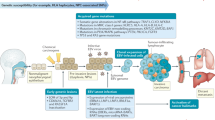
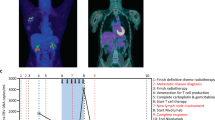
Abstract
Nasopharyngeal carcinoma (NPC) is a major disease burden in endemic regions, where Epstein–Barr virus (EBV) infection has a key aetiological role in this malignancy. Both plasma EBV DNA and serum antibodies targeting EBV antigens have been validated independently in large-scale prospective trials as effective biomarkers for early detection of NPC. Plasma EBV DNA analysis by PCR could identify patients with early-stage, asymptomatic NPC. Emergent studies have shown that fragmentomics analysis of plasma EBV DNA can further enhance the specificity of NPC detection at the time of testing and better predict the future risk of NPC. Initial antibody-based NPC screening approaches were based on the detection of immunoglobulin A antibodies targeting EBV viral capsid antigen or Epstein–Barr nuclear antigen 1, which resulted in a subsequent reduction in NPC-specific mortality in a population screening trial. Subsequently, the detection of anti-BNLF2b antibodies alone has been reported to achieve higher sensitivity and specificity relative to the dual antibody approach. Cost-effectiveness analyses support the implementation of NPC screening in endemic regions using either EBV DNA or antibodies. Ongoing research initiatives are focusing on developing prophylactic and therapeutic vaccines as preventive measures against EBV-associated diseases, including NPC. In this Review, we discuss these advances as well as their relevance for the implementation of prevention strategies such as population-wide NPC screening and vaccination in endemic areas of NPC prevalence. We also highlight valuable insights from plasma EBV DNA studies that might facilitate optimization of liquid biopsy-based screening strategies for other types of cancer.
Key points
-
The prevalence of nasopharyngeal carcinoma (NPC) is characterized by marked geographical and ethnic disparities, and is particularly high in southern China and Southeast Asia. NPC is strongly associated with Epstein–Barr virus (EBV) infection, prompting a focus on early detection and prevention, including strategies for screening using EBV-based biomarkers or the development of EBV vaccines, to alleviate the burden of NPC.
-
The quantification of plasma EBV DNA using real-time PCR assays or other sequencing-based methods provides a sensitive and specific biomarker for NPC screening, and evidence suggests that the initial test results can predict future risk of NPC development within the subsequent few years.
-
Quantification of serum antibodies targeting EBV antigens, including EBV viral capsid antigen, Epstein–Barr nuclear antigen 1 (EBNA1) and BNLF2b, has demonstrated promising screening performance, improving early diagnosis of NPC and reducing NPC-specific mortality by 30% in a population screening trial.
-
Further research and the development of risk models based on other EBV-based and human biomarkers, including specific EBV variants and human HLA genotypes, could refine NPC screening and the identification of populations at high risk, thereby enhancing the predictive value and cost-effectiveness of screening programmes.
-
Current efforts in EBV vaccine development focus on administering EBV envelope glycoproteins, such as gp350, gH–gL, gp42 and gB, or EBV proteins expressed in infected NPC cells that promote oncogenic properties, including EBNA1 and latent membrane protein 2. Nanoparticle-based and mRNA-based vaccines have demonstrated promising results in preclinical studies and are being tested in ongoing clinical trials.
-
Plasma EBV DNA-based and serological antibody-based approaches for NPC screening provide numerous insights for the development of blood-based multi-cancer early detection tests, highlighting the importance of longitudinal follow-up, increasing participation in screening programmes and leveraging multimodal-based detection methods.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wong, K. C. W. et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat. Rev. Clin. Oncol. 18, 679–695 (2021).
Chang, E. T. & Adami, H. O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 15, 1765–1777 (2006).
Young, L. S., Yap, L. F. & Murray, P. G. Epstein-Barr virus: more than 50 years old and still providing surprises. Nat. Rev. Cancer 16, 789–802 (2016).
zur Hausen, H. et al. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 228, 1056–1058 (1970).
Su, Z. Y., Siak, P. Y., Leong, C. O. & Cheah, S. C. The role of Epstein-Barr virus in nasopharyngeal carcinoma. Front. Microbiol. 14, 1116143 (2023).
