Abstract
Cell plasticity is a crucial trait for cancer progression towards metastasis and treatment resistance. Research efforts from the past 20–30 years have revealed that the dynamic flux of the epithelial–mesenchymal transition (EMT) programme is one of the major underlying processes enabling cancer cell plasticity and greatly facilitates these major causes of cancer mortality. The spectrum of evidence ranges from extensive data from cell line and animal model studies across multiple cancer types through a rapidly expanding body of work demonstrating associations between EMT biomarkers and disease progression and mortality in patients. EMT is also implicated in resistance to most of the major treatment modalities, yet our efforts to harness this knowledge to improve therapeutic outcomes are currently in their early stages. In this Review, we describe clinical evidence supporting a role of EMT and the associated epithelial–mesenchymal plasticity in various stages of cancer in patients and discuss the subsequent clinical opportunities and challenges associated with attempts to implement this knowledge as novel therapies or clinical management approaches.
Key points
-
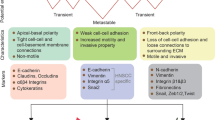
Cell plasticity is a crucial trait that supports the progression of a tumour towards metastatic dissemination and treatment resistance.
-
Partial and transient activation of the epithelial–mesenchymal transition (EMT) programme has an important role in enabling cancer cell plasticity.
-
Clinical data and analyses of human tumour tissue samples support a role of EMT and epithelial–mesenchymal plasticity (EMP) in many human cancer types.
-
EMT and EMP can influence all stages of cancer progression, from tumour initiation to the development of treatment resistance and metastases.
-
Interference with EMT and EMP is anticipated to offer various clinical opportunities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Yang, J. et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020).
Hay, E. D. An overview of epithelio-mesenchymal transformation. Acta Anat. 154, 8–20 (1995).
Nieto, M. A., Huang, R. Y., Jackson, R. A. & Thiery, J. P. EMT: 2016. Cell 166, 21–45 (2016).
Haerinck, J., Goossens, S. & Berx, G. The epithelial-mesenchymal plasticity landscape: principles of design and mechanisms of regulation. Nat. Rev. Genet. 24, 590–609 (2023).
Brabletz, S., Schuhwerk, H., Brabletz, T. & Stemmler, M. P. Dynamic EMT: a multi-tool for tumor progression. EMBO J. 40, e108647 (2021).
Stemmler, M. P., Eccles, R. L., Brabletz, S. & Brabletz, T. Non-redundant functions of EMT transcription factors. Nat. Cell Biol. 21, 102–112 (2019).
Perelli, L. et al. Evolutionary fingerprints of epithelial-to-mesenchymal transition. Nature https://doi.org/10.1038/s41586-025-08671-2 (2025).
Celia-Terrassa, T. & Kang, Y. How important is EMT for cancer metastasis? PLoS Biol. 22, e3002487 (2024).
Fontana, R., Mestre-Farrera, A. & Yang, J. Update on epithelial-mesenchymal plasticity in cancer progression. Annu. Rev. Pathol. 19, 133–156 (2024).
Jonckheere, S. et al. Epithelial-mesenchymal transition (EMT) as a therapeutic target. Cell Tissues Organs 211, 157–182 (2022).
Shibue, T. & Weinberg, R. A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 14, 611–629 (2017).
Redfern, A. D., Spalding, L. J. & Thompson, E. W. The Kraken wakes: induced EMT as a driver of tumour aggression and poor outcome. Clin. Exp. Metastasis 35, 285–308 (2018).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 (2019).
Youssef, K. K. & Nieto, M. A. Epithelial-mesenchymal transition in tissue repair and degeneration. Nat. Rev. Mol. Cell Biol. 25, 720–739 (2024).
Lambert, A. W., Zhang, Y. & Weinberg, R. A. Cell-intrinsic and microenvironmental determinants of metastatic colonization. Nat. Cell Biol. 26, 687–697 (2024).
Tan, E. J., Olsson, A. K. & Moustakas, A. Reprogramming during epithelial to mesenchymal transition under the control of TGFβ. Cell Adh. Migr. 9, 233–246 (2015).
Tan, T. Z. et al. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol. Med. 6, 1279–1293 (2014).
Yoshimura, A. et al. Epithelial-mesenchymal transition status is a remarkable biomarker for the combination treatment with avutometinib and defactinib in KRAS-mutated non-small cell lung cancer. Br. J. Cancer https://doi.org/10.1038/s41416-024-02727-2 (2024).
Barkley, D. et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat. Genet. 54, 1192–1201 (2022).
Cook, D. P. & Vanderhyden, B. C. Transcriptional census of epithelial-mesenchymal plasticity in cancer. Sci. Adv. 8, eabi7640 (2022).
Gavish, A. et al. Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature 618, 598–606 (2023).
Malagoli Tagliazucchi, G., Wiecek, A. J., Withnell, E. & Secrier, M. Genomic and microenvironmental heterogeneity shaping epithelial-to-mesenchymal trajectories in cancer. Nat. Commun. 14, 789 (2023).
Lin, J. R. et al. Multiplexed 3D atlas of state transitions and immune interaction in colorectal cancer. Cell 186, 363–381.e19 (2023).
Jolly, M. K. et al. Measuring and modelling the epithelial- mesenchymal hybrid state in cancer: clinical implications. Cell Tissues Organs 211, 110–133 (2022).
Beerling, E. et al. Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep. 14, 2281–2288 (2016).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Ishay-Ronen, D. et al. Gain fat-lose metastasis: converting invasive breast cancer cells into adipocytes inhibits cancer metastasis. Cancer Cell 35, 17–32.e6 (2019).
Lambert, A. W. et al. ΔNp63/p73 drive metastatic colonization by controlling a regenerative epithelial stem cell program in quasi-mesenchymal cancer stem cells. Dev. Cell 57, 2714–2730.e8 (2022).
Luond, F. et al. Distinct contributions of partial and full EMT to breast cancer malignancy. Dev. Cell 56, 3203–3221.e11 (2021).
Aiello, N. M. et al. EMT subtype influences epithelial plasticity and mode of cell migration. Dev. Cell 45, 681–695.e4 (2018).
Krebs, A. M. et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 19, 518–529 (2017).
Reichert, M. et al. Regulation of epithelial plasticity determines metastatic organotropism in pancreatic cancer. Dev. Cell 45, 696–711.e8 (2018).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Pastushenko, I. et al. Identification of the tumour transition states occurring during EMT. Nature 556, 463–468 (2018).
Tsai, J. H., Donaher, J. L., Murphy, D. A., Chau, S. & Yang, J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22, 725–736 (2012).
Verstappe, J. et al. ZEB2 drives intra-tumor heterogeneity and skin squamous cell carcinoma formation with distinct EMP transition states. iScience 27, 111169 (2024).
