Abstract
Chimeric antigen receptor (CAR) T cell therapy is revolutionizing the management of haematological malignancies but faces particular hurdles in the treatment of solid tumours. In this Review, we discuss important advances in refining CAR T cell therapy to provide practical clinical insights to address these challenges. We describe key strategies, including target antigen selection to enhance efficacy while minimizing on-target, off-tumour toxicities; early apheresis, rapid manufacturing and frontline application to preserve T cell fitness and ensure timely treatment; lymphodepletion to augment CAR T cell expansion; locoregional delivery to maximize local therapeutic concentrations and reduce systemic toxicity; and repeat infusions to prolong therapeutic effects. Furthermore, we discuss advanced response evaluation frameworks that will be essential for accurate assessment of the efficacy of CAR T cell therapies, and we highlight the need for robust toxicity management approaches to mitigate severe adverse events. By systematically addressing these multifaceted challenges, this Review provides a comprehensive guide for the optimization of CAR T cell therapy for solid tumours to enhance both efficacy and safety.
Key points
-
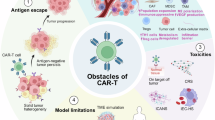
Chimeric antigen receptor (CAR) T cell therapy is effective for the treatment of haematological malignancies, although implementing this therapeutic modality in solid tumours faces substantial challenges.
-
Key hurdles include tumour heterogeneity, the immunosuppressive tumour microenvironment and a paucity of tumour-specific antigens.
-
Target selection should prioritize antigens with high tumour specificity, broad tumour coverage and stable expression to minimize on-target, off-tumour toxicity and ensure durable antitumour efficacy.
-
Strategies such as early apheresis, rapid manufacturing and frontline application of CAR T cell products aim to improve T cell fitness and expedite therapy, which will be crucial for patients with aggressive and/or rapidly progressing solid tumours.
-
Lymphodepletion and locoregional delivery methods can enhance CAR T cell expansion, trafficking and local efficacy, with the potential to reduce the risks of systemic adverse effects.
-
Novel approaches to efficacy evaluation (beyond RECIST) and proactive safety management protocols are essential for accurate assessment of response, distinguishing pseudoprogression and mitigating severe CAR T cell-related adverse events.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Brudno, J. N., Maus, M. V. & Hinrichs, C. S. CAR T cells and T-cell therapies for cancer: a translational science review. JAMA 332, 1924–1935 (2024).
Lu, Z. H. et al. The landscape of cancer research and cancer care in China. Nat. Med. 29, 3022–3032 (2023).
Saez-Ibañez, A. R. et al. The changing landscape of cancer cell therapies: clinical trials and real-world data. Nat. Rev. Drug Discov. 23, 736–737 (2024).
Martínez-Gamboa, D. A. et al. CAR T-cell therapy landscape in pediatric, adolescent and young adult oncology – a comprehensive analysis of clinical trials. Crit. Rev. Oncol. Hematol. 209, 104648 (2025).
Schaft, N. The landscape of CAR-T cell clinical trials against solid tumors — a comprehensive overview. Cancers 12, 2567 (2020).
Albelda, S. M. CAR T cell therapy for patients with solid tumours: key lessons to learn and unlearn. Nat. Rev. Clin. Oncol. 21, 47–66 (2023).
Du, B. et al. CAR-T therapy in solid tumors. Cancer Cell 43, 665–679 (2025).
Rosenberg, S. A. Finding suitable targets is the major obstacle to cancer gene therapy. Cancer Gene Ther. 21, 45–47 (2014).
Flugel, C. L. et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat. Rev. Clin. Oncol. 20, 49–62 (2022).
Smith, C. C. et al. Alternative tumour-specific antigens. Nat. Rev. Cancer 19, 465–478 (2019).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Poorebrahim, M. et al. TCR-like CARs and TCR-CARs targeting neoepitopes: an emerging potential. Cancer Gene Ther. 28, 581–589 (2021).
Liu, X. et al. Development of a TCR-like antibody and chimeric antigen receptor against NY-ESO-1/HLA-A2 for cancer immunotherapy. J. Immunother. Cancer 10, e004035 (2022).