National Cancer Registry Department. Summary of the Malaysia National Cancer Registry report 2017-2021. Ministry of Health https://nci.moh.gov.my/images/pdf_folder/SUMMARY-OF-MALAYSIA-NATIONAL-CANCER-REGISTRY-REPORT-2017-2021.pdf (2025).
Singapore Cancer Registry. Singapore Cancer Registry annual report 2022. National Registry of Diseases Office https://www.nrdo.gov.sg/publications/cancer (2024).
Au, K. H. et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral. Oncol. 77, 16–21 (2018).
Zou, X. et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur. J. Cancer 77, 117–126 (2017).
Liao, C.-H. et al. Quality of life as a mediator between cancer stage and long-term mortality in nasopharyngeal cancer patients treated with intensity-modulated radiotherapy. Cancers 13, 5063 (2021).
Hong, J. S., Tian, J., Han, Q. F. & Ni, Q. Y. Quality of life of nasopharyngeal cancer survivors in China. Curr. Oncol. 22, e142–e147 (2015).
Hong Kong Cancer Registry. Nasopharyngeal cancer in 2022. Hong Kong Cancer Registry, Hospital Authority. https://www3.ha.org.hk/cancereg/pdf/factsheet/2022/npc_2022.pdf (2022).
Mao, Y. P. et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 73, 1326–1334 (2009).
Alsavaf, M. B. et al. Patient characteristics and treatment outcomes of nasopharyngeal carcinoma in nonendemic regions. JAMA Netw. Open 8, e251895 (2025).
Blanchard, P. et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 16, 645–655 (2015).
Chang, E. T., Ye, W., Zeng, Y. X. & Adami, H. O. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 30, 1035–1047 (2021).
Jia, W. H. et al. Familial risk and clustering of nasopharyngeal carcinoma in Guangdong, China. Cancer 101, 363–369 (2004).
Liu, Z. et al. Quantification of familial risk of nasopharyngeal carcinoma in a high-incidence area. Cancer 123, 2716–2725 (2017).
Hsu, W. L. et al. Familial tendency and risk of nasopharyngeal carcinoma in Taiwan: effects of covariates on risk. Am. J. Epidemiol. 173, 292–299 (2011).
Loh, K. S., Goh, B. C., Lu, J., Hsieh, W. S. & Tan, L. Familial nasopharyngeal carcinoma in a cohort of 200 patients. Arch. Otolaryngol. Head Neck Surg. 132, 82–85 (2006).
Ng, W. T. et al. Familial nasopharyngeal carcinoma in Hong Kong: epidemiology and implication in screening. Fam. Cancer 8, 103–108 (2009).
Choi, C. W. et al. An analysis of the efficacy of serial screening for familial nasopharyngeal carcinoma based on Markov chain models. Fam. Cancer 10, 133–139 (2011).
Friborg, J. et al. Cancer susceptibility in nasopharyngeal carcinoma families — a population-based cohort study. Cancer Res. 65, 8567–8572 (2005).
Noel, C. W. et al. Association of immigration status and Chinese and South Asian ethnicity with incidence of head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 146, 1125–1135 (2020).
Wang, Q. et al. Racial and ethnic disparities in nasopharyngeal cancer with an emphasis among Asian Americans. Int. J. Cancer 151, 1291–1303 (2022).
Tse, K. P. et al. Genome-wide association study reveals multiple nasopharyngeal carcinoma-associated loci within the HLA region at chromosome 6p21.3. Am. J. Hum. Genet. 85, 194–203 (2009).
Bei, J. X. et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat. Genet. 42, 599–603 (2010).
Tang, M. et al. The principal genetic determinants for nasopharyngeal carcinoma in China involve the HLA class I antigen recognition groove. PLoS Genet. 8, e1003103 (2012).
Chin, Y. M. et al. HLA-A SNPs and amino acid variants are associated with nasopharyngeal carcinoma in Malaysian Chinese. Int. J. Cancer 136, 678–687 (2015).
He, Y. Q. et al. A polygenic risk score for nasopharyngeal carcinoma shows potential for risk stratification and personalized screening. Nat. Commun. 13, 1966 (2022).
Wang, T. M. et al. Whole-exome sequencing study of familial nasopharyngeal carcinoma and its implication for identifying high-risk individuals. J. Natl Cancer Inst. 114, 1689–1697 (2022).