Yang, D. et al. Lineage tracing reveals the phylodynamics, plasticity, and paths of tumor evolution. Cell 185, 1905–1923.e25 (2022).
Brabletz, T., Jung, A., Spaderna, S., Hlubek, F. & Kirchner, T. Opinion: migrating cancer stem cells — an integrated concept of malignant tumour progression. Nat. Rev. Cancer 5, 744–749 (2005).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Morel, A. P. et al. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 3, e2888 (2008).
Puisieux, A., Brabletz, T. & Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 16, 488–494 (2014).
De Craene, B. et al. Epidermal snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 21, 310–320 (2014).
Schuhwerk, H. & Brabletz, T. Mutual regulation of TGFβ-induced oncogenic EMT, cell cycle progression and the DDR. Semin. Cancer Biol. 97, 86–103 (2023).
Fujiwara, S. et al. Expression of SNAIL in accompanying PanIN is a key prognostic indicator in pancreatic ductal adenocarcinomas. Cancer Med. 8, 1671–1678 (2019).
Goebel, L. et al. CD4+ T cells potently induce epithelial-mesenchymal-transition in premalignant and malignant pancreatic ductal epithelial cells-novel implications of CD4+ T cells in pancreatic cancer development. Oncoimmunology 4, e1000083 (2015).
Karamitopoulou, E. et al. Loss of Raf-1 kinase inhibitor protein (RKIP) is strongly associated with high-grade tumor budding and correlates with an aggressive phenotype in pancreatic ductal adenocarcinoma (PDAC). J. Transl. Med. 11, 311 (2013).
Kuvendjiska, J. et al. Circulating epithelial cells in patients with intraductal papillary mucinous neoplasm of the pancreas. Life 13, 1570 (2023).
Morris, H. T., Bamlet, W. R., Razidlo, G. L. & Machesky, L. M. FSCN1 and epithelial mesenchymal transformation transcription factor expression in human pancreatic intraepithelial neoplasia and ductal adenocarcinoma. Pathol. Res. Pract. 251, 154836 (2023).
Wartenberg, M. et al. Accumulation of FOXP3+T-cells in the tumor microenvironment is associated with an epithelial-mesenchymal-transition-type tumor budding phenotype and is an independent prognostic factor in surgically resected pancreatic ductal adenocarcinoma. Oncotarget 6, 4190–4201 (2015).
Jun, Y. K. et al. Molecular activity of inflammation and epithelial-mesenchymal transition in the microenvironment of ulcerative colitis. Gut Liver 18, 1037–1047 (2024).
Zhao, X. et al. Mobilization of epithelial mesenchymal transition genes distinguishes active from inactive lesional tissue in patients with ulcerative colitis. Hum. Mol. Genet. 24, 4615–4624 (2015).
Kroepil, F. et al. Down-regulation of CDH1 is associated with expression of SNAI1 in colorectal adenomas. PLoS ONE 7, e46665 (2012).
Kurmyshkina, O., Kovchur, P., Schegoleva, L. & Volkova, T. Markers of angiogenesis, lymphangiogenesis, and epithelial-mesenchymal transition (plasticity) in CIN and early invasive carcinoma of the cervix: exploring putative molecular mechanisms involved in early tumor invasion. Int. J. Mol. Sci. 21, 6515 (2020).
Liu, Y. et al. The indicative function of Twist2 and E-cadherin in HPV oncogene-induced epithelial-mesenchymal transition of cervical cancer cells. Oncol. Rep. 33, 639–650 (2015).
Popiel-Kopaczyk, A. et al. The immunohistochemical expression of epithelial-mesenchymal transition markers in precancerous lesions and cervical cancer. Int. J. Mol. Sci. 24, 8063 (2023).
Smith, C. M. et al. miR-200 family expression is downregulated upon neoplastic progression of Barrett’s esophagus. World J. Gastroenterol. 17, 1036–1044 (2011).
De, A. et al. Forkhead box F1 induces columnar phenotype and epithelial-to-mesenchymal transition in esophageal squamous cells to initiate Barrett’s like metaplasia. Lab. Invest. 101, 745–759 (2021).
Wei, L. et al. Overexpression and oncogenic function of HMGA2 in endometrial serous carcinogenesis. Am. J. Cancer Res. 6, 249–259 (2016).
Tuhkanen, H. et al. Nuclear expression of Snail1 in borderline and malignant epithelial ovarian tumours is associated with tumour progression. BMC Cancer 9, 289 (2009).
Li, H. et al. The tumor microenvironment: an irreplaceable element of tumor budding and epithelial-mesenchymal transition-mediated cancer metastasis. Cell Adh. Migr. 10, 434–446 (2016).
Fluegen, G. et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat. Cell Biol. 19, 120–132 (2017).
Brabletz, T. et al. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl Acad. Sci. USA 98, 10356–10361 (2001).
Grigore, A. D., Jolly, M. K., Jia, D., Farach-Carson, M. C. & Levine, H. Tumor budding: the name is EMT. Partial EMT. J. Clin. Med. 5, https://doi.org/10.3390/jcm5050051 (2016).
Kohler, I. et al. Detailed analysis of epithelial-mesenchymal transition and tumor budding identifies predictors of long-term survival in pancreatic ductal adenocarcinoma. J. Gastroenterol. Hepatol. 30, 78–84 (2015).
Rees, J. R., Onwuegbusi, B. A., Save, V. E., Alderson, D. & Fitzgerald, R. C. In vivo and in vitro evidence for transforming growth factor-β1-mediated epithelial to mesenchymal transition in esophageal adenocarcinoma. Cancer Res. 66, 9583–9590 (2006).
Krstic, M. et al. TBX3 promotes progression of pre-invasive breast cancer cells by inducing EMT and directly up-regulating SLUG. J. Pathol. 248, 191–203 (2019).
Volinia, S. et al. Levels of miR-126 and miR-218 are elevated in ductal carcinoma in situ (DCIS) and inhibit malignant potential of DCIS derived cells. Oncotarget 9, 23543–23553 (2018).
Szynglarewicz, B. et al. Biological aggressiveness of subclinical no-mass ductal carcinoma in situ (DCIS) can be reflected by the expression profiles of epithelial-mesenchymal transition triggers. Int. J. Mol. Sci. 19, 3941 (2018).
Lo, P. K. et al. Tumor-associated myoepithelial cells promote the invasive progression of ductal carcinoma in situ through activation of TGFβ signaling. J. Biol. Chem. 292, 11466–11484 (2017).
Makki, J., Myint, O., Wynn, A. A., Samsudin, A. T. & John, D. V. Expression distribution of cancer stem cells, epithelial to mesenchymal transition, and telomerase activity in breast cancer and their association with clinicopathologic characteristics. Clin. Med. Insights Pathol. 8, 1–16 (2015).