Alsalloum, A. et al. Exploring TCR-like CAR-engineered lymphocyte cytotoxicity against MAGE-A4. Int. J. Mol. Sci. 24, 15134 (2023).
Tokatlian, T. et al. Chimeric antigen receptors directed at mutant KRAS exhibit an inverse relationship between functional potency and neoantigen selectivity. Cancer Res. Commun. 2, 58–65 (2022).
Liu, B. N., Yan, L. L. & Zhou, M. Target selection of CAR T cell therapy in accordance with the TME for solid tumors. Am. J. Cancer Res. 9, 228–241 (2019).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Haas, A. R. et al. Two cases of severe pulmonary toxicity from highly active mesothelin-directed CAR T cells. Mol. Ther. 31, 2309–2325 (2023).
Horna, P., Nowakowski, G., Endell, J. & Boxhammer, R. Comparative assessment of surface CD19 and CD20 expression on B-cell lymphomas from clinical biopsies: implications for targeted therapies. Blood 134, 5345 (2019).
Qi, C. S. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial final results. Nat. Med. 30, 2224–2234 (2024).
Chen, N., Li, X. Y., Chintala, N. K., Tano, Z. E. & Adusumilli, P. S. Driving CARs on the uneven road of antigen heterogeneity in solid tumors. Curr. Opin. Immunol. 51, 103–110 (2018).
Choi, E., Shin, J., Ryu, M.-H., Kim, H.-D. & Park, Y. S. Heterogeneity of claudin 18.2 expression in metastatic gastric cancer. Sci. Rep. 14, 17648 (2024).
Wei, J. S., Han, X., Bo, J. & Han, W. D. Target selection for CAR-T therapy. J. Hematol. Oncol. 12, 62 (2019).
Hirabayashi, K. et al. Dual-targeting CAR-T cells with optimal co-stimulation and metabolic fitness enhance antitumor activity and prevent escape in solid tumors. Nat. Cancer 2, 904–918 (2021).
Jiang, G. et al. Dual CAR-T cells to treat cancers co-expressing NKG2D and PD1 ligands in xenograft models of peritoneal metastasis. Cancer Immunol. Immunother. 72, 223–234 (2022).
Li, D. & Wang, W. Targeting hepatocellular carcinoma heterogeneity with FAP and GPC3-specific tandem CAR-T cells. Mol. Ther. Oncol. 32, 200859 (2024).
Han, X., Wang, Y., Wei, J. & Han, W. Multi-antigen-targeted chimeric antigen receptor T cells for cancer therapy. J. Hematol. Oncol. 12, 128 (2019).
Klampatsa, A. et al. Analysis and augmentation of the immunologic bystander effects of CAR T cell therapy in a syngeneic mouse cancer model. Mol. Ther. Oncolytics 18, 360–371 (2020).
Albelda, S. M. Tumor antigen heterogeneity: the “Elephant in the Room” of adoptive T-cell therapy for solid tumors. Cancer Immunol. Res. 8, 2 (2020).
Upadhyay, R. et al. A critical role for fas-mediated off-target tumor killing in T-cell immunotherapy. Cancer Discov. 11, 599–613 (2021).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Krenciute, G. et al. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res. 5, 571–581 (2017).
Qi, C. S. et al. 68 Ga-NC-BCH whole-body PET imaging rapidly targets claudin18.2 in lesions in gastrointestinal cancer patients. J. Nucl. Med. 65, 856–863 (2024).
Beatty, G. L. et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunol. Res. 2, 112–120 (2014).
Morello, A., Sadelain, M. & Adusumilli, P. S. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 6, 133–146 (2016).
Adusumilli, P. S. et al. A phase I trial of regional mesothelin-targeted CAR T-cell therapy in patients with malignant pleural disease, in combination with the anti-PD-1 agent pembrolizumab. Cancer Discov. 11, 2748–2763 (2021).
Punekar, S. R. et al. EVEREST-2: initial data of the logic-gated tmod chimeric antigen receptor T-cell (CAR T) therapy A2B694 for patients with solid tumors associated with mesothelin (MSLN) expression and with human leukocyte antigen (HLA) loss of heterozygosity (LOH). J. Clin. Oncol. 43, 3040–3040 (2025).
