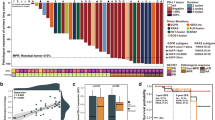
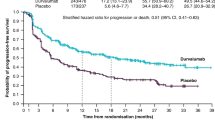
Abstract
Despite advances in immunotherapy, unresectable stage III non-small-cell lung cancer (NSCLC) remains a highly challenging disease, with only around one-third of patients remaining disease-free at 5 years. The PACIFIC trial established consolidation with the anti-PD-L1 antibody durvalumab after concurrent chemoradiotherapy as the standard-of-care approach. Furthermore, the LAURA trial has redefined the treatment of patients with stage III unresectable EGFR-mutant NSCLC, demonstrating unprecedented progression-free survival durations with osimertinib consolidation. Despite these advances, novel approaches are urgently needed. Circulating tumour DNA-based monitoring of minimal residual disease is emerging as a personalized method of tailoring treatment duration and escalation strategies. Novel radiotherapy techniques have the potential to provide synergy with immunotherapy while minimizing toxicities. Additionally, ongoing trials evaluating chemoimmunotherapy combinations adapted from the neoadjuvant setting with the potential for conversion to resectable disease might, in the near future, redefine the boundary of surgical resectability. In this Review, we describe the rapidly evolving field of unresectable stage III NSCLC, providing a state-of-the-art overview that includes challenging topics such as biomarkers, personalization of therapy and the role of immunotherapy rechallenge.
Key points
-
The PACIFIC regimen, comprising concurrent chemoradiotherapy followed by consolidation with the anti-PD-L1 antibody durvalumab, remains the standard-of-care treatment for patients with unresectable EGFR and ALK wild-type stage III non-small-cell lung cancer (NSCLC).
-
In patients with stage III unresectable EGFR-mutant NSCLC, consolidation with a third-generation EGFR tyrosine kinase inhibitor improves progression-free survival and reduces the risk of central nervous system progression.
-
Current data do not support the addition of an immune-checkpoint inhibitor to concurrent chemoradiotherapy owing to the increased risk of toxicities as well as limited evidence of improved efficacy.
-
Limited data are available on the efficacy of induction chemoimmunotherapy in patients with unresectable stage III NSCLC, although prospective data from large-cohort phase III trials will be needed before implementation in clinical practice.
-
Strategies for patients with unresectable stage III NSCLC who are ineligible for chemotherapy are being explored in phase II trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 74, 12–49 (2024).
Postmus, P. E. et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 28, iv1–iv21 (2017).
Forde, P. M. et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386, 1973–1985 (2022).
Spicer, J. D. et al. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Lond. Engl. 404, 1240–1252 (2024).
Heymach, J. V. et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N. Engl. J. Med. 389, 1672–1684 (2023).
Cascone, T. et al. Perioperative nivolumab in resectable lung cancer. N. Engl. J. Med. 390, 1756–1769 (2024).
O’Brien, M. et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 23, 1274–1286 (2022).
Felip, E. et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet Lond. Engl. 398, 1344–1357 (2021).
Dingemans, A.-M. et al. OA06.05 consensual definition of stage III NSCLC resectability: EORTC-Lung Cancer Group Initiative with other scientific societies. J. Thorac. Oncol. 18, S57–S58 (2023).
Seung, S. J., Hurry, M., Walton, R. N. & Evans, W. K. Retrospective cohort study of unresectable stage III non-small-cell lung cancer in Canada. Curr. Oncol. 27, e354–e360 (2020).
Agbarya, A. et al. Real-world journey of unresectable stage III NSCLC patients: current dilemmas for disease staging and treatment. J. Clin. Med. 11, 1738 (2022).
Aupérin, A. et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 28, 2181–2190 (2010).
Cheema, P. K., Rothenstein, J., Melosky, B., Brade, A. & Hirsh, V. Perspectives on treatment advances for stage III locally advanced unresectable non-small-cell lung cancer. Curr. Oncol. 26, 37–42 (2019).
Bradley, J. D. et al. Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J. Clin. Oncol. 38, 706–714 (2020).
Kelly, K. et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J. Clin. Oncol. 26, 2450–2456 (2008).
Ready, N. et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: Cancer and Leukemia Group B (CALEB) 30106, a CALGB-stratified phase II trial. J. Thorac. Oncol. 5, 1382–1390 (2010).