Schulte, J. et al. Expression of the E-cadherin repressors Snail, Slug and Zeb1 in urothelial carcinoma of the urinary bladder: relation to stromal fibroblast activation and invasive behaviour of carcinoma cells. Histochem. Cell Biol. 138, 847–860 (2012).
Morelli, M. B. et al. Correlation between High PD-L1 and EMT/invasive genes expression and reduced recurrence-free survival in blood-circulating tumor cells from patients with non-muscle-invasive bladder cancer. Cancers 13, 5989 (2021).
Jethwa, P. et al. Overexpression of slug is associated with malignant progression of esophageal adenocarcinoma. World J. Gastroenterol. 14, 1044–1052 (2008).
Jang, T. J. Epithelial to mesenchymal transition in cutaneous squamous cell carcinoma is correlated with COX-2 expression but not with the presence of stromal macrophages or CD10-expressing cells. Virchows Arch. 460, 481–487 (2012).
Verneuil, L. et al. Donor-derived stem-cells and epithelial mesenchymal transition in squamous cell carcinoma in transplant recipients. Oncotarget 6, 41497–41507 (2015).
Murata, M. et al. OVOL2-mediated ZEB1 downregulation may prevent promotion of actinic keratosis to cutaneous squamous cell carcinoma. J. Clin. Med. 9, 618 (2020).
Youssef, K. K. et al. Two distinct epithelial-to-mesenchymal transition programs control invasion and inflammation in segregated tumor cell populations. Nat. Cancer 5, 1660–1680 (2024).
Zhang, Y. et al. Genome-wide CRISPR screen identifies PRC2 and KMT2D-COMPASS as regulators of distinct EMT trajectories that contribute differentially to metastasis. Nat. Cell Biol. 24, 554–564 (2022).
Aceto, N., Toner, M., Maheswaran, S. & Haber, D. A. En route to metastasis: circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer 1, 44–52 (2015).
Pastushenko, I. & Blanpain, C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 29, 212–226 (2019).
Sinha, D., Saha, P., Samanta, A. & Bishayee, A. Emerging concepts of hybrid epithelial-to-mesenchymal transition in cancer progression. Biomolecules 10, 1561 (2020).
Douma, S. et al. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 430, 1034–1039 (2004).
Smit, M. A. & Peeper, D. S. Zeb1 is required for TrkB-induced epithelial-mesenchymal transition, anoikis resistance and metastasis. Oncogene 30, 3735–3744 (2011).
Raimondi, C. et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res. Treat. 130, 449–455 (2011).
Kallergi, G. et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 13, R59 (2011).
Armstrong, A. J. et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 9, 997–1007 (2011).
Hassan, S., Blick, T., Wood, J., Thompson, E. W. & Williams, E. D. Circulating tumour cells indicate the presence of residual disease post-castration in prostate cancer patient-derived xenograft models. Front. Cell Dev. Biol. 10, 858013 (2022).
Tachtsidis, A. et al. Human-specific RNA analysis shows uncoupled epithelial-mesenchymal plasticity in circulating and disseminated tumour cells from human breast cancer xenografts. Clin. Exp. Metastasis 36, 393–409 (2019).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Han, M. et al. Lymph liquid biopsy for detection of cancer stem cells. Cytom. A 99, 496–502 (2021).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Cohen, E. N. et al. Phenotypic plasticity in circulating tumor cells is associated with poor response to therapy in metastatic breast cancer patients. Cancers 15, 1616 (2023).
Ting, D. T. et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 8, 1905–1918 (2014).
Sun, Y. F. et al. Dissecting spatial heterogeneity and the immune-evasion mechanism of CTCs by single-cell RNA-seq in hepatocellular carcinoma. Nat. Commun. 12, 4091 (2021).
Balcik-Ercin, P., Cayrefourcq, L., Soundararajan, R., Mani, S. A. & Alix-Panabières, C. Epithelial-to-mesenchymal plasticity in circulating tumor cell lines sequentially derived from a patient with colorectal cancer. Cancers 13, 5408 (2021).
Grillet, F. et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 66, 1802–1810 (2017).
Hassan, S., Blick, T., Thompson, E. W. & Williams, E. D. Diversity of epithelial-mesenchymal phenotypes in circulating tumour cells from prostate cancer patient-derived xenograft models. Cancers 13, 2750 (2021).
Wang, X. et al. μ-opioid receptor agonist facilitates circulating tumor cell formation in bladder cancer via the MOR/AKT/slug pathway: a comprehensive study including randomized controlled trial. Cancer Commun. 43, 365–386 (2023).
Hamilton, G., Hochmair, M., Rath, B., Klameth, L. & Zeillinger, R. Small cell lung cancer: circulating tumor cells of extended stage patients express a mesenchymal-epithelial transition phenotype. Cell Adh. Migr. 10, 360–367 (2016).
Zhang, Q. et al. Prognostic value of stem-like circulating tumor cells in patients with cancer: a systematic review and meta-analysis. Clin. Exp. Med. 23, 1933–1944 (2023).
Aouad, P., Quinn, H. M., Berger, A. & Brisken, C. Tumor dormancy: EMT beyond invasion and metastasis. Genesis 62, e23552 (2024).
So, K. W. L., Su, Z., Cheung, J. P. Y. & Choi, S. W. Single-cell analysis of bone-marrow-disseminated tumour cells. Diagnostics 14, 2172 (2024).
Korpal, M. et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 17, 1101–1108 (2011).
Aouad, P. et al. Epithelial-mesenchymal plasticity determines estrogen receptor positive breast cancer dormancy and epithelial reconversion drives recurrence. Nat. Commun. 13, 4975 (2022).
Nobre, A. R. et al. ZFP281 drives a mesenchymal-like dormancy program in early disseminated breast cancer cells that prevents metastatic outgrowth in the lung. Nat. Cancer 3, 1165–1180 (2022).
Lawson, D. A. et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 526, 131–135 (2015).
Francescangeli, F. et al. A pre-existing population of ZEB2+ quiescent cells with stemness and mesenchymal features dictate chemoresistance in colorectal cancer. J. Exp. Clin. Cancer Res. 39, 2 (2020).
Kreso, A. et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science 339, 543–548 (2013).
Conzelmann, M., Linnemann, U. & Berger, M. R. Detection of disseminated tumour cells in the liver of cancer patients. Eur. J. Surg. Oncol. 31, 977–985 (2005).
Pantel, K. & Alix-Panabières, C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. BoneKEy Rep. 3, 584 (2014).
Yang, Y. et al. Comprehensive analysis of EMT-related genes and lncRNAs in the prognosis, immunity, and drug treatment of colorectal cancer. J. Transl. Med. 19, 391 (2021).
Lawlor, R. T. et al. Prognostic role of high-grade tumor budding in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis with a focus on epithelial to mesenchymal transition. Cancers 11, 113 (2019).