Cheng, W.-L. et al. The role of EREG/EGFR pathway in tumor progression. Int. J. Mol. Sci. 22, 12828 (2021).
Sigismund, S., Avanzato, D. & Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 12, 3–20 (2018).
Friedlaender, A. et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat. Rev. Clin. Oncol. 19, 51–69 (2022).
Feng, K. et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci. China Life Sci. 59, 468–479 (2016).
Liu, Y. et al. Anti-EGFR chimeric antigen receptor-modified T cells in metastatic pancreatic carcinoma: a phase I clinical trial. Cytotherapy 22, 573–580 (2020).
Pelloski, C. E. et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J. Clin. Oncol. 25, 2288–2294 (2007).
Zheng, L. et al. Intragenic EGFR::EGFR.E1E8 fusion (EGFRvIII) in 4331 solid tumors. Cancers 16, 6 (2023).
Choi, B. D. et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N. Engl. J. Med. 390, 1290–1298 (2024).
Bagley, S. J. et al. Repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab show no efficacy in glioblastoma: a phase 1 trial. Nat. Cancer 5, 517–531 (2024).
Gust, J. et al. Locoregional infusion of EGFR806-CAR T cells for recurrent or refractory pediatric CNS tumors: results of the completed BrainChild02 phase 1 clinical trial. Neuro-Oncol. 27, 2170–2181 (2025).
Goff, S. L. et al. Pilot trial of adoptive transfer of chimeric antigen receptor–transduced T cells targeting EGFRvIII in patients with glioblastoma. J. Immunother. 42, 126–135 (2019).
Nathanson, D. A. et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 343, 72–76 (2014).
Nazha, B., Inal, C. & Owonikoko, T. K. Disialoganglioside GD2 expression in solid tumors and role as a target for cancer therapy. Front. Oncol. 10, 1000 (2020).
Monje, M. et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature 637, 708–715 (2025).
Del Bufalo, F. et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N. Engl. J. Med. 388, 1284–1295 (2023).
Locatelli, F. et al. GD2-targeting CAR T cells in high-risk neuroblastoma: a phase 1/phase 2 trial. Nat. Med. https://doi.org/10.1038/s41591-025-03874-6 (2025).
Brown, C. E. et al. Glioma IL13Rα2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS ONE 8, e77769 (2013).
Thaci, B. et al. Significance of interleukin-13 receptor alpha 2–targeted glioblastoma therapy. Neuro-Oncol. 16, 1304–1312 (2014).
Brown, C. E. et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8+ T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 21, 4062–4072 (2015).
Brown, C. E. et al. Off-the-shelf, steroid-resistant, IL13Rα2-specific CAR T cells for treatment of glioblastoma. Neuro-Oncol. 24, 1318–1330 (2022).
Brown, C. E. et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: a phase 1 trial. Nat. Med. 30, 1001–1012 (2024).
Nakayama, I. et al. Claudin 18.2 as a novel therapeutic target. Nat. Rev. Clin. Oncol. 21, 354–369 (2024).
Wöll, S. et al. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int. J. Cancer 134, 731–739 (2013).
Qi, C. S. et al. Clinicopathological significance and immunotherapeutic outcome of claudin 18.2 expression in advanced gastric cancer: a retrospective study. Chin. J. Cancer Res. 36, 78–89 (2024).
Qi, C. S. et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat. Med. 28, 1189 (2022).
Qi, C. et al. Safety and efficacy of CT041 in patients with refractory metastatic pancreatic cancer: a pooled analysis of two early-phase trials. J. Clin. Oncol. 42, 2565–2577 (2024).
Qi, C. et al. Claudin-18 isoform 2-specific CAR T-cell therapy (satri-cel) versus treatment of physician’s choice for previously treated advanced gastric or gastro-oesophageal junction cancer (CT041-ST-01): a randomised, open-label, phase 2 trial. Lancet 405, 2049–2060 (2025).
Ushiku, T., Shinozaki-Ushiku, A., Maeda, D., Morita, S. & Fukayama, M. Distinct expression pattern of claudin-6, a primitive phenotypic tight junction molecule, in germ cell tumours and visceral carcinomas. Histopathology 61, 1043–1056 (2012).
