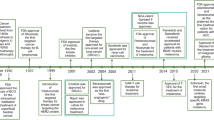
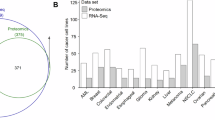
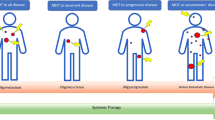
Abstract
Cancer evolution can engender tumours with the ability to resist multiple treatments with distinct chemical structures and mechanisms of action, and this multidrug resistance (MDR) phenotype has long been a substantial challenge in cancer therapy. Despite the established benefits of systemic treatments including chemotherapies, molecularly targeted therapies and immunotherapies across various cancers, MDR inevitably occurs at some point during the course of the disease and its treatment in most patients. Since the discovery of MDR in the 1960s, our understanding of the underlying mechanisms has deepened. However, few strategies are currently available to combat MDR in the clinical setting, and approaches to systematically translate knowledge of new MDR mechanisms and treatments from the laboratory into the clinic are lacking. In this Review, we focus on preclinical and clinical advances in understanding MDR, with an emphasis on resistance to chemotherapy and targeted therapy. We also summarize progress made in translating these findings from bench to bedside through the development of potential strategies to overcome MDR and thus improve patient outcomes.
Key points
-
Multidrug resistance (MDR) is driven first and foremost by ATP-binding cassette efflux pumps and is further reinforced by tumour heterogeneity, epigenetic changes, signalling pathway interactions and the tumour microenvironment.
-
Many drug resistance mechanisms, despite clear evidence that they confer cross-resistance to multiple agents, are often discussed only in general terms of ‘drug resistance’; emphasizing contributions of these mechanisms to MDR is important to the clinical translation of MDR-reversal strategies.
-
Robust preclinical models are vital to bridge the gap between basic research and clinical application. Traditional xenograft models often fail to reflect the complexities of cancer in patients; genetically engineered mouse models and patient-derived xenograft models are typically more representative but still do not fully recapitulate tumour heterogeneity and immune interactions, necessitating further refinement.
-
Addressing MDR will require combinations of molecularly targeted agents and other therapies, immune modulation and innovative drug delivery methods to target multiple resistance pathways simultaneously.
-
Incorporating molecular profiling and biomarker-based diagnostics will facilitate progress in MDR research by connecting preclinical findings with clinical outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cancer facts and figures 2024. American Cancer Society https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf (2024).
Ashique, S. et al. Multi drug resistance in colorectal cancer — approaches to overcome, advancements and future success. Adv. Cancer Biol. Metastasis 10, 100114 (2024).
Cancer multidrug resistance. Nat. Biotechnol. 18, IT18–IT20 (2000).
Mansoori, B., Mohammadi, A., Davudian, S., Shirjang, S. & Baradaran, B. The different mechanisms of cancer drug resistance: a brief review. Adv. Pharm. Bull. 7, 339–348 (2017).
Kessel, D. & Bosmann, H. B. On the characteristics of actinomycin D resistance in L5178Y cells. Cancer Res. 30, 2695–2701 (1970).
Kessel, D. & Wodinsky, I. Uptake in vivo and in vitro of actinomycin D by mouse leukemias as factors in survival. Biochem. Pharmacol. 17, 161–164 (1968).
Kessel, D., Botterill, V. & Wodinsky, I. Uptake and retention of daunomycin by mouse leukemic cells as factors in drug response. Cancer Res. 28, 938–941 (1968).
Bellamy, W. T. & Dalton, W. S. Multidrug resistance in the laboratory and clinic. Adv. Clin. Chem. 31, 1–61 (1994).
Kurre, D., Dang, P. X., Le, L. T. M., Gadkari, V. V. & Alam, A. Structural insights into binding-site access and ligand recognition by human ABCB1. EMBO J. https://doi.org/10.1038/s44318-025-00361-z (2025).
Shinde, O. et al. Structures of ATP-binding cassette transporter ABCC1 reveal the molecular basis of cyclic dinucleotide cGAMP export. Immunity 58, 59–73 (2025).
Lei, Z. N. et al. Understanding and targeting resistance mechanisms in cancer. MedComm 4, e265 (2023).
Tippett, V. L. et al. The strategy and clinical relevance of in vitro models of MAP resistance in osteosarcoma: a systematic review. Oncogene 42, 259–277 (2023).
McDermott, M. et al. In vitro development of chemotherapy and targeted therapy drug-resistant cancer cell lines: a practical guide with case studies. Front. Oncol. 4, 40 (2014).
Wu, Z. X. et al. Establishment and characterization of an irinotecan-resistant human colon cancer cell line. Front. Oncol. 10, 624954 (2020).
Watson, M. B., Lind, M. J. & Cawkwell, L. Establishment of in-vitro models of chemotherapy resistance. Anticancer Drugs 18, 749–754 (2007).
Beraldo-Neto, E. et al. Proteomic dynamics of multidrug resistance mechanisms in lucena 1 cell line. Cells 13, 1427 (2024).
Rosch, L. et al. ERBB and P-glycoprotein inhibitors break resistance in relapsed neuroblastoma models through P-glycoprotein. Mol. Oncol. 17, 37–58 (2023).
Berrieman, H. K., Lind, M. J. & Cawkwell, L. Do beta-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol. 5, 158–164 (2004).
Klotz, D. M. et al. Establishment and molecular characterization of an in vitro model for PARPi-resistant ovarian cancer. Cancers 15, 3774 (2023).
Bell, C. C. & Gilan, O. Principles and mechanisms of non-genetic resistance in cancer. Br. J. Cancer 122, 465–472 (2020).
Gillet, J. P. et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc. Natl Acad. Sci. USA 108, 18708–18713 (2011).
Aldonza, M. B., Hong, J. Y. & Lee, S. K. Paclitaxel-resistant cancer cell-derived secretomes elicit ABCB1-associated docetaxel cross-resistance and escape from apoptosis through FOXO3a-driven glycolytic regulation. Exp. Mol. Med. 49, e286 (2017).
Alalawy, A. I. Key genes and molecular mechanisms related to paclitaxel resistance. Cancer Cell Int. 24, 244 (2024).
Vaghari-Tabari, M. et al. CRISPR/Cas9 gene editing: a new approach for overcoming drug resistance in cancer. Cell. Mol. Biol. Lett. 27, 49 (2022).
Lei, Z. N. et al. Overcoming multidrug resistance by knockout of ABCB1 gene using CRISPR/Cas9 system in SW620/Ad300 colorectal cancer cells. MedComm 2, 765–777 (2021).
Liu, T. et al. Targeting ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the CRISPR–Cas9 system to reverse drug resistance. Oncotarget 7, 83502 (2016).
Wang, K. et al. Targeting uPAR by CRISPR/Cas9 system attenuates cancer malignancy and multidrug resistance. Front. Oncol. 9, 80 (2019).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Kim, M. A. et al. Tousled-like kinase loss confers PARP inhibitor resistance in BRCA1-mutated cancers by impeding non-homologous end joining repair. Mol. Med. 31, 18 (2025).
Ibrahim, K., Abdul Murad, N. A., Harun, R. & Jamal, R. Knockdown of Tousled-like kinase 1 inhibits survival of glioblastoma multiforme cells. Int. J. Mol. Med. 46, 685–699 (2020).
Balon, K., Sheriff, A., Jacków, J. & Łaczmański, Ł. Targeting cancer with CRISPR/Cas9-based therapy. Int. J. Mol. Sci. 23, 573 (2022).
Lavi, O. Redundancy: a critical obstacle to improving cancer therapy. Cancer Res. 75, 808–812 (2015).