Pal, A., Barrett, T. F., Paolini, R., Parikh, A. & Puram, S. V. Partial EMT in head and neck cancer biology: a spectrum instead of a switch. Oncogene 40, 5049–5065 (2021).
Kolijn, K., Verhoef, E. I. & van Leenders, G. J. Morphological and immunohistochemical identification of epithelial-to-mesenchymal transition in clinical prostate cancer. Oncotarget 6, 24488–24498 (2015).
Grosse-Wilde, A. et al. Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS ONE 10, e0126522 (2015).
Xue, W. et al. Establishment and analysis of an individualized EMT-related gene signature for the prognosis of breast cancer in female patients. Dis. Markers 2022, 1289445 (2022).
Tao, H. et al. Identification of an EMT-related lncRNA signature and LINC01116 as an immune-related oncogene in hepatocellular carcinoma. Aging 14, 1473–1491 (2022).
Song, J., Wei, R., Huo, S., Gao, J. & Liu, X. Metastasis related epithelial-mesenchymal transition signature predicts prognosis and response to immunotherapy in gastric cancer. Front. Immunol. 13, 920512 (2022).
Gibbons, D. L. & Creighton, C. J. Pan-cancer survey of epithelial-mesenchymal transition markers across the cancer genome atlas. Dev. Dyn. 247, 555–564 (2018).
Spaderna, S. et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 131, 830–840 (2006).
Ueno, H. et al. Predictors of extrahepatic recurrence after resection of colorectal liver metastases. Br. J. Surg. 91, 327–333 (2004).
Ueno, H., Murphy, J., Jass, J. R., Mochizuki, H. & Talbot, I. C. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40, 127–132 (2002).
Imani, S., Hosseinifard, H., Cheng, J., Wei, C. & Fu, J. Prognostic value of EMT-inducing transcription factors (EMT-TFs) in metastatic breast cancer: a systematic review and meta-analysis. Sci. Rep. 6, 28587 (2016).
Chen, Z. et al. Clinical value of octamer-binding transcription factor 4 as a prognostic marker in patients with digestive system cancers: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 32, 567–576 (2017).
Wan, T., Zhang, T., Si, X. & Zhou, Y. Overexpression of EMT-inducing transcription factors as a potential poor prognostic factor for hepatocellular carcinoma in Asian populations: a meta-analysis. Oncotarget 8, 59500–59508 (2017).
Ahmadiankia, N. & Khosravi, A. Significance of epithelial-to-mesenchymal transition inducing transcription factors in predicting distance metastasis and survival in patients with colorectal cancer: a systematic review and meta-analysis. J. Res. Med. Sci. 25, 60 (2020).
Natsugoe, S. et al. Snail plays a key role in E-cadherin-preserved esophageal squamous cell carcinoma. Oncol. Rep. 17, 517–523 (2007).
Breyer, J. et al. Epithelial-mesenchymal transformation markers E-cadherin and survivin predict progression of stage pTa urothelial bladder carcinoma. World J. Urol. 34, 709–716 (2016).
Otto, W. et al. WHO 1973 grade 3 and infiltrative growth pattern proved, aberrant E-cadherin expression tends to be of predictive value for progression in a series of stage T1 high-grade bladder cancer after organ-sparing approach. Int. Urol. Nephrol. 49, 431–437 (2017).
Xie, X. et al. Prognostic significance of β-catenin expression in osteosarcoma: a meta-analysis. Front. Oncol. 10, 402 (2020).
Gou, Y. et al. Snail is an independent prognostic indicator for predicting recurrence and progression in non-muscle-invasive bladder cancer. Int. Urol. Nephrol. 47, 289–293 (2015).
Creighton, C. J. et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl Acad. Sci. USA 106, 13820–13825 (2009).
Hennessy, B. T. et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 69, 4116–4124 (2009).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Collisson, E. A. et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 17, 500–503 (2011).
Moffitt, R. A. et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat. Genet. 47, 1168–1178 (2015).
Saxena, M., Stephens, M. A., Pathak, H. & Rangarajan, A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2, e179 (2011).
Giles, K. M. et al. Axl mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol. Cancer Ther. 12, 2541–2558 (2013).
Bhangu, A. et al. The role of epithelial mesenchymal transition and resistance to neoadjuvant therapy in locally advanced rectal cancer. Colorectal Dis. 16, O133–O143 (2014).
Porter, R. L. et al. Epithelial to mesenchymal plasticity and differential response to therapies in pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA 116, 26835–26845 (2019).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Wang, D. et al. Zeb1-controlled metabolic plasticity enables remodeling of chromatin accessibility in the development of neuroendocrine prostate cancer. Cell Death Differ. 31, 779–791 (2024).
Tang, Y., Feinberg, T., Keller, E. T., Li, X. Y. & Weiss, S. J. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat. Cell Biol. 18, 917–929 (2016).
Ruh, M. et al. The EMT transcription factor ZEB1 blocks osteoblastic differentiation in bone development and osteosarcoma. J. Pathol. 254, 199–211 (2021).
Kahlert, U. D., Joseph, J. V. & Kruyt, F. A. E. EMT- and MET-related processes in nonepithelial tumors: importance for disease progression, prognosis, and therapeutic opportunities. Mol. Oncol. 11, 860–877 (2017).
Rowe, R. G. et al. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J. Cell Biol. 184, 399–408 (2009).
Siebzehnrubl, F. A. et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol. Med. 5, 1196–1212 (2013).
Xiao, Z. et al. Prognosis and clinical features analysis of EMT-related signature and tumor immune microenvironment in glioma. J. Med. Biochem. 42, 122–137 (2023).
Zhu, X. et al. P4HA1 as an unfavorable prognostic marker promotes cell migration and invasion of glioblastoma via inducing EMT process under hypoxia microenvironment. Am. J. Cancer Res. 11, 590–617 (2021).
Liu, Y. et al. Silencing IKBKE inhibits the migration and invasion of glioblastoma by promoting Snail1 degradation. Clin. Transl. Oncol. 24, 816–828 (2022).
El-Naggar, A. M. et al. Translational activation of HIF1α by YB-1 promotes sarcoma metastasis. Cancer Cell 27, 682–697 (2015).
Lamhamedi-Cherradi, S. E. et al. Transcriptional activators YAP/TAZ and AXL orchestrate dedifferentiation, cell fate, and metastasis in human osteosarcoma. Cancer Gene Ther. 28, 1325–1338 (2021).
Yang, L. et al. CCNDBP1, a prognostic marker regulated by DNA methylation, inhibits aggressive behavior in dedifferentiated liposarcoma via repressing epithelial mesenchymal transition. Front. Oncol. 11, 687012 (2021).