Du, H., Yang, X., Fan, J. & Du, X. Claudin 6: therapeutic prospects for tumours, and mechanisms of expression and regulation (Review). Mol. Med. Rep. 24, 677 (2021).
Reinhard, K. et al. An RNA vaccine drives expansion and efficacy of claudin-CAR-T cells against solid tumors. Science 367, 446–453 (2020).
Mackensen, A. et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat. Med. 29, 2844–2853 (2023).
Carrithers, S. L. et al. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc. Natl Acad. Sci. USA 93, 14827–14832 (1996).
Birbe, R. et al. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum. Pathol. 36, 170–179 (2005).
Qi, C. S. et al. Antigen-independent activation is critical for the durable antitumor effect of GUCY2C-targeted CAR-T cells. J. Immunother. Cancer 12, e009960 (2024).
Qi, C. S. et al. Phase I study of GUCY2C CAR-T therapy IM96 in patients with metastatic colorectal cancer. J. Clin. Oncol. 42, 2518–2518 (2024).
Zhang, Q. et al. Novel GUCY2C targeting CAR-T therapy: efficacy in advanced colorectal cancer. J. Clin. Oncol. 41, 3559–3559 (2023).
Chen, N. F. et al. Chimeric antigen receptor T cells targeting CD19 and GCC in metastatic colorectal cancer a nonrandomized clinical trial. JAMA Oncol. 10, 1532–1536 (2024).
Baumhoer, D. et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am. J. Clin. Pathol. 129, 899–906 (2008).
Nakatsura, T., Ofuji, K., Saito, K. & Yoshikawa, T. Critical analysis of the potential of targeting GPC3 in hepatocellular carcinoma. J. Hepatocell. Carcinoma 1, 35–42 (2014).
Shi, D. H. et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin. Cancer Res. 26, 3979–3989 (2020).
Steffin, D. et al. Interleukin-15-armoured GPC3 CAR T cells for patients with solid cancers. Nature 637, 940–946 (2025).
Zhao, Z. et al. An armored GPC3-directed CAR-T for refractory or relapsed hepatocellular carcinoma in China: a phase I trial. J. Clin. Oncol. 39, 4095–4095 (2021).
Fu, Q. et al. RUNX-3-expressing CAR T cells targeting glypican-3 in patients with heavily pretreated advanced hepatocellular carcinoma: a phase I trial. eClinicalMedicine 63, 102175 (2023).
Zhang, Q. et al. Phase I study of C-CAR031, a GPC3-specific TGFβRIIDN armored autologous CAR-T, in patients with advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 42, 4019–4019 (2024).
Nakajima, T. E. et al. Updated results from first-in-human phase 1 dose-escalation trial of TAK-102, a GPC3-targeted armored CAR T cells, in patients with advanced solid tumors. J. Clin. Oncol. 42, 2543–2543 (2024).
Lin, X., Lee, S., Sharma, P., George, B. & Scott, J. Summary of US Food and Drug Administration chimeric antigen receptor (CAR) T-cell biologics license application approvals from a statistical perspective. J. Clin. Oncol. 40, 3501–3509 (2022).
Chen, S. & Liu, D. Rapid manufacturing of CAR-T therapy: strategies and impact. Trends Biotechnol. 43, 745–748 (2025).
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Harrer, D. C. et al. Apheresis for chimeric antigen receptor T-cell production in adult lymphoma patients. Transfusion 62, 1602–1611 (2022).
Baguet, C., Larghero, J. & Mebarki, M. Early predictive factors of failure in autologous CAR T-cell manufacturing and/or efficacy in hematologic malignancies. Blood Adv. 8, 337–342 (2024).
Das, R. K., Vernau, L., Grupp, S. A. & Barrett, D. M. Naive T-cell deficits at diagnosis and after chemotherapy impair cell therapy potential in pediatric cancers. Cancer Discov. 9, 492–499 (2019).
Dubnikov Sharon, T. et al. Early lymphocyte collection for anti-CD19 CART production improves T-cell fitness in patients with relapsed/refractory diffuse large B-cell lymphoma. Br. J. Haematol. 202, 74–85 (2023).