Senan, S. et al. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 34, 953–962 (2016).
Higgins, K. A., Puri, S. & Gray, J. E. Systemic and radiation therapy approaches for locally advanced non-small-cell lung cancer. J. Clin. Oncol. 40, 576–585 (2022).
Spigel, D. R. et al. Five-year survival outcomes from the PACIFIC Trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 40, 1301–1311 (2022).
Lu, S. et al. Osimertinib after chemoradiotherapy in stage III EGFR-mutated NSCLC. N. Engl. J. Med. 391, 585–597 (2024).
Yu, J. et al. PL04.13 aumolertinib maintenance after chemoradiotherapy in stage III non-small-cell lung cancer: interim results of the phase III study (POLESTAR). J. Thorac. Oncol. 19, S6–S7 (2024).
Lynch, C., Pitroda, S. P. & Weichselbaum, R. R. Radiotherapy, immunity, and immune checkpoint inhibitors. Lancet Oncol. 25, e352–e362 (2024).
Mondini, M., Levy, A., Meziani, L., Milliat, F. & Deutsch, E. Radiotherapy-immunotherapy combinations — perspectives and challenges. Mol. Oncol. 14, 1529–1537 (2020).
Spaas, M. & Lievens, Y. Is the combination of immunotherapy and radiotherapy in non-small cell lung cancer a feasible and effective approach? Front. Med. 6, 244 (2019).
McLaughlin, M. et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 20, 203–217 (2020).
Deng, L. et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 41, 843–852 (2014).
Durante, M. & Formenti, S. C. Radiation-induced chromosomal aberrations and immunotherapy: micronuclei, cytosolic DNA, and interferon-production pathway. Front. Oncol. 8, 192 (2018).
Formenti, S. C. & Demaria, S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J. Natl Cancer Inst. 105, 256–265 (2013).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Deutsch, E. & Levy, A. Eradicating gross tumor disease: a prerequisite for efficient radioimmunotherapy? J. Natl Cancer Inst. 116, 1008–1011 (2024).
Telarovic, I. et al. Delayed tumor-draining lymph node irradiation preserves the efficacy of combined radiotherapy and immune checkpoint blockade in models of metastatic disease. Nat. Commun. 15, 5500 (2024).
Vanpouille-Box, C. et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 8, 15618 (2017).
Laurent, P. A. et al. Low-dose radiotherapy enhances the efficacy of PD-L1 blockade and induces the abscopal effect. J. Immunother. Cancer 13, e011487 (2025).
Schoenfeld, J. D. et al. Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 23, 279–291 (2022).
Venkatesulu, B. P., Mallick, S., Lin, S. H. & Krishnan, S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit. Rev. Oncol. Hematol. 123, 42–51 (2018).
Deutsch, E., Chargari, C., Galluzzi, L. & Kroemer, G. Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol. 20, e452–e463 (2019).
Peters, S. et al. 65O CheckMate 73L: phase III study comparing nivolumab (N) + concurrent chemoradiotherapy (CCRT) followed by N ± ipilimumab (I) v CCRT followed by durvalumab (D) for previously untreated, locally advanced stage (stg) III NSCLC. Immuno-Oncol. Technol. 24, 100808 (2024).
Cortiula, F. et al. Proton and photon radiotherapy in stage III NSCLC: effects on hematological toxicity and adjuvant immune therapy. Radiother. Oncol. 190, 110019 (2024).
Durm, G. A. et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer 126, 4353–4361 (2020).
Naidoo, J. et al. Characterizing immune-mediated adverse events with durvalumab in patients with unresectable stage III NSCLC: a post-hoc analysis of the PACIFIC trial. Lung Cancer Amst. Neth. 166, 84–93 (2022).
Hui, R. et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 20, 1670–1680 (2019).
Faivre-Finn, C. et al. Impact of prior chemoradiotherapy-related variables on outcomes with durvalumab in unresectable stage III NSCLC (PACIFIC). Lung Cancer Amst. Neth. 151, 30–38 (2021).
Paz-Ares, L. et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 31, 798–806 (2020).
Garassino, M. C. et al. Durvalumab after sequential chemoradiotherapy in unresectable stage III non-small-cell lung cancer-final analysis from the phase II PACIFIC-6 trial. ESMO Open 10, 105071 (2025).