Harada, H. et al. Carcinosarcoma of the esophagus: a report of 6 cases associated with zinc finger E-box-binding homeobox 1 expression. Oncol. Lett. 17, 578–586 (2019).
Ho, G. Y. et al. Epithelial-to-mesenchymal transition supports ovarian carcinosarcoma tumorigenesis and confers sensitivity to microtubule targeting with eribulin. Cancer Res. 82, 4457–4473 (2022).
Sertier, A. S. et al. Dissecting the origin of heterogeneity in uterine and ovarian carcinosarcomas. Cancer Res. Commun. 3, 830–841 (2023).
Zhang, M. et al. A gut microbiota rheostat forecasts responsiveness to PD-L1 and VEGF blockade in mesothelioma. Nat. Commun. 15, 7187 (2024).
Almotiri, A. et al. Zeb1 modulates hematopoietic stem cell fates required for suppressing acute myeloid leukemia. J. Clin. Invest. 131, e129115 (2021).
Radhakrishnan, K., Truong, L. & Carmichael, C. L. An “unexpected” role for EMT transcription factors in hematological development and malignancy. Front. Immunol. 14, 1207360 (2023).
Wang, J. et al. Interplay between the EMT transcription factors ZEB1 and ZEB2 regulates hematopoietic stem and progenitor cell differentiation and hematopoietic lineage fidelity. PLoS Biol. 19, e3001394 (2021).
van Helden, M. J. et al. Terminal NK cell maturation is controlled by concerted actions of T-bet and Zeb2 and is essential for melanoma rejection. J. Exp. Med. 212, 2015–2025 (2015).
Scott, C. L. et al. The transcription factor Zeb2 regulates development of conventional and plasmacytoid DCs by repressing Id2. J. Exp. Med. 213, 897–911 (2016).
Lemma, S. et al. Biological roles and prognostic values of the epithelial-mesenchymal transition-mediating transcription factors Twist, ZEB1 and Slug in diffuse large B-cell lymphoma. Histopathology 62, 326–333 (2013).
Mishra, A. B. & Nishank, S. S. Therapeutic targeting approach on epithelial-mesenchymal plasticity to combat cancer metastasis. Med. Oncol. 40, 190 (2023).
Yang, J. Z. et al. High nuclear expression of Twist1 in the skeletal extramedullary disease of myeloma patients predicts inferior survival. Pathol. Res. Pract. 212, 210–216 (2016).
Wang, N. et al. TWIST-1 promotes cell growth, drug resistance and progenitor clonogenic capacities in myeloid leukemia and is a novel poor prognostic factor in acute myeloid leukemia. Oncotarget 6, 20977–20992 (2015).
Percio, S., Coltella, N., Grisanti, S., Bernardi, R. & Pattini, L. A HIF-1 network reveals characteristics of epithelial-mesenchymal transition in acute promyelocytic leukemia. Genome Med. 6, 84 (2014).
Dongre, A. et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 77, 3982–3989 (2017).
Pang, Q. Y., Chiu, Y. C. & Huang, R. Y. Regulating epithelial-mesenchymal plasticity from 3D genome organization. Commun. Biol. 7, 750 (2024).
Lehmann, B. D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121, 2750–2767 (2011).
Masuda, H. et al. Changes in triple-negative breast cancer molecular subtypes in patients without pathologic complete response after neoadjuvant systemic chemotherapy. JCO Precis. Oncol. 6, e2000368 (2022).
Pitroda, S. P. et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat. Commun. 9, 1793 (2018).
Lugli, A., Zlobec, I., Berger, M. D., Kirsch, R. & Nagtegaal, I. D. Tumour budding in solid cancers. Nat. Rev. Clin. Oncol. 18, 101–115 (2021).
Levine, H. et al. 2354P computational pathology-derived features capture varied epithelial-mesenchymal transition (EMT) states. Ann. Oncol. 34, S1197 (2023).
Bagheri, M. et al. Pharmacological induction of chromatin remodeling drives chemosensitization in triple-negative breast cancer. Cell Rep. Med. 5, 101504 (2024).
Twelves, C. et al. “New” metastases are associated with a poorer prognosis than growth of pre-existing metastases in patients with metastatic breast cancer treated with chemotherapy. Breast Cancer Res. 17, 150 (2015).
Guillen, K. P. et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat. Cancer 3, 232–250 (2022).
Demetri, G. D. et al. Activity of eribulin in patients with advanced liposarcoma demonstrated in a subgroup analysis from a randomized phase III study of eribulin versus dacarbazine. J. Clin. Oncol. 35, 3433–3439 (2017).
Kitahara, H. et al. Eribulin sensitizes oral squamous cell carcinoma cells to cetuximab via induction of mesenchymal-to-epithelial transition. Oncol. Rep. 36, 3139–3144 (2016).
Zhang, X. et al. Netrin-1 elicits metastatic potential of non-small cell lung carcinoma cell by enhancing cell invasion, migration and vasculogenic mimicry via EMT induction. Cancer Gene Ther. 25, 18–26 (2018).
Cassier, P. A. et al. Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature 620, 409–416 (2023).
Cassier, P. et al. A first in human, phase I trial of NP137, a first-in-class antibody targeting netrin-1, in patients with advanced refractory solid tumors. Ann. Oncol. 30, v159 (2019).
Yadav, M. et al. AXL signaling in cancer: from molecular insights to targeted therapies. Signal Transduct. Target. Ther. 10, 37 (2025).
Engelsen, A. S. T. et al. AXL is a driver of stemness in normal mammary gland and breast cancer. iScience 23, 101649 (2020).
Antony, J. & Huang, R. Y. AXL-driven EMT state as a targetable conduit in cancer. Cancer Res. 77, 3725–3732 (2017).
Antony, J. et al. The GAS6-AXL signaling network is a mesenchymal (Mes) molecular subtype-specific therapeutic target for ovarian cancer. Sci. Signal. 9, ra97 (2016).
Arner, E. N. et al. AXL-TBK1 driven AKT3 activation promotes metastasis. Sci. Signal. 17, eado6057 (2024).
Goyette, M. A. et al. Targeting Axl favors an antitumorigenic microenvironment that enhances immunotherapy responses by decreasing Hif-1α levels. Proc. Natl Acad. Sci. USA 118, e2023868118 (2021).
Kollmannsberger, C. et al. Phase I study evaluating glesatinib (MGCD265), an inhibitor of MET and AXL, in patients with non-small cell lung cancer and other advanced solid tumors. Target. Oncol. 18, 105–118 (2023).
Yau, T. C. C. et al. A phase I/II multicenter study of single-agent foretinib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 23, 2405–2413 (2017).
Li, H. et al. AXL targeting restores PD-1 blockade sensitivity of STK11/LKB1 mutant NSCLC through expansion of TCF1+ CD8 T cells. Cell Rep. Med. 3, 100554 (2022).