Xu, M. et al. Host genetic variants, Epstein-Barr virus subtypes, and the risk of nasopharyngeal carcinoma: assessment of interaction and mediation. Cell Genom. 4, 100474 (2024).
Xu, M. et al. Genome sequencing analysis identifies Epstein-Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat. Genet. 51, 1131–1136 (2019).
Hui, K. F. et al. High risk Epstein-Barr virus variants characterized by distinct polymorphisms in the EBER locus are strongly associated with nasopharyngeal carcinoma. Int. J. Cancer 144, 3031–3042 (2019).
Barrett, D. et al. Past and recent salted fish and preserved food intakes are weakly associated with nasopharyngeal carcinoma risk in adults in Southern China. J. Nutr. 149, 1596–1605 (2019).
Feng, H. et al. Consumption of processed food and risk of nasopharyngeal carcinoma: a systematic review and meta-analysis. Transl. Cancer Res. 11, 872–879 (2022).
Zheng, Y. M. et al. Environmental and dietary risk factors for nasopharyngeal carcinoma: a case-control study in Zangwu county, Guangxi, China. Br. J. Cancer 69, 508–514 (1994).
Guo, X. et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of southern China. Int. J. Cancer 124, 2942–2947 (2009).
Armstrong, R. W. et al. Nasopharyngeal carcinoma in Malaysian Chinese: salted fish and other dietary exposures. Int. J. Cancer 77, 228–235 (1998).
Jia, W. H. et al. Traditional Cantonese diet and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer 10, 446 (2010).
Yong, S. K. et al. Associations of lifestyle and diet with the risk of nasopharyngeal carcinoma in Singapore: a case-control study. Chin. J. Cancer 36, 3 (2017).
Mai, Z. M. et al. Dietary fiber intake from fresh and preserved food and risk of nasopharyngeal carcinoma: observational evidence from a Chinese population. Nutr. J. 20, 14 (2021).
Lin, C. et al. Chinese nonmedicinal herbal diet and risk of nasopharyngeal carcinoma: a population-based case-control study. Cancer 125, 4462–4470 (2019).
Liu, Y. T. et al. Greater intake of fruit and vegetables is associated with lower risk of nasopharyngeal carcinoma in Chinese adults: a case-control study. Cancer Causes Control. 23, 589–599 (2012).
Polesel, J. et al. Consumption of fruit, vegetables, and other food groups and the risk of nasopharyngeal carcinoma. Cancer Causes Control. 24, 1157–1165 (2013).
Friborg, J. T. et al. A prospective study of tobacco and alcohol use as risk factors for pharyngeal carcinomas in Singapore Chinese. Cancer 109, 1183–1191 (2007).
Feng, R. et al. Intake of alcohol and tea and risk of nasopharyngeal carcinoma: a population-based case-control study in southern China. Cancer Epidemiol. Biomark. Prev. 30, 545–553 (2021).
Chen, L. et al. Alcohol consumption and the risk of nasopharyngeal carcinoma: a systematic review. Nutr. Cancer 61, 1–15 (2009).
Du, T. et al. Association between alcohol consumption and risk of nasopharyngeal carcinoma: a comprehensive meta-analysis of epidemiological studies. Alcohol. Clin. Exp. Res. 43, 2262–2273 (2019).
Lin, J. H. et al. Smoking and nasopharyngeal cancer: individual data meta-analysis of six prospective studies on 334 935 men. Int. J. Epidemiol. 50, 975–986 (2021).
Xue, W. Q., Qin, H. D., Ruan, H. L., Shugart, Y. Y. & Jia, W. H. Quantitative association of tobacco smoking with the risk of nasopharyngeal carcinoma: a comprehensive meta-analysis of studies conducted between 1979 and 2011. Am. J. Epidemiol. 178, 325–338 (2013).
Chang, E. T. et al. Active and passive smoking and risk of nasopharyngeal carcinoma: a population-based case-control study in southern China. Am. J. Epidemiol. 185, 1272–1280 (2017).
Hu, T. et al. Smoking can increase nasopharyngeal carcinoma risk by repeatedly reactivating Epstein-Barr virus: an analysis of a prospective study in southern China. Cancer Med. 8, 2561–2571 (2019).
Long, M., Fu, Z., Li, P. & Nie, Z. Cigarette smoking and the risk of nasopharyngeal carcinoma: a meta-analysis of epidemiological studies. BMJ Open 7, e016582 (2017).