Che, L. et al. Computer-assisted engineering of programmed drug releasing multilayer nanomedicine via indomethacin-mediated ternary complex for therapy against a multidrug resistant tumor. Acta Biomater. 97, 461–473 (2019).
Qin, S. et al. CCT251545 enhances drug delivery and potentiates chemotherapy in multidrug-resistant cancers by Rac1-mediated macropinocytosis. Drug. Resist. Updat. 66, 100906 (2023).
Cho, S. Y. Patient-derived xenografts as compatible models for precision oncology. Lab. Anim. Res. 36, 14 (2020).
Goto, T. Patient-derived tumor xenograft models: toward the establishment of precision cancer medicine. J. Pers. Med. 10, 64 (2020).
Lubachowski, M. et al. Activation of the anaphase promoting complex restores impaired mitotic progression and chemosensitivity in multiple drug-resistant human breast cancer. Cancers 16, 1755 (2024).
Lim, S. M. et al. Exploration of immune-modulatory effects of amivantamab in combination with pembrolizumab in lung and head and neck squamous cell carcinoma. Cancer Res. Commun. 4, 1748–1764 (2024).
Dickins, R. A. et al. Tissue-specific and reversible RNA interference in transgenic mice. Nat. Genet. 39, 914–921 (2007).
Hooijkaas, A. I., Gadiot, J., van der Valk, M., Mooi, W. J. & Blank, C. U. Targeting BRAFV600E in an inducible murine model of melanoma. Am. J. Pathol. 181, 785–794 (2012).
Politi, K., Fan, P. D., Shen, R., Zakowski, M. & Varmus, H. Erlotinib resistance in mouse models of epidermal growth factor receptor-induced lung adenocarcinoma. Dis. Model. Mech. 3, 111–119 (2010).
Zhang, Z., Yang, S. & Wang, Q. Impact of MET alterations on targeted therapy with EGFR-tyrosine kinase inhibitors for EGFR-mutant lung cancer. Biomark. Res. 7, 27 (2019).
Lee, J. B., Shim, J. S. & Byoung, C. C. Evolving roles of MET as a therapeutic target in NSCLC and beyond. Nat. Rev. Clin. Oncol. https://doi.org/10.1038/s41571-025-01051-9 (2025).
Ben-David, U. et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 49, 1567–1575 (2017).
Abate-Shen, C. & Pandolfi, P. P. Effective utilization and appropriate selection of genetically engineered mouse models for translational integration of mouse and human trials. Cold Spring Harb. 2013, https://doi.org/10.1101/pdb.top078774 (2013).
Yang, Y. et al. Comprehensive landscape of resistance mechanisms for neoadjuvant therapy in esophageal squamous cell carcinoma by single-cell transcriptomics. Signal. Transduct. Target. Ther. 8, 298 (2023).
Sahu, D., Shi, J., Segura Rueda, I. A., Chatrath, A. & Dutta, A. Development of a polygenic score predicting drug resistance and patient outcome in breast cancer. NPJ Precis. Oncol. 8, 219 (2024).
Yu, S. et al. LncRNA AGPG confers endocrine resistance in breast cancer by promoting E2F1 activity. Cancer Res. 83, 3220–3236 (2023).
Bartolomucci, A. et al. Circulating tumor DNA to monitor treatment response in solid tumors and advance precision oncology. NPJ Precis. Oncol. 9, 84 (2025).
Thress, K. S. et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 21, 560–562 (2015).
Otsuji, K. et al. Serial single-cell RNA sequencing unveils drug resistance and metastatic traits in stage IV breast cancer. NPJ Precis. Oncol. 8, 222 (2024).
Martinelli, E. et al. Cetuximab rechallenge plus avelumab in pretreated patients with RAS wild-type metastatic colorectal cancer: the phase 2 single-arm clinical CAVE trial. JAMA Oncol. 7, 1529–1535 (2021).
Wu, H. et al. Single-cell RNA sequencing reveals tumor heterogeneity, microenvironment, and drug-resistance mechanisms of recurrent glioblastoma. Cancer Sci. 114, 2609–2621 (2023).
Zhang, Y. et al. Single-cell RNA sequencing in cancer research. J. Exp. Clin. Cancer Res. 40, 81 (2021).
Xu, M., Zhao, X., Wen, T. & Qu, X. Unveiling the role of KRAS in tumor immune microenvironment. Biomed. Pharmacother. 171, 116058 (2024).
Nussinov, R., Tsai, C. J. & Jang, H. Anticancer drug resistance: an update and perspective. Drug. Resist. Updat. 59, 100796 (2021).
Glaviano, A. et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 22, 138 (2023).
Chen, Y. et al. CEA-induced PI3K/AKT pathway activation through the binding of CEA to KRT1 contributes to oxaliplatin resistance in gastric cancer. Drug. Resist. Updat. 78, 101179 (2025).
Liu, R. et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 11, 797 (2020).
Zhang, L. et al. CDK6–PI3K signaling axis is an efficient target for attenuating ABCB1/P-gp mediated multi-drug resistance (MDR) in cancer cells. Mol. Cancer 21, 103 (2022).
Yang, Q., Jiang, W. & Hou, P. Emerging role of PI3K/AKT in tumor-related epigenetic regulation. Semin. Cancer Biol. 59, 112–124 (2019).
Haselager, M. et al. Regulation of Bcl-XL by non-canonical NF-κB in the context of CD40-induced drug resistance in CLL. Cell Death Differ. 28, 1658–1668 (2021).
Chen, W., Liu, X., Yuan, S. & Qiao, T. HSPA12B overexpression induces cisplatin resistance in non-small-cell lung cancer by regulating the PI3K/Akt/NF-κB signaling pathway. Oncol. Lett. 15, 3883–3889 (2018).
Hernandez, L. et al. Activation of NF-kappaB signaling by inhibitor of NF-kappaB kinase beta increases aggressiveness of ovarian cancer. Cancer Res. 70, 4005–4014 (2010).
Kuo, M. T. et al. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene 21, 1945–1954 (2002).
Li, X. et al. PI3K/Akt/mTOR signaling orchestrates the phenotypic transition and chemo-resistance of small cell lung cancer. J. Genet. Genom. 48, 640–651 (2021).
Kim, E. K. & Choi, E. J. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et. Biophysica Acta 1802, 396–405 (2010).
Lee, S., Rauch, J. & Kolch, W. Targeting MAPK signaling in cancer: mechanisms of drug resistance and sensitivity. Int. J. Mol. Sci. 21, 1102 (2020).
Yu, D., Zhao, W., Vallega, K. A. & Sun, S. Y. Managing acquired resistance to third-generation EGFR tyrosine kinase inhibitors through co-targeting MEK/ERK signaling. Lung Cancer 12, 1–10 (2021).
Eberlein, C. A. et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res. 75, 2489–2500 (2015).
Peters, T. L. et al. Evolution of MET and NRAS gene amplification as acquired resistance mechanisms in EGFR mutant NSCLC. NPJ Precis. Oncol. 5, 91 (2021).
Ercan, D. et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2, 934–947 (2012).
Ciardiello, D. et al. Comprehensive genomic profiling by liquid biopsy portrays metastatic colorectal cancer mutational landscape to predict antitumor efficacy of FOLFIRI plus cetuximab in the CAPRI-2 GOIM trial. ESMO Open. 10, 104511 (2025).
Ahronian, L. G. et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov. 5, 358–367 (2015).
Rizzolio, S. et al. Neuropilin-1 upregulation elicits adaptive resistance to oncogene-targeted therapies. J. Clin. Invest. 128, 3976–3990 (2018).
Guo, G. et al. A TNF-JNK-Axl-ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat. Neurosci. 20, 1074–1084 (2017).