Levine, B. L., Miskin, J., Wonnacott, K. & Keir, C. Global manufacturing of CAR T cell therapy. Mol. Ther. Methods Clin. Dev. 4, 92–101 (2017).
Ayala Ceja, M., Khericha, M., Harris, C. M., Puig-Saus, C. & Chen, Y. Y. CAR-T cell manufacturing: major process parameters and next-generation strategies. J. Exp. Med. 221, e20230903 (2024).
Dickinson, M. J. et al. A novel autologous CAR-T therapy, YTB323, with preserved T-cell stemness shows enhanced CAR T-cell efficacy in preclinical and early clinical development. Cancer Discov. 13, 1982–1997 (2023).
Zhang, C. et al. Novel CD19 chimeric antigen receptor T cells manufactured next-day for acute lymphoblastic leukemia. Blood Cancer J. 12, 96 (2022).
Ghassemi, S. et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 6, 118 (2022).
Bui, T. A., Mei, H., Sang, R., Ortega, D. G. & Deng, W. Advancements and challenges in developing in vivo CAR T cell therapies for cancer treatment. eBioMedicine 106, 105266 (2024).
Short, L., Holt, R. A., Cullis, P. R. & Evgin, L. Direct in vivo CAR T cell engineering. Trends Pharmacol. Sci. 45, 406–418 (2024).
Xu, J. et al. In-vivo B-cell maturation antigen CAR T-cell therapy for relapsed or refractory multiple myeloma. Lancet 406, 228–231 (2025).
Hay, K. A. et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 130, 2295–2306 (2017).
Cohen, A. D. et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 12, 32 (2022).
Greenbaum, U. et al. CRP and ferritin in addition to the EASIX score predict CAR-T-related toxicity. Blood Adv. 5, 2799–2806 (2021).
Locke, F. L. et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N. Engl. J. Med. 386, 640–654 (2022).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Neelapu, S. S. et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat. Med. 28, 735–742 (2022).
Locke, F. L. et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 4, 4898–4911 (2020).
Meacham, C. E. & Morrison, S. J. Tumour heterogeneity and cancer cell plasticity. Nature 501, 328–337 (2013).
Yuan, Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb. Perspect. Med. 6, a026583 (2016).
Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018).
Pantel, K. & Alix-Panabières, C. Minimal residual disease as a target for liquid biopsy in patients with solid tumours. Nat. Rev. Clin. Oncol. 22, 65–77 (2024).
Uslu, U. et al. Chimeric antigen receptor T cells as adjuvant therapy for unresectable adenocarcinoma. Sci. Adv. 9, eade2526 (2023).
Hirayama, A. V. et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 133, 1876–1887 (2019).
Amini, L. et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat. Rev. Clin. Oncol. 19, 342–355 (2022).
Lickefett, B. et al. Lymphodepletion — an essential but undervalued part of the chimeric antigen receptor T-cell therapy cycle. Front. Immunol. 14, 1303935 (2023).
Ju, A., Choi, S., Jeon, Y. & Kim, K. Lymphodepletion in chimeric antigen receptor T-cell therapy for solid tumors: a focus on brain tumors. Brain Tumor Res. Treat. 12, 208–220 (2024).
Medina, T. et al. Long-term efficacy and safety of lifileucel tumor-infiltrating lymphocyte cell therapy in patients with advanced melanoma: a 5-year analysis of the C-144-01 study. J. Clin. Oncol. https://doi.org/10.1200/JCO-25-00765 (2025).
D’Angelo, S. P. et al. Afamitresgene autoleucel for advanced synovial sarcoma and myxoid round cell liposarcoma (SPEARHEAD-1): an international, open-label, phase 2 trial. Lancet 403, 1460–1471 (2024).
Heczey, A. et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol. Ther. 25, 2214–2224 (2017).
Haas, A. R. et al. Phase I study of lentiviral-transduced chimeric antigen receptor-modified T cells recognizing mesothelin in advanced solid cancers. Mol. Ther. 27, 1919–1929 (2019).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Dorff, T. B. et al. PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 30, 1636–1644 (2024).
Turtle, C. J. et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 8, 355ra116 (2016).
Gardner, R. A. et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129, 3322–3331 (2017).