Hsu, W. L. et al. Cigarette smoking increases the risk of nasopharyngeal carcinoma through the elevated level of IgA antibody against Epstein-Barr virus capsid antigen: a mediation analysis. Cancer Med. 9, 1867–1876 (2020).
Xu, F. H. et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein-Barr virus activation. J. Natl Cancer Inst. 104, 1396–1410 (2012).
Xie, S. H., Yu, I. T., Tse, L. A., Au, J. S. & Lau, J. S. Tobacco smoking, family history, and the risk of nasopharyngeal carcinoma: a case-referent study in Hong Kong Chinese. Cancer Causes Control. 26, 913–921 (2015).
Ji, X. et al. Nasopharyngeal carcinoma risk by histologic type in central China: impact of smoking, alcohol and family history. Int. J. Cancer 129, 724–732 (2011).
He, Y. Q. et al. Household inhalants exposure and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer 15, 1022 (2015).
Chen, Y. et al. Occupational exposures and risk of nasopharyngeal carcinoma in a high-risk area: a population-based case-control study. Cancer 127, 2724–2735 (2021).
E, M. et al. Wood dust exposure and risks of nasopharyngeal carcinoma: a meta-analysis. Eur. J. Public Health 30, 817–822 (2020).
Chen, Y. et al. Residence characteristics and risk of nasopharyngeal carcinoma in southern China: a population-based case-control study. Env. Int. 151, 106455 (2021).
Yang, T., Liu, Y., Zhao, W., Chen, Z. & Deng, J. Association of ambient air pollution with nasopharyngeal carcinoma incidence in ten large Chinese cities, 2006-2013. Int. J. Environ. Res. Public Health 17, 1824 (2020).
Fan, H. C. et al. Increased risk of incident nasopharyngeal carcinoma with exposure to air pollution. PLoS ONE 13, e0204568 (2018).
Huang, H. C. et al. Association between coarse particulate matter (PM(10-2.5)) and nasopharyngeal carcinoma among Taiwanese men. J. Investig. Med. 68, 419–424 (2020).
Chen, B. et al. Long-term trends in the burden of nasopharyngeal carcinoma in China: a comprehensive analysis from 1990 to 2021 and projections to 2030 based on the Global Burden of Disease Study 2021. Radiother. Oncol. 202, 110613 (2025).
Lo, Y. M. et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 59, 1188–1191 (1999).
Lam, W. K. J., Chan, K. C. A. & Lo, Y. M. D. Plasma Epstein-Barr virus DNA as an archetypal circulating tumour DNA marker. J. Pathol. 247, 641–649 (2019).
Chan, A. T. C. et al. Plasma Epstein-Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J. Natl Cancer Inst. 94, 1614–1619 (2002).
Lin, J. C. et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N. Engl. J. Med. 350, 2461–2470 (2004).
Leung, S. F. et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J. Clin. Oncol. 24, 5414–5418 (2006).
Chai, S. J. et al. Clinical significance of plasma Epstein-Barr virus DNA loads in a large cohort of Malaysian patients with nasopharyngeal carcinoma. J. Clin. Virol. 55, 34–39 (2012).
Lee, V. H. et al. Prognostication of serial post-intensity-modulated radiation therapy undetectable plasma EBV DNA for nasopharyngeal carcinoma. Oncotarget 8, 5292–5308 (2017).
Ma, B. B. et al. Relationship between pretreatment level of plasma Epstein-Barr virus DNA, tumor burden, and metabolic activity in advanced nasopharyngeal carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 66, 714–720 (2006).
Chan, K. C. et al. Investigation into the origin and tumoral mass correlation of plasma Epstein-Barr virus DNA in nasopharyngeal carcinoma. Clin. Chem. 51, 2192–2195 (2005).
Chan, K. C. A. et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N. Engl. J. Med. 377, 513–522 (2017).
Lee, A. W. et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int. J. Radiat. Oncol. Biol. Phys. 61, 1107–1116 (2005).
Chan, K. C. A. et al. Plasma Epstein-Barr virus DNA and risk of future nasopharyngeal cancer. NEJM Evid. 2, EVIDoa2200309 (2023).