Xu, L., Fu, Y., Li, Y. & Han, X. Cisplatin induces expression of drug resistance-related genes through c-jun N-terminal kinase pathway in human lung cancer cells. Cancer Chemother. Pharmacol. 80, 235–242 (2017).
Seino, M. et al. Requirement of JNK signaling for self-renewal and tumor-initiating capacity of ovarian cancer stem cells. Anticancer. Res. 34, 4723–4731 (2014).
Matsuda, K. et al. Targeting JNK for therapeutic depletion of stem-like glioblastoma cells. Sci. Rep. 2, 516 (2012).
Liu, J. et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal. Transduct. Target. Ther. 7, 3 (2022).
Bu, H., Liu, D., Cui, J., Cai, K. & Shen, F. Wnt/β-catenin signaling pathway is involved in induction of apoptosis by oridonin in colon cancer COLO205 cells. Transl. Cancer Res. 8, 1782–1794 (2019).
Wu, X., Luo, F., Li, J., Zhong, X. & Liu, K. Tankyrase 1 inhibitior XAV939 increases chemosensitivity in colon cancer cell lines via inhibition of the Wnt signaling pathway. Int. J. Oncol. 48, 1333–1340 (2016).
Zhong, Z. & Virshup, D. M. Wnt signaling and drug resistance in cancer. Mol. Pharmacol. 97, 72–89 (2020).
Lepore Signorile, M. et al. Pharmacological targeting of the novel β-catenin chromatin-associated kinase p38α in colorectal cancer stem cell tumorspheres and organoids. Cell Death Dis. 12, 316 (2021).
de Sousa, E. M. F. & Vermeulen, L. Wnt signaling in cancer stem cell biology. Cancers 8, 60 (2016).
Tammela, T. et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 545, 355–359 (2017).
Nagaraj, A. B. et al. Critical role of Wnt/β-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget 6, 23720–23734 (2015).
Daisy Precilla, S., Biswas, I., Kuduvalli, S. S. & Anitha, T. S. Crosstalk between PI3K/AKT/mTOR and WNT/beta-catenin signaling in GBM - could combination therapy checkmate the collusion? Cell Signal. 95, 110350 (2022).
Chien, A. J. et al. Targeted BRAF inhibition impacts survival in melanoma patients with high levels of Wnt/beta-catenin signaling. PLoS ONE 9, e94748 (2014).
Tabernero, J. et al. A phase Ib/II study of WNT974 + encorafenib + cetuximab in patients with BRAF V600E-mutant KRAS wild-type metastatic colorectal cancer. Oncologist 28, 230–238 (2023).
Shen, W., Huang, J. & Wang, Y. Biological significance of Notch signaling strength. Front. Cell Dev. Biol. 9, 652273 (2021).
Wang, Z., Li, Y., Banerjee, S. & Sarkar, F. H. Emerging role of Notch in stem cells and cancer. Cancer Lett. 279, 8–12 (2009).
Wang, X. et al. miR-181b/Notch2 overcome chemoresistance by regulating cancer stem cell-like properties in NSCLC. Stem Cell Res. Ther. 9, 327 (2018).
Jiang, N. et al. HIF-1α-regulated miR-1275 maintains stem cell-like phenotypes and promotes the progression of LUAD by simultaneously activating Wnt/β-catenin and Notch signaling. Theranostics 10, 2553–2570 (2020).
Lin, X. et al. Notch4+ cancer stem-like cells promote the metastatic and invasive ability of melanoma. Cancer Sci. 107, 1079–1091 (2016).
Christiansen, J. J. & Rajasekaran, A. K. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 66, 8319–8326 (2006).
Shao, S. et al. Notch1 signaling regulates the epithelial-mesenchymal transition and invasion of breast cancer in a Slug-dependent manner. Mol. Cancer 14, 28 (2015).
Sun, X., Huang, T., Liu, Z., Sun, M. & Luo, S. LncRNA SNHG7 contributes to tumorigenesis and progression in breast cancer by interacting with miR-34a through EMT initiation and the Notch-1 pathway. Eur. J. Pharmacol. 856, 172407 (2019).
Yuan, X. et al. Notch signaling and EMT in non-small cell lung cancer: biological significance and therapeutic application. J. Hematol. Oncol. 7, 87 (2014).
Wang, Z. et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 69, 2400–2407 (2009).
Zhang, J. et al. Notch signalling induces epithelial-mesenchymal transition to promote metastasis in oral squamous cell carcinoma. Int. J. Mol. Med. 42, 2276–2284 (2018).
Clark, A. G., Bertrand, F. E. & Sigounas, G. A potential requirement for Smad3 phosphorylation in Notch-mediated EMT in colon cancer. Adv. Biol. Regul. 88, 100957 (2023).
Zhang, J., Kuang, Y., Wang, Y., Xu, Q. & Ren, Q. Notch-4 silencing inhibits prostate cancer growth and EMT via the NF-κB pathway. Int. J. Program. Cell Death 22, 877–884 (2017).
Lailler, C. et al. PrPC controls epithelial-to-mesenchymal transition in EGFR-mutated NSCLC: implications for TKI resistance and patient follow-up. Oncogene 43, 2781–2794 (2024).
Hu, X., Li, J., Fu, M., Zhao, X. & Wang, W. The JAK/STAT signaling pathway: from bench to clinic. Signal. Transduct. Target. Ther. 6, 402 (2021).
Xue, C. et al. Evolving cognition of the JAK–STAT signaling pathway: autoimmune disorders and cancer. Signal. Transduct. Target. Ther. 8, 204 (2023).
Jin, W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells 9, 217 (2020).
Bauvois, B. et al. Activation of interferon signaling in chronic lymphocytic leukemia cells contributes to apoptosis resistance via a JAK-Src/STAT3/Mcl-1 signaling pathway. Biomedicines 9, 188 (2021).
Ho, J. N. et al. Bcl-XL and STAT3 mediate malignant actions of gamma-irradiation in lung cancer cells. Cancer Sci. 101, 1417–1423 (2010).
Huang, C. Y., Chen, L. J., Chen, C. S., Wang, C. Y. & Hong, S. Y. MCL1 inhibition: a promising approach to augment the efficacy of sorafenib in NSCLC through ferroptosis induction. Cell Death Discov. 10, 137 (2024).
Kim, S. Y. et al. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell. Signal. 25, 961–969 (2013).
Puris, E., Fricker, G. & Gynther, M. The role of solute carrier transporters in efficient anticancer drug delivery and therapy. Pharmaceutics 15, 364 (2023).
Marin, J. J. G. et al. Impact of genetic variants in the solute carrier (SLC) genes encoding drug uptake transporters on the response to anticancer chemotherapy. Cancer Drug. Resist. 7, 27 (2024).
Huang, Y. & Sadée, W. Membrane transporters and channels in chemoresistance and sensitivity of tumor cells. Cancer Lett. 239, 168–182 (2006).
Obaidat, A., Roth, M. & Hagenbuch, B. The expression and function of organic anion transporting polypeptides in normal tissues and in cancer. Annu. Rev. Pharmacol. Toxicol. 52, 135–151 (2012).
de Morree, E. S. et al. Loss of SLCO1B3 drives taxane resistance in prostate cancer. Br. J. Cancer 115, 674–681 (2016).
König, J., Seithel, A., Gradhand, U. & Fromm, M. F. Pharmacogenomics of human OATP transporters. Naunyn-Schmiedeberg’s Arch. Pharmacol. 372, 432–443 (2006).
Kiyotani, K. et al. Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci. 99, 967–972 (2008).
