following radiation injury. BMC Microbiol. 21, 93 (2021).
Li, Y. et al. Alterations of the gut microbiome composition and lipid metabolic profile in radiation enteritis. Front. Cell Infect. Microbiol. 10, 541178 (2020).
Teng, H. et al. Gut microbiota-mediated nucleotide synthesis attenuates the response to neoadjuvant chemoradiotherapy in rectal cancer. Cancer Cell 41, 124–138 (2023).
Casero, D. et al. Space-type radiation induces multimodal responses in the mouse gut microbiome and metabolome. Microbiome 5, 105 (2017).
Ruan, J. L. et al. Irradiation at ultra-high (FLASH) dose rates reduces acute normal tissue toxicity in the mouse gastrointestinal system. Int. J. Radiat. Oncol. Biol. Phys. 111, 1250–1261 (2021).
Nam, Y. D., Kim, H. J., Seo, J. G., Kang, S. W. & Bae, J. W. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS ONE 8, e82659 (2013).
Touchefeu, Y. et al. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis — current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 40, 409–421 (2014).
Egan, L. J. et al. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc. Natl Acad. Sci. USA 101, 2452–2457 (2004).
Riehl, T., Cohn, S., Tessner, T., Schloemann, S. & Stenson, W. F. Lipopolysaccharide is radioprotective in the mouse intestine through a prostaglandin-mediated mechanism. Gastroenterology 118, 1106–1116 (2000).
Burdelya, L. G. et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 320, 226–230 (2008).
Vijay-Kumar, M. et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J. Immunol. 180, 8280–8285 (2008).
Riehl, T. E. et al. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut 68, 1003–1013 (2019).
Gerassy-Vainberg, S. et al. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut 67, 97–107 (2018).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Beltran-Visiedo, M., Soler-Agesta, R., Sarosiek, K. A., Green, D. R. & Galluzzi, L. Regulation of inflammatory processes by caspases. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-025-00869-6 (2025).
Leibowitz, B. J. et al. Interferon b drives intestinal regeneration after radiation. Sci. Adv. 7, eabi5253 (2021).
Shi, X. et al. FLASH X-ray spares intestinal crypts from pyroptosis initiated by cGAS-STING activation upon radioimmunotherapy. Proc. Natl Acad. Sci. USA 119, e2208506119 (2022).
Barturen, G. & Alarcon-Riquelme, M. E. Revisiting the heterogeneity of interferon-related autoimmune diseases. Nat. Rev. Rheumatol. 21, 7–8 (2025).
Galluzzi, L., Vanpouille-Box, C., Bakhoum, S. F. & Demaria, S. SnapShot: cGAS-STING signaling. Cell 173, 276–276 (2018).
Musella, M. et al. Type I IFNs promote cancer cell stemness by triggering the epigenetic regulator KDM1B. Nat. Immunol. 23, 1379–1392 (2022).
Vanpouille-Box, C., Demaria, S., Formenti, S. C. & Galluzzi, L. Cytosolic DNA sensing in organismal tumor control. Cancer Cell 34, 361–378 (2018).
Guo, H. et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 370, eaay9097 (2020).
Al-Qadami, G. et al. Antibiotic-induced gut microbiota depletion accelerates the recovery of radiation-induced oral mucositis in rats. Int. J. Radiat. Oncol. Biol. Phys. 113, 845–858 (2022).
Zhao, T. S. et al. Dysbiosis of gut microbiota is associated with the progression of radiation-induced intestinal injury and is alleviated by oral compound probiotics in mouse model. Front. Cell Infect. Microbiol. 11, 717636 (2021).
He, K. Y. et al. Akkermansia muciniphila protects the intestine from irradiation-induced injury by secretion of propionic acid. Gut Microbes 15, 2293312 (2023).
Lapiere, A. et al. Prophylactic Faecalibacterium prausnitzii treatment prevents the acute breakdown of colonic epithelial barrier in a preclinical model of pelvic radiation disease. Gut Microbes 12, 1–15 (2020).
Xie, L. W. et al. Probiotic consortia protect the intestine against radiation injury by improving intestinal epithelial homeostasis. Int. J. Radiat. Oncol. Biol. Phys. 120, 189–204 (2024).
Xie, L. W. et al. Microbiota-derived I3A protects the intestine against radiation injury by activating AhR/IL-10/Wnt signaling and enhancing the abundance of probiotics. Gut Microbes 16, 2347722 (2024).