Lam, W. K. J. et al. Recommendations for Epstein-Barr virus-based screening for nasopharyngeal cancer in high- and intermediate-risk regions. J. Natl Cancer Inst. 115, 355–364 (2023).
Kanakry, J. & Ambinder, R. The biology and clinical utility of EBV monitoring in blood. Curr. Top. Microbiol. Immunol. 391, 475–499 (2015).
Chan, K. C. et al. Early detection of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA analysis in a surveillance program. Cancer 119, 1838–1844 (2013).
Lam, W. K. J. et al. Methylation analysis of plasma DNA informs etiologies of Epstein-Barr virus-associated diseases. Nat. Commun. 10, 3256 (2019).
Chan, K. C. et al. Molecular characterization of circulating EBV DNA in the plasma of nasopharyngeal carcinoma and lymphoma patients. Cancer Res. 63, 2028–2032 (2003).
Chan, K. C. A., Chu, S. W. I. & Lo, Y. M. D. Ambient temperature and screening for nasopharyngeal cancer. N. Engl. J. Med. 378, 962–963 (2018).
Wang, H. Y. et al. Cancers screening in an asymptomatic population by using multiple tumour markers. PLoS ONE 11, e0158285 (2016).
Lam, W. K. J. et al. Sequencing-based counting and size profiling of plasma Epstein-Barr virus DNA enhance population screening of nasopharyngeal carcinoma. Proc. Natl Acad. Sci. USA 115, E5115–E5124 (2018).
Zhou, Q. et al. Epigenetic analysis of cell-free DNA by fragmentomic profiling. Proc. Natl Acad. Sci. USA 119, e2209852119 (2022).
Shaw, J. E., Levinger, L. F. & Carter, C. W. Jr. Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J. Virol. 29, 657–665 (1979).
Dyson, P. J. & Farrell, P. J. Chromatin structure of Epstein-Barr virus. J. Gen. Virol. 66(Pt 9), 1931–1940 (1985).
Arvey, A. et al. An atlas of the Epstein-Barr virus transcriptome and epigenome reveals host-virus regulatory interactions. Cell Host Microbe 12, 233–245 (2012).
Arvey, A., Tempera, I. & Lieberman, P. M. Interpreting the Epstein-Barr virus (EBV) epigenome using high-throughput data. Viruses 5, 1042–1054 (2013).
Johannsen, E. et al. Proteins of purified Epstein-Barr virus. Proc. Natl Acad. Sci. USA 101, 16286–16291 (2004).
Fernandez, A. F. et al. The dynamic DNA methylomes of double-stranded DNA viruses associated with human cancer. Genome Res. 19, 438–451 (2009).
Tempera, I. & Lieberman, P. M. Epigenetic regulation of EBV persistence and oncogenesis. Semin. Cancer Biol. 26, 22–29 (2014).
Woellmer, A. & Hammerschmidt, W. Epstein-Barr virus and host cell methylation: regulation of latency, replication and virus reactivation. Curr. Opin. Virol. 3, 260–265 (2013).
Lam, W. K. J. et al. Fragmentomics profiling and quantification of plasma Epstein-Barr virus DNA enhance prediction of future nasopharyngeal carcinoma. Cancer Cell 43, 728–739.e5 (2025).
Le, Q. T. et al. An international collaboration to harmonize the quantitative plasma Epstein-Barr virus DNA assay for future biomarker-guided trials in nasopharyngeal carcinoma. Clin. Cancer Res. 19, 2208–2215 (2013).
Yip, T. T., Ngan, R. K., Fong, A. H. & Law, S. C. Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral. Oncol. 50, 527–538 (2014).
Kim, K. Y. et al. Current state of PCR-based Epstein-Barr virus DNA testing for nasopharyngeal cancer. J. Natl Cancer Inst. 109, djx007 (2017).
Zeng, Y. et al. Prospective studies on nasopharyngeal carcinoma in Epstein-Barr virus IgA/VCA antibody-positive persons in Wuzhou city, China. Int. J. Cancer 36, 545–547 (1985).
Ji, M. F. et al. Sustained elevation of Epstein-Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br. J. Cancer 96, 623–630 (2007).
Cao, S. M. et al. Fluctuations of epstein-barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS ONE 6, e19100 (2011).
Zeng, Y. et al. Serological mass survey for early detection of nasopharyngeal carcinoma in Wuzhou city, China. Int. J. Cancer 29, 139–141 (1982).