Feng, Y. L., Chen, D. Q., Vaziri, N. D., Guo, Y. & Zhao, Y. Y. Small molecule inhibitors of epithelial-mesenchymal transition for the treatment of cancer and fibrosis. Med. Res. Rev. 40, 54–78 (2020).
Gu, Y., Zhang, Z. & Ten Dijke, P. Harnessing epithelial-mesenchymal plasticity to boost cancer immunotherapy. Cell Mol. Immunol. 20, 318–340 (2023).
Ramesh, V., Brabletz, T. & Ceppi, P. Targeting EMT in cancer with repurposed metabolic inhibitors. Trends Cancer 6, 942–950 (2020).
Zhang, N. et al. Novel therapeutic strategies: targeting epithelial-mesenchymal transition in colorectal cancer. Lancet Oncol. 22, e358–e368 (2021).
Zhou, Y. et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 9, 132 (2024).
Huber, M. A., Kraut, N. & Beug, H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558 (2005).
Waryah, C. et al. Synthetic epigenetic reprogramming of mesenchymal to epithelial states using the CRISPR/dCas9 platform in triple negative breast cancer. Adv. Sci. 10, e2301802 (2023).
Ahmadi-Hadad, A., de Queiroz, P. C. C., Schettini, F. & Giuliano, M. Reawakening the master switches in triple-negative breast cancer: a strategic blueprint for confronting metastasis and chemoresistance via microRNA-200/205: a systematic review. Crit. Rev. Oncol. Hematol. 204, 104516 (2024).
Laney, V. et al. MR molecular image guided treatment of pancreatic cancer with targeted ECO/miR-200c nanoparticles in immunocompetent mouse tumor models. Pharm. Res. 41, 1811–1825 (2024).
Plumber, S. A. et al. Rosiglitazone and trametinib exhibit potent anti-tumor activity in a mouse model of muscle invasive bladder cancer. Nat. Commun. 15, 6538 (2024).
Friedmann Angeli, J. P., Krysko, D. V. & Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 19, 405–414 (2019).
Schwab, A. et al. Zeb1 mediates EMT/plasticity-associated ferroptosis sensitivity in cancer cells by regulating lipogenic enzyme expression and phospholipid composition. Nat. Cell Biol. https://doi.org/10.1038/s41556-024-01464-1 (2024).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Wang, Y. et al. ACSL4 and polyunsaturated lipids support metastatic extravasation and colonization. Cell https://doi.org/10.1016/j.cell.2024.10.047 (2024).
Jonckheere, S. et al. Development and validation of a high-throughput screening pipeline of compound libraries to target EMT. Cell Death Differ. https://doi.org/10.1038/s41418-025-01515-6 (2025).
Terry, S. et al. New insights into the role of EMT in tumor immune escape. Mol. Oncol. 11, 824–846 (2017).
Wang, H. & Yee, D. I-SPY 2: a neoadjuvant adaptive clinical trial designed to improve outcomes in high-risk breast cancer. Curr. Breast Cancer Rep. 11, 303–310 (2019).
Twelves, C. et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res. Treat. 148, 553–561 (2014).
Shichiri, K., Oshi, M., Ziazadeh, D., Endo, I. & Takabe, K. High miR-200c expression is associated with suppressed epithelial-mesenchymal transition, TGF-β signaling and better survival despite enhanced cell proliferation in gastric cancer patients. Am. J. Cancer Res. 13, 3027–3040 (2023).
Oshi, M. et al. Enhanced epithelial-mesenchymal transition signatures are linked with adverse tumor microenvironment, angiogenesis and worse survival in gastric cancer. Cancer Gene Ther. 31, 746–754 (2024).
Pascual, T. et al. Neoadjuvant eribulin in HER2-negative early-stage breast cancer (SOLTI-1007-NeoEribulin): a multicenter, two-cohort, non-randomized phase II trial. NPJ Breast Cancer 7, 145 (2021).
Filho, O. M. et al. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov. 11, 2474–2487 (2021).
Michalski, K. et al. Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [(177)Lu]PSMA radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 48, 2024–2030 (2021).
Kahlert, C. et al. Overexpression of ZEB2 at the invasion front of colorectal cancer is an independent prognostic marker and regulates tumor invasion in vitro. Clin. Cancer Res. 17, 7654–7663 (2011).
Al Bakir, M. et al. The evolution of non-small cell lung cancer metastases in TRACERx. Nature 616, 534–542 (2023).
el Ghouzzi, V. et al. Mutations of the TWIST gene in the Saethre-Chotzen syndrome. Nat. Genet. 15, 42–46 (1997).
Hudson, L. G. et al. Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2). J. Dermatol. Sci. 56, 19–26 (2009).
Sánchez-Martín, M. et al. SLUG (SNAI2) deletions in patients with Waardenburg disease. Hum. Mol. Genet. 11, 3231–3236 (2002).
Takagi, T., Moribe, H., Kondoh, H. & Higashi, Y. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development 125, 21–31 (1998).
Van de Putte, T. et al. Mice lacking ZFHX1B, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease-mental retardation syndrome. Am. J. Hum. Genet. 72, 465–470 (2003).
Xu, Y. et al. Inducible knockout of Twist1 in young and adult mice prolongs hair growth cycle and has mild effects on general health, supporting Twist1 as a preferential cancer target. Am. J. Pathol. 183, 1281–1292 (2013).
Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282 (2021).
McNiel, E. A. & Tsichlis, P. N. Analyses of publicly available genomics resources define FGF-2-expressing bladder carcinomas as EMT-prone, proliferative tumors with low mutation rates and high expression of CTLA-4, PD-1 and PD-L1. Signal Transduct. Target. Ther. 2, 16045 (2017).
Wang, L. et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat. Commun. 9, 3503 (2018).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Knudsen, E. S. et al. Progression of ductal carcinoma in situ to invasive breast cancer is associated with gene expression programs of EMT and myoepithelia. Breast Cancer Res. Treat. 133, 1009–1024 (2012).
Rios, A. C. et al. Intraclonal plasticity in mammary tumors revealed through large-scale single-cell resolution 3D imaging. Cancer Cell 35, 618–632.e6 (2019).
Horimoto, Y. et al. Analysis of circulating tumour cell and the epithelial mesenchymal transition (EMT) status during eribulin-based treatment in 22 patients with metastatic breast cancer: a pilot study. J. Transl. Med. 16, 287 (2018).
Papadaki, M. A. et al. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol. Cancer Ther. 18, 437–447 (2019).
Guan, X. et al. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. Cancer Commun. 39, 1 (2019).
Asiedu, M. K., Ingle, J. N., Behrens, M. D., Radisky, D. C. & Knutson, K. L. TGFβ/TNFα-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 71, 4707–4719 (2011).
Harper, K. L. et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592 (2016).