Xiao, H. W. et al. Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein. Microbiome 8, 69 (2020).
Cui, M. et al. Faecal microbiota transplantation protects against radiation-induced toxicity. EMBO Mol. Med. 9, 448–461 (2017).
Jang, B. S. et al. Gut microbiome composition is associated with a pathologic response after preoperative chemoradiation in patients with rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 107, 736–746 (2020).
Nenclares, P. et al. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur. J. Cancer 131, 9–15 (2020).
Qiu, B. et al. Gut microbiome is associated with the response to chemoradiotherapy in patients with non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 115, 407–418 (2023).
Sasaki, T. et al. Gut microbiome can predict chemoradiotherapy efficacy in patients with esophageal squamous cell carcinoma. Esophagus 20, 691–703 (2023).
Sims, T. T. et al. Gut microbiome diversity is an independent predictor of survival in cervical cancer patients receiving chemoradiation. Commun. Biol. 4, 237 (2021).
Li, Z. et al. Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma Via STING signaling. Gut Microbes 14, 2119055 (2022).
Uribe-Herranz, M. et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Invest. 130, 466–479 (2020).
Yang, K. et al. Suppression of local type I interferon by gut microbiota-derived butyrate impairs antitumor effects of ionizing radiation. J. Exp. Med. 218, e20201915 (2021).
Dong, J. et al. Roseburia intestinalis sensitizes colorectal cancer to radiotherapy through the butyrate/OR51E1/RALB axis. Cell Rep. 43, 113846 (2024).
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, e108863 (2021).
Zhou, H. et al. Methylglyoxal from gut microbes boosts radiosensitivity and radioimmunotherapy in rectal cancer by triggering endoplasmic reticulum stress and cGAS-STING activation. J. Immunother. Cancer 11, e007840 (2023).
Galluzzi, L., Guilbaud, E., Schmidt, D., Kroemer, G. & Marincola, F. M. Targeting immunogenic cell stress and death for cancer therapy. Nat. Rev. Drug Discov. 23, 445–460 (2024).
Iliev, I. D. & Leonardi, I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat. Rev. Immunol. 17, 635–646 (2017).
Leonardi, I. et al. Mucosal fungi promote gut barrier function and social behavior via type 17 immunity. Cell 185, 831–846 (2022).
van Tilburg Bernardes, E. et al. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nat. Commun. 11, 2577 (2020).
Shiao, S. L. et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 39, 1202–1213 (2021).
Colbert, L. E. et al. Tumor-resident Lactobacillus iners confer chemoradiation resistance through lactate-induced metabolic rewiring. Cancer Cell 41, 1945–1962 (2023).
De Martino, M., Rathmell, J. C., Galluzzi, L. & Vanpouille-Box, C. Cancer cell metabolism and antitumour immunity. Nat. Rev. Immunol. 24, 654–669 (2024).
Chen, J. et al. Low-dose irradiation of the gut improves the efficacy of PD-L1 blockade in metastatic cancer patients. Cancer Cell 43, 361–379 (2025).
Tadepalli, S. et al. Rapid recruitment and IFN-I-mediated activation of monocytes dictate focal radiotherapy efficacy. Sci. Immunol. 8, eadd7446 (2023).
Galluzzi, L., Smith, K. N., Liston, A. & Garg, A. D. The diversity of CD8+ T cell dysfunction in cancer and viral infection. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-025-01161-6 (2025).
Ahren, I. L., Bjurberg, M., Steineck, G., Bergmark, K. & Jeppsson, B. Decreasing the adverse effects in pelvic radiation therapy: a randomized controlled trial evaluating the use of probiotics. Adv. Radiat. Oncol. 8, 101089 (2023).
Giralt, J. et al. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: results from multicenter, randomized, placebo-controlled nutritional trial. Int. J. Radiat. Oncol. Biol. Phys. 71, 1213–1219 (2008).
Urbancsek, H., Kazar, T., Mezes, I. & Neumann, K. Results of a double-blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation-induced diarrhoea. Eur. J. Gastroenterol. Hepatol. 13, 391–396 (2001).
Delia, P. et al. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 13, 912–915 (2007).
Jiang, C. et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer 125, 1081–1090 (2019).
Demers, M., Dagnault, A. & Desjardins, J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 33, 761–767 (2014).
Chitapanarux, I. et al. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 5, 31 (2010).