Yao, J. J. et al. Prognostic value of serum Epstein-Barr virus antibodies in patients with nasopharyngeal carcinoma and undetectable pretreatment Epstein-Barr virus DNA. Cancer Sci. 108, 1640–1647 (2017).
Liu, Z. et al. Two Epstein-Barr virus-related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in southern China. Am. J. Epidemiol. 177, 242–250 (2013).
Ji, M. F. et al. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China. Ann. Oncol. 30, 1630–1637 (2019).
Chen, W. J. et al. Impact of an Epstein-Barr virus serology-based screening program on nasopharyngeal carcinoma mortality: a cluster-randomized controlled trial. J. Clin. Oncol. 43, 22–31 (2025).
Li, T. et al. Anti-Epstein-Barr virus BNLF2b for mass screening for nasopharyngeal cancer. N. Engl. J. Med. 389, 808–819 (2023).
Lam, W. K. J. & Chan, A. T. C. Nasopharyngeal cancer screening with an anti-BNLF2b antibody: a new arrow in the quiver? Nat. Rev. Clin. Oncol. 21, 6–7 (2024).
Liu, Z. et al. Multilaboratory assessment of Epstein-Barr virus serologic assays: the case for standardization. J. Clin. Microbiol. 57, e01107-19 (2019).
Lou, P. J. et al. Performance and operational feasibility of Epstein-Barr virus-based screening for detection of nasopharyngeal carcinoma: direct comparison of two alternative approaches. J. Clin. Oncol. 41, 4257–4266 (2023).
Lam, W. K. J. et al. Sequencing analysis of plasma Epstein-Barr virus DNA reveals nasopharyngeal carcinoma-associated single nucleotide variant profiles. Clin. Chem. 66, 598–605 (2020).
Cui, Q. et al. An extended genome-wide association study identifies novel susceptibility loci for nasopharyngeal carcinoma. Hum. Mol. Genet. 25, 3626–3634 (2016).
Zhou, X. et al. A comprehensive risk score for effective risk stratification and screening of nasopharyngeal carcinoma. Nat. Commun. 12, 5189 (2021).
Chen, G. H. et al. Prospective assessment of a nasopharyngeal carcinoma risk score in a population undergoing screening. Int. J. Cancer 148, 2398–2406 (2021).
Chen, G. H. et al. Utility of Epstein-Barr virus DNA in nasopharynx swabs as a reflex test to triage seropositive individuals in nasopharyngeal carcinoma screening programs. Clin. Chem. 68, 953–962 (2022).
Simon, J. et al. Validation of an Epstein-Barr virus antibody risk stratification signature for nasopharyngeal carcinoma by use of multiplex serology. J. Clin. Microbiol. 58, e00077-20 (2020).
Yang, L. et al. Prospective evaluation of the relevance of Epstein-Barr virus antibodies for early detection of nasopharyngeal carcinoma in Chinese adults. Int. J. Epidemiol. 53, dyae098 (2024).
King, A. D. et al. Magnetic resonance imaging for the detection of nasopharyngeal carcinoma. AJNR Am. J. Neuroradiol. 27, 1288–1291 (2006).
King, A. D. et al. Primary nasopharyngeal carcinoma: diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 258, 531–537 (2011).
King, A. D. et al. Detection of nasopharyngeal carcinoma by MR imaging: diagnostic accuracy of MRI compared with endoscopy and endoscopic biopsy based on long-term follow-up. AJNR Am. J. Neuroradiol. 36, 2380–2385 (2015).
King, A. D. et al. Complementary roles of MRI and endoscopic examination in the early detection of nasopharyngeal carcinoma. Ann. Oncol. 30, 977–982 (2019).
Liu, Z. et al. Comparison of new magnetic resonance imaging grading system with conventional endoscopy for the early detection of nasopharyngeal carcinoma. Cancer 127, 3403–3412 (2021).
King, A. D. et al. Early detection of nasopharyngeal carcinoma: performance of a short contrast-free screening magnetic resonance imaging. J. Natl Cancer Inst. 116, 665–672 (2024).
Ke, L. et al. Development of a self-constrained 3D DenseNet model in automatic detection and segmentation of nasopharyngeal carcinoma using magnetic resonance images. Oral. Oncol. 110, 104862 (2020).