Kim, D. H. et al. Clinical relevance of a pharmacogenetic approach using multiple candidate genes to predict response and resistance to imatinib therapy in chronic myeloid leukemia. Clin. Cancer Res. 15, 4750–4758 (2009).
Cargnin, S., Ravegnini, G., Soverini, S., Angelini, S. & Terrazzino, S. Impact of SLC22A1 and CYP3A5 genotypes on imatinib response in chronic myeloid leukemia: a systematic review and meta-analysis. Pharmacol. Res. 131, 244–254 (2018).
White, B. & Swietach, P. What can we learn about acid-base transporters in cancer from studying somatic mutations in their genes? Pflug. Arch. 476, 673–688 (2024).
Lavoro, A. et al. In silico analysis of the solute carrier (SLC) family in cancer indicates a link among DNA methylation, metabolic adaptation, drug response, and immune reactivity. Front. Pharmacol. 14, 1191262 (2023).
Wang, L. et al. Drug resistance in ovarian cancer: from mechanism to clinical trial. Mol. Cancer 23, 66 (2024).
Meng, X. et al. Cell membrane fatty acid composition differs between normal and malignant cell lines. Puerto Rico Health Sci. J. 23, 103–106 (2004).
Peetla, C., Vijayaraghavalu, S. & Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: implications for drug transport and drug delivery with nanoparticles. Adv. Drug. Deliv. Rev. 65, 1686–1698 (2013).
Hendrich, A. B. & Michalak, K. Lipids as a target for drugs modulating multidrug resistance of cancer cells. Curr. Drug. Targets 4, 23–30 (2003).
Casares, D., Escribá, P. V. & Rosselló, C. A. Membrane lipid composition: effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 20, 2167 (2019).
Vijayaraghavalu, S., Peetla, C., Lu, S. & Labhasetwar, V. Epigenetic modulation of the biophysical properties of drug-resistant cell lipids to restore drug transport and endocytic functions. Mol. Pharm. 9, 2730–2742 (2012).
Huang, H. et al. LC–MS based sphingolipidomic study on A2780 human ovarian cancer cell line and its taxol-resistant strain. Sci. Rep. 6, 34684 (2016).
Kristenson, L. et al. Deletion of the TMEM30A gene enables leukemic cell evasion of NK cell cytotoxicity. Proc. Natl Acad. Sci. USA 121, e2316447121 (2024).
Riedl, S. et al. In search of a novel target — phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim. Biophys. Acta 1808, 2638–2645 (2011).
Rivel, T., Ramseyer, C. & Yesylevskyy, S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 9, 5627 (2019).
May, G. L. et al. Plasma membrane lipid composition of vinblastine sensitive and resistant human leukaemic lymphoblasts. Int. J. Cancer 42, 728–733 (1988).
Storch, C. H., Ehehalt, R., Haefeli, W. E. & Weiss, J. Localization of the human breast cancer resistance protein (BCRP/ABCG2) in lipid rafts/caveolae and modulation of its activity by cholesterol in vitro. J. Pharmacol. Exp. Ther. 323, 257–264 (2007).
Gayet, L. et al. Control of P-glycoprotein activity by membrane cholesterol amounts and their relation to multidrug resistance in human CEM leukemia cells. Biochemistry 44, 4499–4509 (2005).
Simon, S., Roy, D. & Schindler, M. Intracellular pH and the control of multidrug resistance. Proc. Natl Acad. Sci. USA 91, 1128–1132 (1994).
Rauch, C. Toward a mechanical control of drug delivery. On the relationship between Lipinski’s 2nd rule and cytosolic pH changes in doxorubicin resistance levels in cancer cells: a comparison to published data. Eur. Biophys. J. 38, 829–846 (2009).
Wojtkowiak, J. W., Verduzco, D., Schramm, K. J. & Gillies, R. J. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol. Pharm. 8, 2032–2038 (2011).
Juliano, R. L. & Ling, V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 455, 152–162 (1976).
Wang, J. Q. et al. ATP-binding cassette (ABC) transporters in cancer: a review of recent updates. J. Evid. Based Med. 14, 232–256 (2021).
Lu, Q., Ambudkar, S. V. & Yang, D. H. Editorial: ABC transporters and drug resistance. Drug. Resist. Updat. 77, 101135 (2024).
van den Heuvel-Eibrink, M. M. et al. Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML). Leukemia 16, 833–839 (2002).
Marzac, C. et al. ATP binding cassette transporters associated with chemoresistance: transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica 96, 1293–1301 (2011).
van den Heuvel-Eibrink, M. M. et al. CD34-related coexpression of MDR1 and BCRP indicates a clinically resistant phenotype in patients with acute myeloid leukemia (AML) of older age. Ann. Hematol. 86, 329–337 (2007).
Burger, H. et al. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin. Cancer Res. 9, 827–836 (2003).
Lheureux, S. et al. EVOLVE: a multicenter open-label single-arm clinical and translational phase II trial of cediranib plus olaparib for ovarian cancer after PARP inhibition progression. Clin. Cancer Res. 26, 4206–4215 (2020).
Haber, M. et al. Association of high-level MRP1 expression with poor clinical outcome in a large prospective study of primary neuroblastoma. J. Clin. Oncol. 24, 1546–1553 (2006).
Hegedus, C. et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br. J. Pharmacol. 158, 1153–1164 (2009).
Fu, H. et al. The resistance of cancer cells to palbociclib, a cyclin-dependent kinase 4/6 inhibitor, is mediated by the ABCB1 transporter. Front. Pharmacol. 13, 861642 (2022).
Eadie, L. N., Hughes, T. P. & White, D. L. The clinical significance of early imatinib induced ABCB1 overexpression in chronic phase CML patients: a TIDEL II sub-study. Blood 126, 348–348 (2015).
Malhotra, H. et al. Molecular response to imatinib & its correlation with mRNA expression levels of imatinib influx & efflux transporters in patients with chronic myeloid leukaemia in chronic phase. Indian. J. Med. Res. 142, 175–182 (2015).
Ieiri, I. Functional significance of genetic polymorphisms in P-glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2). Drug. Metab. Pharmacokinet. 27, 85–105 (2012).
Lewis, L. D. et al. The relationship of polymorphisms in ABCC2 and SLCO1B3 with docetaxel pharmacokinetics and neutropenia: CALGB 60805 (Alliance). Pharmacogenet. Genom. 23, 29–33 (2013).
Corbett, S. et al. The role of specific ATP-binding cassette transporters in the acquired resistance to pyrrolobenzodiazepine dimer-containing antibody-drug conjugates. Mol. Cancer Ther. 19, 1856–1865 (2020).
Kijima, T. et al. Predictive role of ABC transporters in the efficacy of enfortumab vedotin for urothelial carcinoma. BJUI Compass 6, e488 (2025).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Hoxhaj, G. & Manning, B. D. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020).
Zhao, Y. et al. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 71, 4585–4597 (2011).
Muramatsu, H. et al. Targeting lactate dehydrogenase-A promotes docetaxel-induced cytotoxicity predominantly in castration-resistant prostate cancer cells. Oncol. Rep. 42, 224–230 (2019).
Hua, G., Liu, Y., Li, X., Xu, P. & Luo, Y. Targeting glucose metabolism in chondrosarcoma cells enhances the sensitivity to doxorubicin through the inhibition of lactate dehydrogenase-A. Oncol. Rep. 31, 2727–2734 (2014).
Nagamine, A., Araki, T., Nagano, D., Miyazaki, M. & Yamamoto, K. l-Lactate dehydrogenase B may be a predictive marker for sensitivity to anti-EGFR monoclonal antibodies in colorectal cancer cell lines. Oncol. Lett. 17, 4710–4716 (2019).