Garcia-Peris, P. et al. Effect of a mixture of inulin and fructo-oligosaccharide on Lactobacillus and Bifidobacterium intestinal microbiota of patients receiving radiotherapy: a randomised, double-blind, placebo-controlled trial. Nutr. Hosp. 27, 1908–1915 (2012).
Nascimento, M., Aguilar-Nascimento, J. E., Caporossi, C., Castro-Barcellos, H. M. & Motta, R. T. Efficacy of synbiotics to reduce acute radiation proctitis symptoms and improve quality of life: a randomized, double-blind, placebo-controlled pilot trial. Int. J. Radiat. Oncol. Biol. Phys. 90, 289–295 (2014).
Liu, M. M., Li, S. T., Shu, Y. & Zhan, H. Q. Probiotics for prevention of radiation-induced diarrhea: a meta-analysis of randomized controlled trials. PLoS ONE 12, e0178870 (2017).
Peng, X. et al. Streptococcus salivarius K12 alleviates oral mucositis in patients undergoing radiotherapy for malignant head and neck tumors: a randomized controlled trial. J. Clin. Oncol. 42, 1426–1435 (2024).
Ding, X. et al. Fecal microbiota transplantation: a promising treatment for radiation enteritis? Radiother. Oncol. 143, 12–18 (2020).
Schoenfeld, J. D. et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 23, 279–291 (2022).
Lin, D. et al. Microbiome dynamics during chemoradiation therapy for anal cancer. Int. J. Radiat. Oncol. Biol. Phys. 113, 974–984 (2022).
Qiao, H. et al. Association of intratumoral microbiota with prognosis in patients with nasopharyngeal carcinoma from 2 hospitals in China. JAMA Oncol. 8, 1301–1309 (2022).
Medeiros, M. C. et al. Salivary microbiome changes distinguish response to chemoradiotherapy in patients with oral cancer. Microbiome 11, 268 (2023).
Zheng, L. et al. Gut microbiota is associated with response to 131I therapy in patients with papillary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 50, 1453–1465 (2023).
Huang, X. et al. Metagenomic analysis of intratumoral microbiome linking to response to neoadjuvant chemoradiotherapy in rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 117, 1255–1269 (2023).
Yi, Y. et al. Gut microbiome components predict response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a prospective, longitudinal study. Clin. Cancer Res. 27, 1329–1340 (2021).
Serna, G. et al. Fusobacterium nucleatum persistence and risk of recurrence after preoperative treatment in locally advanced rectal cancer. Ann. Oncol. 31, 1366–1375 (2020).
Acknowledgements
The authors would like to thank long-standing collaborations with the Histopathology Department of Gustave Roussy, the Experimental and Translational Pathology (PETRA, AMMICA, INSERM US23/CNRS) Platform at Gustave Roussy, and the Bioinformatics Platform at Gustave Roussy. The work of J.C. is supported by the ARC foundation, the National Natural Science Foundation of China (82173079), the Natural Science Foundation of Guangdong Province of China (2022A1515011220) and GuangDong Basic and Applied Basic Research Foundation-Enterprise Joint Funds (2022A1515220007 and 2024A1515220093, PI: C. Chen). The work of E.D. is supported by F. Hoffmann-La Roche, French National Research Agency under the France 2030 investment plan (grant application N° ANR-21-RHUS-0005) and the imCORE research network (N°SG40863). The work of G.K. is supported by the Ligue contre le Cancer (équipe labellisée), Agence National de la Recherche (ANR-22-CE14-0066 VIVORUSH, ANR-23-CE44-0030 COPPERMAC, ANR-23-R4HC-0006 Ener-LIGHT), Association pour la recherche sur le cancer (ARC), Cancéropôle Ile-de-France, Fondation pour la Recherche Médicale (FRM), European Research Council Advanced Investigator Award (ERC-2021-ADG, Grant No. 101052444; project acronym: ICD-Cancer, project title: Immunogenic cell death (ICD) in the cancer-immune dialogue), the ERA4 Health Cardinoff Grant Ener-LIGHT, European Union Horizon 2020 research and innovation programmes Oncobiome (grant agreement number: 825410, Project Acronym: ONCOBIOME, Project title: Gut OncoMicrobiome Signatures [GOMS] associated with cancer incidence, prognosis and prediction of treatment response), Prevalung (grant agreement number 101095604, Project Acronym: PREVALUNG EU, project title: Biomarkers affecting the transition from cardiovascular disease to lung cancer: towards stratified interception), national support managed by the Agence Nationale de la Recherche under the France 2030 programme (reference number 21-ESRE-0028, ESR/Equipex + Onco-Pheno-Screen), Hevolution Network on Senescence in Aging (reference HF-E Einstein Network), Institut National du Cancer (INCa), Institut Universitaire de France, PAIR-Obésité INCa_1873, the RHUs Immunolife and LUCA-pi (ANR-21-RHUS-0017 and ANR-23-RHUS-0010, both dedicated to France Relance 2030), Seerave Foundation, and SIRIC Cancer Research and Personalized Medicine (CARPEM, SIRIC CARPEM INCa-DGOS-Inserm-ITMO Cancer_18006 supported by INCa, Ministère des Solidarités et de la Santé and INSERM). This study contributes to the IdEx Université de Paris Cité ANR-18-IDEX-0001. The work of L.G. laboratory is/has been supported by one National Institutes of Healt (NIH) R01 grant (#CA271915), by two Breakthrough Level 2 grants from the United States Deprtment of Defense (DoD) Breast Cancer Research Program (BCRP) (#BC180476P1, #BC210945), by a grant from the STARR Cancer Consortium (#I16-0064), by a Transformative Breast Cancer Consortium Grant from the United States DoD BCRP (#W81XWH2120034, PI: Formenti), by a U54 grant from NIH/NCI (#CA274291, PI: Deasy, Formenti, Weichselbaum), by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177, PI: Formenti) from the Stand Up to Cancer (SU2C), by a Mantle Cell Lymphoma Research Initiative (MCL-RI, PI: Chen-Kiang) grant from the Leukaemia and Lymphoma Society (LLS), by a Rapid Response Grant from the Functional Genomics Initiative (New York, USA), by a pre-SPORE grant (PI: Demaria, Formenti), a Collaborative Research Initiative Grant and a Clinical Trials Innovation Grant from the Sandra and Edward Meyer Cancer Center (New York, USA), by startup funds from the Department of Radiation Oncology at Weill Cornell Medicine (New York, USA), and by startup funds from Fox Chase Cancer Center (Philadelphia, USA). The work of L.Z. is supported by the SEERAVE Foundation, European Union Horizon 2020 (Project Number: 825410 and Project Acronym: ONCOBIOME), INCa, ANR Ileobiome – 19-CE15-0029-01, ANR RHU5 “ANR-21-RHUS-0017” IMMUNOLIFE”, MAdCAM INCA_ 16698, Ligue contre le cancer, la Direction generale de l’offre de soins (DGOS), Prevalung (grant agreement number 101095604, Project Acronym: PREVALUNG EU, project title: Biomarkers affecting the transition from cardiovascular disease to lung cancer: towards stratified interception). The funders had no role in data collection and analysis, decision to publish, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.C. researched data for this manuscript. J.C., E.D., L.G. and L.Z. made a substantial contribution to discussions of content. J.C., G.K., L.G. and L.Z. wrote the manuscript, and all authors reviewed and/or edited the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
E.D. has acted as a consultant and/or adviser of Boehringer-Ingelheim and Merck Serono, has received research funding from AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, IMCORE/Roche Genentech, Merck Serono and MSD, and is a founder of Graegis Pharmaceuticals. G.K. is an adviser of Hevolution and Institut Servier, is a scientific co-founder of EverImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio, is on the Board of Directors of the Bristol Myers Squibb Foundation France, is the inventor of patents covering therapeutic targeting of ageing, cancer, cystic fibrosis and metabolic disorders, and has received research funding from Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sutro, Tollys, and Vascage; his brother (Romano Kroemer) was an employee of Sanofi and now consults for Boehringer-Ingelheim. L.G. has acted as a consultant for and/or adviser of AstraZeneca, AbbVie, Boehringer-Ingelheim, EduCom, Inzen, Imvax, the Longevity Labs, the Luke Heller TECPR2 Foundation, Noxopharm, OmniSEQ, Onxeo, Promontory and Sotio, receives research funding from Lytix Biopharma, Promontory and Onxeo, and holds stock options in Promontory. L.Z. is a founder and president of the advisory board of everImmune and receives research funding from 9 meters, Daichi Sankyo, Kaleido and Pileje. J.C. declares no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks C.C. Wong, N. Liu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Deutsch, E., Kroemer, G. et al. The microbiota in radiotherapy-induced cancer immunosurveillance. Nat Rev Clin Oncol 22, 667–679 (2025). https://doi.org/10.1038/s41571-025-01052-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01052-8