Wong, L. M. et al. Convolutional neural network for discriminating nasopharyngeal carcinoma and benign hyperplasia on MRI. Eur. Radiol. 31, 3856–3863 (2021).
Miller, J. A., Le, Q. T., Pinsky, B. A. & Wang, H. Cost-effectiveness of nasopharyngeal carcinoma screening with Epstein-Barr virus polymerase chain reaction or serology in high-incidence populations worldwide. J. Natl Cancer Inst. 113, 852–862 (2021).
Miller, J. A. et al. Optimization and local cost-effectiveness of nasopharyngeal carcinoma screening strategies in southern China: secondary analysis of the guangdong randomized trial. Cancer Epidemiol. Biomark. Prev. 33, 884–895 (2024).
Clark, P. E., Taparra, K. & Miller, J. A. Identification of high-incidence populations in the United States for anti-Epstein-Barr virus serologic screening for nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 33, 1706–1716 (2024).
Zhong, L., Zhao, Q., Zeng, M. S. & Zhang, X. Prophylactic vaccines against Epstein-Barr virus. Lancet 404, 845 (2024).
Sun, C., Chen, X. C., Kang, Y. F. & Zeng, M. S. The status and prospects of Epstein-Barr virus prophylactic vaccine development. Front. Immunol. 12, 677027 (2021).
Jackman, W. T., Mann, K. A., Hoffmann, H. J. & Spaete, R. R. Expression of Epstein-Barr virus gp350 as a single chain glycoprotein for an EBV subunit vaccine. Vaccine 17, 660–668 (1999).
Morgan, A. J., Finerty, S., Lovgren, K., Scullion, F. T. & Morein, B. Prevention of Epstein-Barr (EB) virus-induced lymphoma in cottontop tamarins by vaccination with the EB virus envelope glycoprotein gp340 incorporated into immune-stimulating complexes. J. Gen. Virol. 69(Pt 8), 2093–2096 (1988).
Finerty, S. et al. Immunization of cottontop tamarins and rabbits with a candidate vaccine against the Epstein-Barr virus based on the major viral envelope glycoprotein gp340 and alum. Vaccine 12, 1180–1184 (1994).
Finerty, S. et al. Protective immunization against Epstein-Barr virus-induced disease in cottontop tamarins using the virus envelope glycoprotein gp340 produced from a bovine papillomavirus expression vector. J. Gen. Virol. 73(Pt 2), 449–453 (1992).
Servat, E. et al. Identification of the critical attribute(s) of EBV gp350 antigen required for elicitation of a neutralizing antibody response in vivo. Vaccine 33, 6771–6777 (2015).
Sokal, E. M. et al. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J. Infect. Dis. 196, 1749–1753 (2007).
Rees, L. et al. A phase I trial of Epstein-Barr virus gp350 vaccine for children with chronic kidney disease awaiting transplantation. Transplantation 88, 1025–1029 (2009).
Moutschen, M. et al. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine 25, 4697–4705 (2007).
Gu, S. Y. et al. First EBV vaccine trial in humans using recombinant vaccinia virus expressing the major membrane antigen. Dev. Biol. Stand. 84, 171–177 (1995).
Cui, X. et al. A novel tetrameric gp350 1-470 as a potential Epstein-Barr virus vaccine. Vaccine 31, 3039–3045 (2013).
Bu, W. et al. Immunization with components of the viral fusion apparatus elicits antibodies that neutralize Epstein-Barr virus in B cells and epithelial cells. Immunity 50, 1305–1316.e6 (2019).
Liu, C. et al. GB and gH/gL fusion machinery: a promising target for vaccines to prevent Epstein-Barr virus infection. Arch. Virol. 169, 167 (2024).
Cui, X. et al. Rabbits immunized with Epstein-Barr virus gH/gL or gB recombinant proteins elicit higher serum virus neutralizing activity than gp350. Vaccine 34, 4050–4055 (2016).
Nguyen, B. & Tolia, N. H. Protein-based antigen presentation platforms for nanoparticle vaccines. NPJ Vaccines 6, 70 (2021).
Kanekiyo, M. et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell 162, 1090–1100 (2015).
Malhi, H. et al. Immunization with a self-assembling nanoparticle vaccine displaying EBV gH/gL protects humanized mice against lethal viral challenge. Cell Rep. Med. 3, 100658 (2022).