Quek, L. E. et al. Glutamine addiction promotes glucose oxidation in triple-negative breast cancer. Oncogene 41, 4066–4078 (2022).
Zhou, M. et al. Warburg effect in chemosensitivity: targeting lactate dehydrogenase-A re-sensitizes taxol-resistant cancer cells to taxol. Mol. Cancer 9, 33 (2010).
Liu, H., Liu, Y. & Zhang, J. T. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol. Cancer Ther. 7, 263–270 (2008).
Al-Bahlani, S. et al. Fatty acid synthase regulates the chemosensitivity of breast cancer cells to cisplatin-induced apoptosis. Int. J. Program. Cell Death 22, 865–876 (2017).
Jin, C. & Yuan, P. Implications of lipid droplets in lung cancer: associations with drug resistance. Oncol. Lett. 20, 2091–2104 (2020).
Accioly, M. T. et al. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 68, 1732–1740 (2008).
Cotte, A. K. et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat. Commun. 9, 322 (2018).
Huang, Q. et al. Co-administration of 20-S-protopanaxatriol (g-PPT) and EGFR-TKI overcomes EGFR-TKI resistance by decreasing SCD1 induced lipid accumulation in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 38, 129 (2019).
Zhang, I. et al. Pharmacological inhibition of lipid droplet formation enhances the effectiveness of curcumin in glioblastoma. Eur. J. Pharm. Biopharm. 100, 66–76 (2016).
Schmidt, R. et al. CYP3A4, CYP2C9 and CYP2B6 expression and ifosfamide turnover in breast cancer tissue microsomes. Br. J. Cancer 90, 911–916 (2004).
Miyoshi, Y. et al. Prediction of response to docetaxel by CYP3A4 mRNA expression in breast cancer tissues. Int. J. Cancer 97, 129–132 (2002).
Yao, D., Ding, S., Burchell, B., Wolf, C. R. & Friedberg, T. Detoxication of vinca alkaloids by human P450 CYP3A4-mediated metabolism: implications for the development of drug resistance. J. Pharmacol. Exp. Ther. 294, 387–395 (2000).
Kumarakulasingham, M. et al. Cytochrome p450 profile of colorectal cancer: identification of markers of prognosis. Clin. Cancer Res. 11, 3758–3765 (2005).
Xue, Y. et al. CYP1B1 promotes PARPi-resistance via histone H1.4 interaction and increased chromatin accessibility in ovarian cancer. Drug. Resist. Updat. 77, 101151 (2024).
Gessner, T., Vaughan, L. A., Beehler, B. C., Bartels, C. J. & Baker, R. M. Elevated pentose cycle and glucuronyltransferase in daunorubicin-resistant P388 cells. Cancer Res. 50, 3921–3927 (1990).
Takahashi, T. et al. The role of glucuronidation in 7-ethyl-10-hydroxycamptothecin resistance in vitro. J. Cancer Res. 88, 1211–1217 (1997).
Cummings, J. et al. Enhanced clearance of topoisomerase I inhibitors from human colon cancer cells by glucuronidation. Biochem. Pharmacol. 63, 607–613 (2002).
Zahreddine, H. A. et al. The sonic hedgehog factor GLI1 imparts drug resistance through inducible glucuronidation. Nature 511, 90–93 (2014).
López-Ayllón, B. D. et al. Biomarkers of erlotinib response in non-small cell lung cancer tumors that do not harbor the more common epidermal growth factor receptor mutations. Int. J. Clin. Exp. Pathol. 8, 2888–2898 (2015).
Romero-Lorca, A., Novillo, A., Gaibar, M., Bandrés, F. & Fernández-Santander, A. Impacts of the glucuronidase genotypes UGT1A4, UGT2B7, UGT2B15 and UGT2B17 on tamoxifen metabolism in breast cancer patients. PLoS ONE 10, e0132269 (2015).
Ascierto, M. L. et al. The intratumoral balance between metabolic and immunologic gene expression is associated with anti-PD-1 response in patients with renal cell carcinoma. Cancer Immunol. Res. 4, 726–733 (2016).
Yang, L. et al. EGFR TKIs impair lysosome-dependent degradation of SQSTM1 to compromise the effectiveness in lung cancer. Signal. Transduct. Target. Ther. 4, 25 (2019).
Seebacher, N., Lane, D. J., Richardson, D. R. & Jansson, P. J. Turning the gun on cancer: utilizing lysosomal P-glycoprotein as a new strategy to overcome multi-drug resistance. Free. Radic. Biol. Med. 96, 432–445 (2016).
Rajagopal, A. & Simon, S. M. Subcellular localization and activity of multidrug resistance proteins. Mol. Biol. Cell 14, 3389–3399 (2003).
Halaby, R. Influence of lysosomal sequestration on multidrug resistance in cancer cells. Cancer Drug. Resist. 2, 31–42 (2019).
Hou, D. Y. et al. A lysosome-targeting self-condensation prodrug-nanoplatform system for addressing drug resistance of cancer. Nano Lett. 22, 3983–3992 (2022).
Colla, R. et al. Glutathione-mediated antioxidant response and aerobic metabolism: two crucial factors involved in determining the multi-drug resistance of high-risk neuroblastoma. Oncotarget 7, 70715–70737 (2016).
Potęga, A. Glutathione-mediated conjugation of anticancer drugs: an overview of reaction mechanisms and biological significance for drug detoxification and bioactivation. Molecules 27, 5252 (2022).
Chen, H. H. & Kuo, M. T. Role of glutathione in the regulation of cisplatin resistance in cancer chemotherapy. Met. Based Drugs 2010, 430939 (2010).
Nasr, R. et al. Molecular analysis of the massive GSH transport mechanism mediated by the human multidrug resistant protein 1/ABCC1. Sci. Rep. 10, 7616 (2020).
Min, H. Y. & Lee, H. Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 54, 1670–1694 (2022).
Li, L. Y., Guan, Y. D., Chen, X. S., Yang, J. M. & Cheng, Y. DNA repair pathways in cancer therapy and resistance. Front. Pharmacol. 11, 629266 (2020).
Bonanno, L., Favaretto, A. & Rosell, R. Platinum drugs and DNA repair mechanisms in lung cancer. Anticancer. Res. 34, 493–501 (2014).
Alagoz, M., Gilbert, D. C., El-Khamisy, S. & Chalmers, A. J. DNA repair and resistance to topoisomerase I inhibitors: mechanisms, biomarkers and therapeutic targets. Curr. Med. Chem. 19, 3874–3885 (2012).
Sethy, C. & Kundu, C. N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 137, 111285 (2021).
Crul, M. et al. DNA repair mechanisms involved in gemcitabine cytotoxicity and in the interaction between gemcitabine and cisplatin. Biochem. Pharmacol. 65, 275–282 (2003).
Karnitz, L. M. & Zou, L. Molecular pathways: targeting ATR in cancer therapy. Clin. Cancer Res. 21, 4780–4785 (2015).
Aziz, M. H. & Ahmad, A. Epigenetic basis of cancer drug resistance. Cancer Drug. Resist. 3, 113–116 (2020).
Ma, Y. et al. DNA methyltransferase mediates the hypermethylation of the microRNA 34a promoter and enhances the resistance of patient-derived pancreatic cancer cells to molecular targeting agents. Pharmacol. Res. 160, 105071 (2020).
Wang, Q. et al. DNMT1-mediated methylation of BEX1 regulates stemness and tumorigenicity in liver cancer. J. Hepatol. 75, 1142–1153 (2021).