Kröger, C. et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl Acad. Sci. USA 116, 7353–7362 (2019).
Bado, I. L. et al. The bone microenvironment increases phenotypic plasticity of ER+ breast cancer cells. Dev. Cell 56, 1100–1117.e9 (2021).
Werner-Klein, M. et al. Interleukin-6 trans-signaling is a candidate mechanism to drive progression of human DCCs during clinical latency. Nat. Commun. 11, 4977 (2020).
Lesniak, D. et al. Spontaneous epithelial-mesenchymal transition and resistance to HER-2-targeted therapies in HER-2-positive luminal breast cancer. PLoS ONE 8, e71987 (2013).
Kawamoto, A. et al. Radiation induces epithelial-mesenchymal transition in colorectal cancer cells. Oncol. Rep. 27, 51–57 (2012).
Gasinska, A. et al. Biomarkers of epithelial-mesenchymal transition in localized, surgically treated clear-cell renal cell carcinoma. Folia Histochem. Cytobiol. 56, 195–206 (2018).
Tao, Z. H. et al. miR-612 suppresses the invasive-metastatic cascade in hepatocellular carcinoma. J. Exp. Med. 210, 789–803 (2013).
Lei, X. et al. Ack1 overexpression promotes metastasis and indicates poor prognosis of hepatocellular carcinoma. Oncotarget 6, 40622–40641 (2015).
Peng, R. et al. Elevated TRIM44 promotes intrahepatic cholangiocarcinoma progression by inducing cell EMT via MAPK signaling. Cancer Med. 7, 796–808 (2018).
Critelli, R. et al. Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study. Cell Death Dis. 8, e3017 (2017).
Yang, R. et al. Low PRRX1 expression and high ZEB1 expression are significantly correlated with epithelial-mesenchymal transition and tumor angiogenesis in non-small cell lung cancer. Medicine 100, e24472 (2021).
Shintani, Y. et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann. Thorac. Surg. 92, 1794–1804 (2011).
Elamin, Y. Y. et al. Poziotinib for EGFR exon 20-mutant NSCLC: clinical efficacy, resistance mechanisms, and impact of insertion location on drug sensitivity. Cancer Cell 40, 754–767.e6 (2022).
Kim, S. et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum. Pathol. 58, 7–14 (2016).
Lou, Y. et al. Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin. Cancer Res. 22, 3630–3642 (2016).
Gonzalez, V. D. et al. Commonly occurring cell subsets in high-grade serous ovarian tumors identified by single-cell mass cytometry. Cell Rep. 22, 1875–1888 (2018).
Mao, Y. et al. The role of nuclear β-catenin accumulation in the Twist2-induced ovarian cancer EMT. PLoS ONE 8, e78200 (2013).
Meidhof, S. et al. ZEB1-associated drug resistance in cancer cells is reversed by the class I HDAC inhibitor mocetinostat. EMBO Mol. Med. 7, 831–847 (2015).
Sharma, S. et al. Secreted protein acidic and rich in cysteine (SPARC) mediates metastatic dormancy of prostate cancer in bone. J. Biol. Chem. 291, 19351–19363 (2016).
Yu-Lee, L. Y. et al. Osteoblast-secreted factors mediate dormancy of metastatic prostate cancer in the bone via activation of the TGFβRIII-p38MAPK-pS249/T252RB pathway. Cancer Res. 78, 2911–2924 (2018).
Marín-Aguilera, M. et al. Epithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Mol. Cancer Ther. 13, 1270–1284 (2014).
Martin, S. K. et al. Multinucleation and mesenchymal-to-epithelial transition alleviate resistance to combined cabazitaxel and antiandrogen therapy in advanced prostate cancer. Cancer Res. 76, 912–926 (2016).
Cmero, M. et al. Loss of SNAI2 in prostate cancer correlates with clinical response to androgen deprivation therapy. JCO Precis. Oncol. 5, https://doi.org/10.1200/po.20.00337 (2021).
Lefevre, M. et al. Epithelial to mesenchymal transition and HPV infection in squamous cell oropharyngeal carcinomas: the papillophar study. Br. J. Cancer 116, 362–369 (2017).
Kong, Y. H. et al. Co-expression of TWIST1 and ZEB2 in oral squamous cell carcinoma is associated with poor survival. PLoS ONE 10, e0134045 (2015).
Genenger, B., Perry, J. R., Ashford, B. & Ranson, M. A tEMTing target? Clinical and experimental evidence for epithelial-mesenchymal transition in the progression of cutaneous squamous cell carcinoma (a scoping systematic review). Discov. Oncol. 13, 42 (2022).
Tomizawa, Y., Wu, T. T. & Wang, K. K. Epithelial mesenchymal transition and cancer stem cells in esophageal adenocarcinoma originating from Barrett’s esophagus. Oncol. Lett. 3, 1059–1063 (2012).
Valenzano, M. C. et al. Zinc gluconate induces potentially cancer chemopreventive activity in Barrett’s esophagus: a phase 1 pilot study. Dig. Dis. Sci. 66, 1195–1211 (2021).
Puram, S. V. et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624.e24 (2017).
Biddle, A., Gammon, L., Liang, X., Costea, D. E. & Mackenzie, I. C. Phenotypic plasticity determines cancer stem cell therapeutic resistance in oral squamous cell carcinoma. eBioMedicine 4, 138–145 (2016).
Guo, Q. et al. ADMA mediates gastric cancer cell migration and invasion via Wnt/β-catenin signaling pathway. Clin. Transl. Oncol. 23, 325–334 (2021).
Yang, Z. et al. Acquisition of resistance to trastuzumab in gastric cancer cells is associated with activation of IL-6/STAT3/Jagged-1/Notch positive feedback loop. Oncotarget 6, 5072–5087 (2015).
Hara, J. et al. Mesenchymal phenotype after chemotherapy is associated with chemoresistance and poor clinical outcome in esophageal cancer. Oncol. Rep. 31, 589–596 (2014).
Denecker, G. et al. Identification of a ZEB2-MITF-ZEB1 transcriptional network that controls melanogenesis and melanoma progression. Cell Death Differ. 21, 1250–1261 (2014).
Kudo-Saito, C., Shirako, H., Takeuchi, T. & Kawakami, Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 15, 195–206 (2009).
Yao, J. et al. Altered expression and splicing of ESRP1 in malignant melanoma correlates with epithelial-mesenchymal status and tumor-associated immune cytolytic activity. Cancer Immunol. Res. 4, 552–561 (2016).
Chen, L. et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 5, 5241 (2014).
Simeonov, K. P. et al. Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states. Cancer Cell 39, 1150–1162.e9 (2021).
Zhao, S. et al. Ningetinib plus gefitinib in EGFR-mutant non-small-cell lung cancer with MET and AXL dysregulations: a phase 1b clinical trial and biomarker analysis. Lung Cancer 188, 107468 (2024).