Wei, C. J. et al. A bivalent Epstein-Barr virus vaccine induces neutralizing antibodies that block infection and confer immunity in humanized mice. Sci. Transl. Med. 14, eabf3685 (2022).
Sun, C. et al. A gB nanoparticle vaccine elicits a protective neutralizing antibody response against EBV. Cell Host Microbe 31, 1882–1897.e10 (2023).
Edwards, K. R. et al. A gH/gL-encoding replicon vaccine elicits neutralizing antibodies that protect humanized mice against EBV challenge. NPJ Vaccines 9, 120 (2024).
Kang, M. S. & Kieff, E. Epstein-Barr virus latent genes. Exp. Mol. Med. 47, e131 (2015).
Hui, E. P. et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 73, 1676–1688 (2013).
Zhao, G. X. et al. mRNA-based vaccines targeting the T-cell epitope-rich domain of Epstein Barr virus latent proteins elicit robust anti-tumor immunity in mice. Adv. Sci. 10, e2302116 (2023).
Oxman, M. N. et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 352, 2271–2284 (2005).
Rubinstein, W. S. et al. Cancer screening with multicancer detection tests: a translational science review. CA Cancer J. Clin. 74, 368–382 (2024).
Wan, J. C. M. et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat. Rev. Cancer 17, 223–238 (2017).
Lennon, A. M. et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 369, eabb9601 (2020).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Lo, Y. M. D., Han, D. S. C., Jiang, P. & Chiu, R. W. K. Epigenetics, fragmentomics, and topology of cell-free DNA in liquid biopsies. Science 372, eaaw3616 (2021).
Thierry, A. R. Circulating DNA fragmentomics and cancer screening. Cell Genom. 3, 100242 (2023).
Jiang, P. et al. Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc. Natl Acad. Sci. USA 112, E1317–E1325 (2015).
Chan, K. C. et al. Second generation noninvasive fetal genome analysis reveals de novo mutations, single-base parental inheritance, and preferred DNA ends. Proc. Natl Acad. Sci. USA 113, E8159–E8168 (2016).
Jiang, P. et al. Preferred end coordinates and somatic variants as signatures of circulating tumor DNA associated with hepatocellular carcinoma. Proc. Natl Acad. Sci. USA 115, E10925–E10933 (2018).
Jiang, P. et al. Plasma DNA end-motif profiling as a fragmentomic marker in cancer, pregnancy, and transplantation. Cancer Discov. 10, 664–673 (2020).
Nicholson, B. D. et al. Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): a large-scale, observational cohort study. Lancet Oncol. 24, 733–743 (2023).
Schrag, D. et al. Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study. Lancet 402, 1251–1260 (2023).
Castle, P. E. & Han, P. K. J. On the nose: reducing nasopharyngeal cancer-related mortality using risk-based Epstein-Barr virus serology screening. J. Clin. Oncol. 43, 1–3 (2025).
Ng, C. C. et al. A genome-wide association study identifies ITGA9 conferring risk of nasopharyngeal carcinoma. J. Hum. Genet. 54, 392–397 (2009).
Wang, T. M. et al. High-throughput identification of regulatory elements and functional assays to uncover susceptibility genes for nasopharyngeal carcinoma. Am. J. Hum. Genet. 110, 1162–1176 (2023).
Acknowledgements
W.K.J.L., Y.M., and K.C.A.C. receive research support from the Innovation and Technology Fund under the InnoHK Initiative, a major initiative of the Hong Kong Special Administrative Region Government, and the Research Grants Council of the Hong Kong SAR Government under the NSFC/RGC Joint Research Scheme (N_CUHK495/22).
Author information
Authors and Affiliations
Contributions
All authors contributed to all aspects of preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
W.K.J.L. holds equity in Illumina. K.C.A.C. holds equity in DRA, Illumina, Insighta and Take2. B.B.Y.M., A.D.K., Y.M. and A.T.C.C. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks S. Franceschi and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lam, W.K.J., Ma, B.B.Y., King, A.D. et al. Achieving control of nasopharyngeal carcinoma: the role of Epstein–Barr virus-based screening and vaccines. Nat Rev Clin Oncol 23, 7–21 (2026). https://doi.org/10.1038/s41571-025-01079-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01079-x