Dong, B., Qiu, Z. & Wu, Y. Tackle epithelial-mesenchymal transition with epigenetic drugs in cancer. Front. Pharmacol. 11, 596239 (2020).
Hervouet, E., Cheray, M., Vallette, F. M. & Cartron, P. F. DNA methylation and apoptosis resistance in cancer cells. Cells 2, 545–573 (2013).
Jahangiri, R., Mosaffa, F., Emami Razavi, A., Teimoori-Toolabi, L. & Jamialahmadi, K. Altered DNA methyltransferases promoter methylation and mRNA expression are associated with tamoxifen response in breast tumors. J. Cell. Physiol. 233, 7305–7319 (2018).
Kang, K. A. et al. Epigenetic modification of Nrf2 in 5-fluorouracil-resistant colon cancer cells: involvement of TET-dependent DNA demethylation. Cell Death Dis. 5, e1183 (2014).
Huang, R. et al. Restoration of TET2 deficiency inhibits tumor growth in head neck squamous cell carcinoma. Ann. Transl. Med. 8, 329 (2020).
Guo, X. J. et al. Loss of 5-hydroxymethylcytosine induces chemotherapy resistance in hepatocellular carcinoma via the 5-hmC/PCAF/AKT axis. Cell Death Dis. 14, 79 (2023).
Zhou, X. et al. Non-coding RNA in cancer drug resistance: underlying mechanisms and clinical applications. Front. Oncol. 12, 951864 (2022).
Zhang, J. et al. Tet methylcytosine dioxygenase 2 (TET2) deficiency elicits EGFR-TKI (tyrosine kinase inhibitors) resistance in non-small cell lung cancer. Signal. Transduct. Target. Ther. 9, 65 (2024).
Sun, L. et al. Understanding and targeting the epigenetic regulation to overcome EGFR-TKIs resistance in human cancer. Recent. Pat. Anti Cancer Drug. Discov. 18, 506–516 (2023).
Chen, J. et al. EZH2 mediated metabolic rewiring promotes tumor growth independently of histone methyltransferase activity in ovarian cancer. Mol. Cancer 22, 85 (2023).
Yang, Y., Zhang, M. & Wang, Y. The roles of histone modifications in tumorigenesis and associated inhibitors in cancer therapy. J. Natl Cancer Cent. 2, 277–290 (2022).
Zhang, X. et al. The role of trimethylation on histone H3 lysine 27 (H3K27me3) in temozolomide resistance of glioma. Brain Res. 1846, 149252 (2025).
Tiwari, A., Trivedi, R. & Lin, S. Y. Tumor microenvironment: barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 29, 83 (2022).
Hinshaw, D. C. & Shevde, L. A. The tumor microenvironment innately modulates cancer progression. Cancer Res. 79, 4557–4566 (2019).
Anderson, N. M. & Simon, M. C. The tumor microenvironment. Curr. Biol. 30, R921–r925 (2020).
Wei, R., Liu, S., Zhang, S., Min, L. & Zhu, S. Cellular and extracellular components in tumor microenvironment and their application in early diagnosis of cancers. Anal. Cell. Pathol. 2020, 6283796 (2020).
Chen, Z., Han, F., Du, Y., Shi, H. & Zhou, W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal. Transduct. Target. Ther. 8, 70 (2023).
Jing, X. et al. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 18, 157 (2019).
Huang, J. et al. Extracellular matrix and its therapeutic potential for cancer treatment. Signal. Transduct. Target. Ther. 6, 153 (2021).
Rizzolio, S., Giordano, S. & Corso, S. The importance of being CAFs (in cancer resistance to targeted therapies). J. Exp. Clin. Cancer Res. 41, 319 (2022).
Huang, T. X., Guan, X. Y. & Fu, L. Therapeutic targeting of the crosstalk between cancer-associated fibroblasts and cancer stem cells. Am. J. Cancer Res. 9, 1889–1904 (2019).
Mitchell, M. I. & Engelbrecht, A. M. Metabolic hijacking: a survival strategy cancer cells exploit? Crit. Rev. Oncol. Hematol. 109, 1–8 (2017).
Wang, H. et al. Cancer-associated fibroblasts secreted miR-103a-3p suppresses apoptosis and promotes cisplatin resistance in non-small cell lung cancer. Aging 13, 14456–14468 (2021).
Ingham, J., Ruan, J. L. & Coelho, M. A. Breaking barriers: we need a multidisciplinary approach to tackle cancer drug resistance. BJC Rep. 3, 11 (2025).
Tamaki, A., Ierano, C., Szakacs, G., Robey, R. W. & Bates, S. E. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 50, 209–232 (2011).
Lai, J. I., Tseng, Y. J., Chen, M. H., Huang, C. F. & Chang, P. M. Clinical perspective of FDA approved drugs with P-glycoprotein inhibition activities for potential cancer therapeutics. Front. Oncol. 10, 561936 (2020).
Milroy, R. A randomised clinical study of verapamil in addition to combination chemotherapy in small cell lung cancer. West of Scotland lung cancer research group, and the Aberdeen oncology group. Br. J. Cancer 68, 813–818 (1993).
Punt, C. J. et al. Phase IB study of doxorubicin in combination with the multidrug resistance reversing agent S9788 in advanced colorectal and renal cell cancer. Br. J. Cancer 76, 1376–1381 (1997).
Lhommé, C. et al. Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J. Clin. Oncol. 26, 2674–2682 (2008).
Wandel, C. et al. P-glycoprotein and cytochrome P-450 3A inhibition: dissociation of inhibitory potencies. Cancer Res. 59, 3944–3948 (1999).
Friedenberg, W. R. et al. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): a trial of the Eastern cooperative oncology group. Cancer 106, 830–838 (2006).
Baer, M. R. et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: cancer and leukemia group B study 9720. Blood 100, 1224–1232 (2002).
Ruff, P. et al. A randomized, placebo-controlled, double-blind phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimen. Cancer Chemother. Pharmacol. 64, 763–768 (2009).
Nobili, S., Landini, I., Giglioni, B. & Mini, E. Pharmacological strategies for overcoming multidrug resistance. Curr. Drug. Targets 7, 861–879 (2006).
Cripe, L. D. et al. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: a randomized, placebo-controlled trial of the Eastern cooperative oncology group 3999. Blood 116, 4077–4085 (2010).
Libby, E. & Hromas, R. Dismounting the MDR horse. Blood 116, 4037–4038 (2010).
Fukuda, Y., Lian, S. & Schuetz, J. D. Leukemia and ABC transporters. Adv. Cancer Res. 125, 171–196 (2015).
Khongorzul, P., Ling, C. J., Khan, F. U., Ihsan, A. U. & Zhang, J. Antibody-drug conjugates: a comprehensive review. Mol. Cancer Res. 18, 3–19 (2020).
Powles, T. et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 384, 1125–1135 (2021).
Gandullo-Sanchez, L., Ocana, A. & Pandiella, A. Generation of antibody-drug conjugate resistant models. Cancers 13, 4631 (2021).
Kovtun, Y. V. et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 70, 2528–2537 (2010).
Murakami, M. et al. Improving drug potency and efficacy by nanocarrier-mediated subcellular targeting. Sci. Transl. Med. 3, 64ra62 (2011).
Zhang, S. et al. pH and redox dual-responsive nanoparticles based on disulfide-containing poly(β-amino ester) for combining chemotherapy and COX-2 inhibitor to overcome drug resistance in breast cancer. J. Nanobiotechnol. 17, 109 (2019).
Borsoi, C. et al. Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma. Cancer Lett. 403, 296–304 (2017).
Godin, B., Tasciotti, E., Liu, X., Serda, R. E. & Ferrari, M. Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc. Chem. Res. 44, 979–989 (2011).