Goto, K. et al. Phase 1 study of DS-1205c combined with gefitinib for EGFR mutation-positive non-small cell lung cancer. Cancer Med. 12, 7090–7104 (2023).
Melisi, D. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 119, 1208–1214 (2018).
Melisi, D. et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Immunother. Cancer 9, e002068 (2021).
Nadal, E. et al. A phase Ib/II study of galunisertib in combination with nivolumab in solid tumors and non-small cell lung cancer. BMC Cancer 23, 708 (2023).
Sardesai, S. D. et al. Inhibiting fatty acid synthase in operable triple negative breast cancer. J. Clin. Oncol. 38, 584–584 (2020).
Blais, N. et al. A phase II trial to evaluate the epithelial-to-mesenchymal (EMT) inhibitor sotevtamab combined with docetaxel in patients with metastatic non-small cell lung cancer following failure of primary chemoimmunotherapy. J. Clin. Oncol. 42, 8577–8577 (2024).
Yamashita, T. et al. Trastuzumab-pertuzumab plus eribulin or taxane as first-line chemotherapy for human epidermal growth factor 2-positive locally advanced/metastatic breast cancer: the randomized noninferiority phase III EMERALD trial. J. Clin. Oncol. 43, 1302–1313 (2025).
Lengrand, J. et al. Pharmacological targeting of netrin-1 inhibits EMT in cancer. Nature 620, 402–408 (2023).
Khan, A. Q. et al. Exploiting transcription factors to target EMT and cancer stem cells for tumor modulation and therapy. Semin. Cancer Biol. 100, 1–16 (2024).
Metge, B. J. et al. Targeting EMT using low-dose teniposide by downregulating ZEB2-driven activation of RNA polymerase I in breast cancer. Cell Death Dis. 15, 322 (2024).
Wu, Y. et al. Identification of a cisplatin resistant-based prognostic immune related gene signature in MIBC. Transl. Oncol. 44, 101942 (2024).
Hsu, D. S. et al. Regulation of excision repair cross-complementation group 1 by Snail contributes to cisplatin resistance in head and neck cancer. Clin. Cancer Res. 16, 4561–4571 (2010).
Wei, L. Y. et al. A six-epithelial-mesenchymal transition gene signature may predict metastasis of triple-negative breast cancer. Onco Targets Ther. 13, 6497–6509 (2020).
Chang, Y. C. et al. RAB31 drives extracellular vesicle fusion and cancer-associated fibroblast formation leading to oxaliplatin resistance in colorectal cancer. J. Extracell. Biol. 3, e141 (2024).
Sun, Y. et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 72, 527–536 (2012).
Hanrahan, K. et al. The role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in mediating docetaxel-resistant prostate cancer. Mol. Oncol. 11, 251–265 (2017).
Stark, T. W. et al. Predictive value of epithelial-mesenchymal-transition (EMT) signature and PARP-1 in prostate cancer radioresistance. Prostate 77, 1583–1591 (2017).
Wu, S. L. et al. 2-Methoxyestradiol inhibits the proliferation and migration and reduces the radioresistance of nasopharyngeal carcinoma CNE-2 stem cells via NF-κB/HIF-1 signaling pathway inactivation and EMT reversal. Oncol. Rep. 37, 793–802 (2017).
Xie, P., Yu, H., Wang, F., Yan, F. & He, X. Inhibition of LOXL2 enhances the radiosensitivity of castration-resistant prostate cancer cells associated with the reversal of the EMT process. Biomed. Res. Int. 2019, 4012590 (2019).
Chae, Y. K. et al. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci. Rep. 8, 2918 (2018).
Plaschka, M. et al. ZEB1 transcription factor promotes immune escape in melanoma. J. Immunother. Cancer 10, e003484 (2022).
Thompson, J. C. et al. Gene signatures of tumor inflammation and epithelial-to-mesenchymal transition (EMT) predict responses to immune checkpoint blockade in lung cancer with high accuracy. Lung Cancer 139, 1–8 (2020).
Zhang, Y. et al. Tumor stemness score to estimate epithelial-to-mesenchymal transition (EMT) and cancer stem cells (CSCs) characterization and to predict the prognosis and immunotherapy response in bladder urothelial carcinoma. Stem Cell Res. Ther. 14, 15 (2023).
Dongre, A. et al. Direct and indirect regulators of epithelial-mesenchymal transition-mediated immunosuppression in breast carcinomas. Cancer Discov. 11, 1286–1305 (2021).
Li, J. et al. Metastasis and immune evasion from extracellular cGAMP hydrolysis. Cancer Discov. 11, 1212–1227 (2021).
Terabe, M. et al. Blockade of only TGF-β 1 and 2 is sufficient to enhance the efficacy of vaccine and PD-1 checkpoint blockade immunotherapy. Oncoimmunology 6, e1308616 (2017).
Wang, C. et al. UGT1A7 altered HER2-positive breast cancer response to trastuzumab by affecting epithelial-to-mesenchymal transition: a potential biomarker to identify patients resistant to trastuzumab treatment. Cancer Gene Ther. 31, 1525–1535 (2024).
Byers, L. A. et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 19, 279–290 (2013).
Lv, L. et al. Anlotinib reverses osimertinib resistance via inhibiting epithelial-to-mesenchymal transition and angiogenesis in non-small cell lung cancer. J. Biomed. Res. https://doi.org/10.7555/jbr.38.20240045 (2024).
Acknowledgements
The authors research is supported by the German Research Foundation (TRR305 TP A03, A04, B01 and B07, SPP2306 project 461704629 and BR1399/17-1, BR4145/1-1, BR4145/2-1 and BR4145/3-1), IZKF-Erlangen (IZKF D39), the Bavarian Cancer Research Center (BZKF:PRe-Ferro 001), the National Breast Cancer Foundation (Australia; RPG0118), and Tour de Cure (Australia; RSP-106-2024).
Author information
Authors and Affiliations
Contributions
E.W.T., A.D.R., S.B., V.A., M.P.S. and T.B. researched data for this manuscript. E.W.T., A.D.R., S.B., K.G., R.Y.H., D.I.-R., P.S., G.S., M.P.S. and T.B. made a substantial contribution to discussions of content. E.W.T., A.D.R., S.B., M.P.S. and T.B. wrote the manuscript. E.W.T., A.D.R., S.B., R.Y.H., D.I.-R., P.S., G.S., M.P.S. and T.B. edited and/or reviewed the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks A. Moustakas, A. Tasdogan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thompson, E.W., Redfern, A.D., Brabletz, S. et al. EMT and cancer: what clinicians should know. Nat Rev Clin Oncol 22, 711–733 (2025). https://doi.org/10.1038/s41571-025-01058-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01058-2