Alvarez, R. et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br. J. Cancer 109, 926–933 (2013).
Von Hoff, D. D. et al. Gemcitabine plusnab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II Trial. J. Clin. Oncol. 29, 4548–4554 (2011).
Feng, K. C. et al. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell 9, 838–847 (2018).
Michels, T. et al. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in a TLR4-independent manner. J. Immunotoxicol. 9, 292–300 (2012).
Zhang, L. et al. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin. Immunol. 129, 219–229 (2008).
Wang, A. X., Ong, X. J., D’Souza, C., Neeson, P. J. & Zhu, J. J. Combining chemotherapy with CAR-T cell therapy in treating solid tumors. Front. Immunol. 14, 1140541 (2023).
Srivastava, S. et al. Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell 39, 193 (2021).
Cherkassky, L., Hou, Z. H., Amador-Molina, A. & Adusumilli, P. S. Regional CAR T cell therapy: an ignition key for systemic immunity in solid tumors. Cancer Cell 40, 569–574 (2022).
Sagnella, S. M. et al. Locoregional delivery of CAR-T cells in the clinic. Pharmacol. Res. 182, 106329 (2022).
Papa, S. et al. Intratumoral pan-ErbB targeted CAR-T for head and neck squamous cell carcinoma: interim analysis of the T4 immunotherapy study. J. Immunother. Cancer 11, e007162 (2023).
Zhang, H. et al. Phase I trial of hypoxia-responsive CEA CAR-T cell therapy in patients with heavily pretreated solid tumor via intraperitoneal or intravenous transfusion. J. Clin. Oncol. 42, 3514–3514 (2024).
Zygogianni, A. et al. From imaging to biology of glioblastoma: new clinical oncology perspectives to the problem of local recurrence. Clin. Transl. Oncol. 20, 989–1003 (2018).
Vitanza, N. A. et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat. Med. 27, 1544–1552 (2021).
Vitanza, N. A. et al. Intracerebroventricular B7-H3-targeting CAR T cells for diffuse intrinsic pontine glioma: a phase 1 trial. Nat. Med. 31, 861–868 (2025).
Gauthier, J. et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood 137, 323–335 (2021).
Locke, F. L. et al. Retreatment (reTx) of patients (pts) with refractory large B-cell lymphoma with axicabtagene ciloleucel (axi-cel) in ZUMA-1. J. Clin. Oncol. 38, 8012–8012 (2020).
Wagner, D. L. et al. Immunogenicity of CAR T cells in cancer therapy. Nat. Rev. Clin. Oncol. 18, 379–393 (2021).
Holland, E. M. et al. Efficacy of second CAR-T (CART2) infusion limited by poor CART expansion and antigen modulation. J. Immunother. Cancer 10, e004483 (2022).
Volpe, A., Adusumilli, P. S., Schöder, H. & Ponomarev, V. Imaging cellular immunotherapies and immune cell biomarkers: from preclinical studies to patients. J. Immunother. Cancer 10, e004902 (2022).
Yu, W. L. & Hua, Z. C. Chimeric antigen receptor T-cell (CAR T) therapy for hematologic and solid malignancies: efficacy and safety-a systematic review with meta-analysis. Cancers 11, 47 (2019).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Boursier, C., Perrin, M., Bordonne, M., Campidelli, A. & Verger, A. Early F-FDG PET flare-up phenomenon after CAR T-cell therapy in lymphoma. Clin. Nucl. Med. 47, E152–E153 (2022).
Wang, J. S. et al. Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non-Hodgkin lymphoma. Biol. Blood Marrow Transplant 25, 1092–1098 (2019).
Danylesko, I. et al. Immune imitation of tumor progression after anti-CD19 chimeric antigen receptor T cells treatment in aggressive B-cell lymphoma. Bone Marrow Transplant. 56, 1134–1143 (2020).
Cohen, D., Beyar-Katz, O., Even-Sapir, E. & Perry, C. Lymphoma pseudoprogression observed on [18F]FDG PET-CT scan 15 days after CAR-T infusion. Eur. J. Nucl. Med. Mol. Imaging 49, 2447–2449 (2022).