Rodríguez, F. et al. Nano-based approved pharmaceuticals for cancer treatment: present and future challenges. Biomolecules 12, 784 (2022).
Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 14, 282–295 (2012).
Alshememry, A. K., Alsaleh, N. B., Alkhudair, N., Alzhrani, R. & Alshamsan, A. Recent nanotechnology advancements to treat multidrug-resistance pancreatic cancer: pre-clinical and clinical overview. Front. Pharmacol. 13, 933457 (2022).
van der Meel, R. et al. Smart cancer nanomedicine. Nat. Nanotechnol. 14, 1007–1017 (2019).
Kinnaird, A., Zhao, S., Wellen, K. E. & Michelakis, E. D. Metabolic control of epigenetics in cancer. Nat. Rev. Cancer 16, 694–707 (2016).
Zhao, Y., Butler, E. B. & Tan, M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 4, e532 (2013).
Hayashi, M. et al. GLUT1 inhibition by BAY-876 induces metabolic changes and cell death in human colorectal cancer cells. BMC Cancer 25, 716 (2025).
Chen, Q., Meng, Y. Q., Xu, X. F. & Gu, J. Blockade of GLUT1 by WZB117 resensitizes breast cancer cells to adriamycin. Anti-Cancer Drugs 28, 880–887 (2017).
Zhang, R. S., Li, Z. K., Liu, J., Deng, Y. T. & Jiang, Y. WZB117 enhanced the anti-tumor effect of apatinib against melanoma via blocking STAT3/PKM2 axis. Front. Pharmacol. 13, 976117 (2022).
Li, Y., Zeng, P., Xiao, J., Huang, P. & Liu, P. Modulation of energy metabolism to overcome drug resistance in chronic myeloid leukemia cells through induction of autophagy. Cell Death Discov. 8, 212 (2022).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug. Discov. 21, 141–162 (2022).
Mansi, J. L. et al. A phase II clinical and pharmacokinetic study of lonidamine in patients with advanced breast cancer. Br. J. Cancer 64, 593–597 (1991).
Raez, L. E. et al. A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 71, 523–530 (2013).
Lemberg, K. M., Vornov, J. J., Rais, R. & Slusher, B. S. We’re not ‘DON’ yet: optimal dosing and prodrug delivery of 6-diazo-5-oxo-l-norleucine. Mol. Cancer Ther. 17, 1824–1832 (2018).
Tenora, L. et al. Tumor-targeted delivery of 6-diazo-5-oxo-l-norleucine (DON) using substituted acetylated lysine prodrugs. J. Med. Chem. 62, 3524–3538 (2019).
Yang, W. H., Qiu, Y., Stamatatos, O., Janowitz, T. & Lukey, M. J. Enhancing the efficacy of glutamine metabolism inhibitors in cancer therapy. Trends Cancer 7, 790–804 (2021).
van Geldermalsen, M. et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35, 3201–3208 (2016).
Schulte, M. L. et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med. 24, 194–202 (2018).
Das, D. S. et al. Anti-myeloma activity of a novel glutaminase inhibitor CB-839. Blood 124, 3439–3439 (2014).
Tannir, N. M. et al. Phase 1 study of glutaminase (GLS) inhibitor CB-839 combined with either everolimus (E) or cabozantinib (Cabo) in patients (pts) with clear cell (cc) and papillary (pap) metastatic renal cell cancer (mRCC). J. Clin. Oncol. https://doi.org/10.1200/JCO.2018.36.6_suppl.603 (2018).
Tannir, N. M. et al. Efficacy and safety of telaglenastat plus cabozantinib vs placebo plus cabozantinib in patients with advanced renal cell carcinoma: the CANTATA randomized clinical trial. JAMA Oncol. 8, 1411–1418 (2022).
Lee, C. H. et al. Telaglenastat plus everolimus in advanced renal cell carcinoma: a randomized, double-blinded, placebo-controlled, phase II ENTRATA trial. Clin. Cancer Res. 28, 3248–3255 (2022).
Falchook, G. et al. First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors. EClinicalMedicine 34, 100797 (2021).
Lv, S. et al. Cerulenin suppresses ErbB2-overexpressing breast cancer by targeting ErbB2/PKM2 pathway. Med. Oncol. 40, 5 (2022).
Aquino, I. G. et al. Anticancer properties of the fatty acid synthase inhibitor TVB-3166 on oral squamous cell carcinoma cell lines. Arch. Oral Biol. 113, 104707 (2020).
Serhan, H. A. et al. Targeting fatty acid synthase in preclinical models of TNBC brain metastases synergizes with SN-38 and impairs invasion. NPJ Breast Cancer 10, 43 (2024).
Puig, T. et al. A novel inhibitor of fatty acid synthase shows activity against HER2+ breast cancer xenografts and is active in anti-HER2 drug-resistant cell lines. Breast Cancer Res. 13, R131 (2011).
Kelly, W. et al. Phase II investigation of TVB-2640 (denifanstat) with bevacizumab in patients with first relapse high-grade astrocytoma. Clin. Cancer Res. 29, 2419–2425 (2023).
Besse, B. et al. Amivantamab plus lazertinib in patients with EGFR-mutant NSCLC after progression on osimertinib and platinum-based chemotherapy: results from CHRYSALIS-2 Cohort A. J. Thorac. Oncol. 20, 651–664 (2025).
Corcoran, R. B. et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 8, 428–443 (2018).
Kopetz, S. et al. Encorafenib, binimetinib, and cetuximab in BRAFV600E-mutated colorectal cancer. N. Engl. J. Med. 381, 1632–1643 (2019).
Kopetz, S. et al. Encorafenib, cetuximab and chemotherapy in BRAF-mutant colorectal cancer: a randomized phase 3 trial. Nat. Med. 31, 901–908 (2025).
Zhang, L. et al. In vitro anti-leukemia activity of dual PI3K/mTOR inhibitor Voxtalisib on HL60 and K562 cells, as well as their multidrug resistance counterparts HL60/ADR and K562/A02 cells. Biomed. Pharmacother. 103, 1069–1078 (2018).
Martinez-Marti, A. et al. Dual MET and ERBB inhibition overcomes intratumor plasticity in osimertinib-resistant-advanced non-small-cell lung cancer (NSCLC). Ann. Oncol. 28, 2451–2457 (2017).
Yang, Y. M., Jang, Y., Lee, S. H., Kang, B. & Lim, S. M. AXL/MET dual inhibitor, CB469, has activity in non-small cell lung cancer with acquired resistance to EGFR TKI with AXL or MET activation. Lung Cancer 146, 70–77 (2020).
Brown, J. R. et al. Voxtalisib (XL765) in patients with relapsed or refractory non-Hodgkin lymphoma or chronic lymphocytic leukaemia: an open-label, phase 2 trial. Lancet Haematol. 5, e170–e180 (2018).
Smith, M. et al. Phase III study of cabozantinib in previously treated metastatic castration-resistant prostate cancer: COMET-1. J. Clin. Oncol. 34, 3005–3013 (2016).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Choueiri, T. K. et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann. Oncol. 25, 1603–1608 (2014).
Mason-Osann, E., Pomeroy, A. E., Palmer, A. C. & Mettetal, J. T. Synergistic drug combinations promote the development of resistance in acute myeloid leukemia. Blood Cancer Discov. 5, 95–105 (2024).
Abbott, M. & Ustoyev, Y. Cancer and the immune system: the history and background of immunotherapy. Semin. Oncol. Nurs. 35, 150923 (2019).
Yamaguchi, T. et al. Efficacy of chemotherapy plus immune checkpoint inhibitors in patients with non-small cell lung cancer who have rare oncogenic driver mutations: a retrospective analysis. BMC Cancer 24, 842 (2024).
Vafaei, S. et al. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 22, 2 (2022).
Li, B., Jin, J., Guo, D., Tao, Z. & Hu, X. Immune checkpoint inhibitors combined with targeted therapy: the recent advances and future potentials. Cancers 15, 2858 (2023).
Chen, N. et al. Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-Driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J. Thorac. Oncol. 10, 910–923 (2015).
Nishida, N. Role of oncogenic pathways on the cancer immunosuppressive microenvironment and its clinical implications in hepatocellular carcinoma. Cancers 13, 3666 (2021).
Walsh, S. R. et al. Endogenous T cells prevent tumor immune escape following adoptive T cell therapy. J. Clin. Invest. 129, 5400–5410 (2019).
Kankeu Fonkoua, L. A., Sirpilla, O., Sakemura, R., Siegler, E. L. & Kenderian, S. S. CAR T cell therapy and the tumor microenvironment: current challenges and opportunities. Mol. Ther. Oncolytics 25, 69–77 (2022).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
FDA. FDA grants accelerated approval to lifileucel for unresectable or metastatic melanoma. US FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-lifileucel-unresectable-or-metastatic-melanoma (2024).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Bishop, M. R. et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N. Engl. J. Med. 386, 629–639 (2022).
Moreau, P. et al. Teclistamab in relapsed or refractory multiple myeloma. N. Engl. J. Med. 387, 495–505 (2022).
FDA. FDA grants accelerated approval to talquetamab-tgvs for relapsed or refractory multiple myeloma. US FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-talquetamab-tgvs-relapsed-or-refractory-multiple-myeloma (2023).
Prieto-Vila, M., Takahashi, R. U., Usuba, W., Kohama, I. & Ochiya, T. Drug resistance driven by cancer stem cells and their niche. Int. J. Mol. Sci. 18, 2574 (2017).
De Francesco, E. M., Sotgia, F. & Lisanti, M. P. Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochem. J. 475, 1611–1634 (2018).
Yang, L. et al. Targeting cancer stem cell pathways for cancer therapy. Signal. Transduct. Target. Ther. 5, 8 (2020).
Catenacci, D. V. et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J. Clin. Oncol. 33, 4284–4292 (2015).
Schott, A. F. et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin. Cancer Res. 19, 1512–1524 (2013).
Locatelli, M. A. et al. Phase I study of the gamma secretase inhibitor PF-03084014 in combination with docetaxel in patients with advanced triple-negative breast cancer. Oncotarget 8, 2320–2328 (2017).
Richter, S. et al. A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575). Invest. N. Drugs 32, 243–249 (2014).
LoConte, N. K. et al. A multicenter phase 1 study of γ -secretase inhibitor RO4929097 in combination with capecitabine in refractory solid tumors. Invest. N. Drugs 33, 169–176 (2015).
Chen, C. L. et al. Profiling of circulating tumor cells for screening of selective inhibitors of tumor-initiating stem-like cells. Adv. Sci. 10, e2206812 (2023).
Walcher, L. et al. Cancer stem cells-origins and biomarkers: perspectives for targeted personalized therapies. Front. Immunol. 11, 1280 (2020).
Guzman, M. L. et al. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 98, 2301–2307 (2001).
Vergez, F. et al. High levels of CD34+CD38low/−CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: a Groupe Ouest-Est des Leucemies Aigues et Maladies du Sang (GOELAMS) study. Haematologica 96, 1792–1798 (2011).
Budde, L. E. et al. Abstract PR14: CD123CAR displays clinical activity in relapsed/refractory (r/r) acute myeloid leukemia (AML) and blastic plasmacytoid dendritic cell neoplasm (BPDCN): safety and efficacy results from a phase 1 study. Cancer Immunol. Res. 8, PR14–PR14 (2020).
Wang, Y. et al. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology 7, e1440169 (2018).
Li, D. et al. EpCAM-targeting CAR-T cell immunotherapy is safe and efficacious for epithelial tumors. Sci. Adv. 9, eadg9721 (2023).
Koseer, A. S., Di Gaetano, S., Arndt, C., Bachmann, M. & Dubrovska, A. Immunotargeting of cancer stem cells. Cancers 15, 1608 (2023).
Yan, Y., Zuo, X. & Wei, D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cell Transl. Med. 4, 1033–1043 (2015).
Menke-van der Houven van Oordt, C. W. et al. First-in-human phase I clinical trial of RG7356, an anti-CD44 humanized antibody, in patients with advanced, CD44-expressing solid tumors. Oncotarget 7, 80046–80058 (2016).
Riechelmann, H. et al. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral. Oncol. 44, 823–829 (2008).
Robinson, A. N. et al. Coexpression of ABCB1 and ABCG2 in a cell line model reveals both independent and additive transporter function. Drug. Metab. Dispos. 47, 715–723 (2019).
Cerovska, E. et al. ABC transporters are predictors of treatment failure in acute myeloid leukaemia. Biomed. Pharmacother. 170, 115930 (2024).
Ebner, J. et al. ABCC1 and glutathione metabolism limit the efficacy of BCL-2 inhibitors in acute myeloid leukemia. Nat. Commun. 14, 5709 (2023).
Garg, P. et al. Emerging therapeutic strategies to overcome drug resistance in cancer cells. Cancers 16, 100754 (2024).
Zhou, Y. et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal. Transduct. Target. Ther. 9, 132 (2024).
El Bairi, K., Atanasov, A. G., Amrani, M. & Afqir, S. The arrival of predictive biomarkers for monitoring therapy response to natural compounds in cancer drug discovery. Biomed. Pharmacother. 109, 2492–2498 (2019).
Sartore-Bianchi, A. et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat. Med. 28, 1612–1618 (2022).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Frei, E. et al. Studies of sequential and combination antimetabolite therapy in acute leukemia: 6-mercaptopurine and methotrexate. Blood 18, 431–454 (1961).
Ling, V. & Thompson, L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J. Cell Physiol. 83, 103–116 (1974).
Gros, P., Croop, J. & Housman, D. Mammalian multidrug resistance gene: complete cDNA sequence indicates strong homology to bacterial transport proteins. Cell 47, 371–380 (1986).
Lowe, S. W., Ruley, H. E., Jacks, T. & Housman, D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74, 957–967 (1993).
Cole, S. P., Downes, H. F. & Slovak, M. L. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br. J. Cancer 59, 42–46 (1989).
Cole, S. P. et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258, 1650–1654 (1992).
Doyle, L. A. et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl Acad. Sci. USA 95, 15665–15670 (1998).
Acknowledgements
X.Y.C. expresses thanks for the teaching fellowship from the Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, St. John’s University. The work of M.G. is supported by the National Natural Science Foundation of China (grant no. 82173855) and the Zhejiang Provincial Science and Technology Program (grant no. 2022R51002).
Author information
Authors and Affiliations
Contributions
M.G, X.-Y.C., P.H. J.S.F. and Z.-F.K. researched data for the article. M.G, X.-Y.C., D.-H.Y, Z.-F.K. and Z.-S.C. contributed substantially to discussion of the content. M.G, X.-Y.C., P.H. and Z.-X.W wrote the article. M.G, X.-Y.C., J.S.F., D.-H.Y, Z.-X.W, Z.-F.K. and Z.-S.C reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks C. Riganti, C.-P. Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ge, M., Chen, XY., Huang, P. et al. Understanding and overcoming multidrug resistance in cancer. Nat Rev Clin Oncol 22, 760–780 (2025). https://doi.org/10.1038/s41571-025-01059-1
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01059-1