Mirza, A. S. et al. Incidence and management of effusions before and after CD19-directed chimeric antigen receptor (CAR) T cell therapy in large B cell lymphoma. Transplant. Cell. Ther. 27, 242.e1–242.e6 (2021).
Zhao, X. et al. Pseudoprogression in CAR-T cell therapy for solid tumor: case report. Front. Immunol. 15, 1504104 (2025).
Mahdi, J. et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 29, 803–810 (2023).
Burger, M. C. et al. Intracranial injection of natural killer cells engineered with a HER2-targeted chimeric antigen receptor in patients with recurrent glioblastoma. Neuro-Oncol. 25, 2058–2071 (2023).
Shao, F. Q. et al. Radionuclide-based molecular imaging allows CAR-T cellular visualization and therapeutic monitoring. Theranostics 11, 6800–6817 (2021).
Beatty, G. L. et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018).
Wang, S. J. et al. First-in-human CLDN18.2 functional diagnostic pet imaging of digestive system neoplasms enables whole-body target mapping and lesion detection. Eur. J. Nucl. Med. Mol. Imaging 50, 2802–2817 (2023).
Wittibschlager, V. et al. CAR T-cell persistence correlates with improved outcome in patients with B-cell lymphoma. Int. J. Mol. Sci. 24, 5688 (2023).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Bao, Y. T., Lv, M., Huang, X. J. & Zhao, X. Y. The mechanisms and countermeasures for CAR-T cell expansion and persistence deficiency. Cancer Lett. 626, 217771 (2025).
Hu, Y. F. & Huang, J. The chimeric antigen receptor detection toolkit. Front. Immunol. 11, 1770 (2020).
Van Hoeck, J., Vanhove, C., De Smedt, S. C. & Raemdonck, K. Non-invasive cell-tracking methods for adoptive T cell therapies. Drug Discov. Today 27, 793–807 (2022).
Skovgard, M. S. et al. Imaging CAR T-cell kinetics in solid tumors: translational implications. Mol. Ther. Oncolytics 22, 355–367 (2021).
Weist, M. R. et al. PET of adoptively transferred chimeric antigen receptor T cells with 89Zr-oxine. J. Nucl. Med. 59, 1531–1537 (2018).
Fröse, J. et al. Development of an antigen-based approach to noninvasively image CAR T cells in real time and as a predictive tool. Sci. Adv. 10, eadn3816 (2024).
Nikanjam, M., Kato, S. & Kurzrock, R. Liquid biopsy: current technology and clinical applications. J. Hematol. Oncol. 15, 131 (2022).
Huang, Z. et al. Liquid biopsy in T-cell lymphoma: biomarker detection techniques and clinical application. Mol. Cancer 23, 36 (2024).
Li, J. et al. Peripheral blood neutrophils contribute to Claudin18.2-specific CAR-T cell treatment resistance in advanced gastric cancer. Br. J. Cancer 132, 1167–1176 (2025).
Lee, J. H. et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti–programmed cell death 1 antibodies. JAMA Oncol. 4, 717–721 (2018).
Kubendran, S. et al. Circulating tumor DNA and association with CAR-T cell therapy response in gastric and pancreatic cancer patients. J. Clin. Oncol. 41, 4053–4053 (2023).
Botta, G. P. et al. Metastatic gastric cancer target lesion complete response with Claudin18.2-CAR T cells. J. Immunother. Cancer 12, e007927 (2024).
Lei, W. et al. Treatment-related adverse events of chimeric antigen receptor T-cell (CAR T) in clinical trials: a systematic review and meta-analysis. Cancers 13, 3912 (2021).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant 25, 625–638 (2019).
Maus, M. V. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J. Immunother. Cancer 8, e001511 (2020).
Schubert, M. L. et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann. Oncol. 32, 34–48 (2021).
Fajgenbaum, D. C. & June, C. H. Cytokine storm. N. Engl. J. Med. 383, 2255–2273 (2020).
Scala, J. J. et al. Treatment strategies for progressive immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome: case series. Haematologica 109, 3439–3445 (2024).
Ortí, G. et al. Less frequent complications following CAR T-cell therapies: hemophagocytic lymphohistiocytosis, graft-versus-host disease, thrombotic microangiopathy, coagulation disorders and secondary malignancies: best practice recommendations from the EBMT practice harmonization and guidelines committee. Bone Marrow Transplant. 60, 751–758 (2025).
Shi, J. et al. CAR-T therapy pulmonary adverse event profile: a pharmacovigilance study based on FAERS database (2017-2023). Front. Pharmacol. 15, 1434231 (2024).
Goldman, A. et al. Adverse cardiovascular and pulmonary events associated with chimeric antigen receptor T-cell therapy. J. Am. Coll. Cardiol. 78, 1800–1813 (2021).
MacKay, M. et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat. Biotechnol. 38, 233–244 (2020).
Jing, Y. et al. Expression of chimeric antigen receptor therapy targets detected by single-cell sequencing of normal cells may contribute to off-tumor toxicity. Cancer Cell 39, 1558–1559 (2021).
Brentjens, R., Yeh, R., Bernal, Y., Riviere, I. & Sadelain, M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol. Ther. 18, 666–668 (2010).
Uslu, U. & June, C. H. Beyond the blood: expanding CAR T cell therapy to solid tumors. Nat. Biotechnol. 43, 506–515 (2025).
Depil, S., Duchateau, P., Grupp, S. A., Mufti, G. & Poirot, L. Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat. Rev. Drug Discov. 19, 185–199 (2020).
Diorio, C., Teachey, D. T. & Grupp, S. A. Allogeneic chimeric antigen receptor cell therapies for cancer: progress made and remaining roadblocks. Nat. Rev. Clin. Oncol. 22, 10–27 (2025).
Wu, Z. et al. Glycan shielding enables TCR-sufficient allogeneic CAR-T therapy. Cell https://doi.org/10.1016/j.cell.2025.07.046 (2025).
Pal, S. K. et al. CD70-targeted allogeneic CAR T-cell therapy for advanced clear cell renal cell carcinoma. Cancer Discov. 14, 1176–1189 (2024).
Quintarelli, C. et al. Donor-derived GD2-specific CAR T cells in relapsed or refractory neuroblastoma. Nat. Med. 31, 849–860 (2025).
Lamers, C. H. J. et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 24, e20–e22 (2006).
Lamers, C. H. J. et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 21, 904–912 (2013).
Parkhurst, M. R. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19, 620–626 (2011).
Thistlethwaite, F. C. et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol. Immunother. 66, 1425–1436 (2017).
Guo, Y. L. et al. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin. Cancer Res. 24, 1277–1286 (2018).
Acknowledgements
The work of the authors is funded by the National Key Research and Development Program of China (No. 2022YFC2505006 and 2023YFC3403700 to L.S., and No. 2022YFA0912400 to C.Q.), the National Natural Science Foundation of China (No. 82522068 to C.Q. and No. U22A20327 to L.S.), the Beijing Natural Science Foundation (L232080 to C.Q.), the Beijing Hospitals Authority Youth Program (QMS20201101 to C.Q.), the Science Foundation of Peking University Cancer Hospital (JC202406 to C.Q.), the Clinical Medicine Plus X – Young Scholars Project of Peking University (to C.Q.), the Peking University Clinical Scientist Training Program (to C.Q.), and the Fundamental Research Funds for the Central Universities (to C.Q.).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
L.S. declares consulting or advisory roles for AstraZeneca, Boehringer Ingelheim, MSD, Servier and Transcenta Holding, and has received research funding (to the institution) from BeiGene and CARsgen. C.Q. declares consulting or advisory roles for AstraZeneca, Bayer, CARsgen, Imunopharm and Legend. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Liu, C., Zhang, P. et al. Optimizing CAR T cell therapy for solid tumours: a clinical perspective. Nat Rev Clin Oncol 22, 953–968 (2025). https://doi.org/10.1038/s41571-025-01075-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01075-1
This article is cited by
-
Impact of concomitant medications on efficacy of CLDN18.2-specific CAR-T cell therapy in advanced gastric cancer
British Journal of Cancer (2026)
-
Optimization of THP-1-CAR monocytes utilizing CD32a signaling phagocytosis for antigen-specific T cell activation
Scientific Reports (2026)