Wu, Y.-L. et al. LBA6 PACIFIC-5: a phase III study of consolidation durvalumab (D) in patients (pts) with unresectable stage III NSCLC and no progression after concurrent or sequential chemoradiotherapy (cCRT or sCRT). Ann. Oncol. 35, S1624 (2024).
Park, C.-K. et al. Korean real-world data on patients with unresectable stage III NSCLC treated with durvalumab after chemoradiotherapy: PACIFIC-KR. J. Thorac. Oncol. 18, 1042–1054 (2023).
Park, J. E. et al. Durvalumab consolidation after chemoradiotherapy in elderly patients with unresectable stage III NSCLC: a real-world multicenter study. Clin. Lung Cancer 25, 354–364 (2024).
S, S. et al. Real world efficacy and toxicity of consolidation durvalumab following chemoradiotherapy in older Australian patients with unresectable stage III non-small cell lung cancer. J. Geriatr. Oncol. 15, 101705 (2024).
Jung, H. A. et al. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer Amst. Neth. 146, 23–29 (2020).
Waterhouse, D. et al. Durvalumab real-world treatment patterns and outcomes in patients with stage III non-small-cell lung cancer treated in a US community setting. Future Oncol. Lond. Engl. 19, 1905–1916 (2023).
Zehentmayr, F. et al. Durvalumab impacts progression-free survival while high-dose radiation >66 Gy improves local control without excess toxicity in unresectable NSCLC stage III: real-world data from the Austrian Radio-oncological Lung Cancer Study Association Registry (ALLSTAR). Radiother. Oncol. 196, 110294 (2024).
Faehling, M. et al. Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non-small cell lung cancer (NSCLC): real-world data on survival and safety from the German Expanded-Access Program (EAP). Lung Cancer Amst. Neth. 150, 114–122 (2020).
Gómez Rueda, A. et al. The S-REAL study: Spanish real-world data on unresectable stage III NSCLC patients treated with durvalumab after chemoradiotherapy. Clin. Transl. Oncol. 26, 1779–1789 (2024).
Sankar, K. et al. Real world outcomes versus clinical trial results of durvalumab maintenance in veterans with stage III non-small cell lung cancer. Cancers 14, 614 (2022).
Kenmotsu, H. et al. Long-term safety and effectiveness of durvalumab in unresectable stage III non-small-cell lung cancer in Japan: a multicenter prospective study (AYAME). J. Thorac. Oncol. https://doi.org/10.1016/j.jtho.2025.08.010 (2025).
Filippi, A. R. et al. Real-world outcomes with durvalumab after chemoradiotherapy in patients with unresectable stage III NSCLC: interim analysis of overall survival from PACIFIC-R. ESMO Open 9, 103464 (2024).
Girard, N. et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J. Thorac. Oncol. 18, 181–193 (2023).
Zhou, Q. et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 23, 209–219 (2022).
Wu, Y.-L. et al. OA02.05 sugemalimab vs placebo after cCRT or sCRT in pts with unresectable stage III NSCLC: final PFS analysis of a phase 3 study. J. Thorac. Oncol. 17, S7–S8 (2022).
Sharma, P. & Allison, J. P. Dissecting the mechanisms of immune checkpoint therapy. Nat. Rev. Immunol. 20, 75–76 (2020).
Zhou, X. et al. Mechanisms of tumor resistance to immune checkpoint blockade and combination strategies to overcome resistance. Front. Immunol. 13, 915094 (2022).
Ramalingam, S. S. et al. OA14.03 six-year survival and HRQoL outcomes with 1L nivolumab + ipilimumab in patients with metastatic NSCLC from CheckMate227. J. Thorac. Oncol. 18, S76–S77 (2023).
Reck, M. et al. Five-year outcomes with first-line nivolumab plus ipilimumab with 2 cycles of chemotherapy versus 4 cycles of chemotherapy alone in patients with metastatic non-small cell lung cancer in the randomized CheckMate 9LA trial. Eur. J. Cancer 211, 114296 (2024).
Peters, S. et al. Durvalumab with or without tremelimumab in combination with chemotherapy in first-line metastatic NSCLC: five-year overall survival outcomes from the phase 3 POSEIDON trial. J. Thorac. Oncol. 20, 76–93 (2025).
Peters, S. et al. Long-term survival outcomes with first-line nivolumab plus ipilimumab-based treatment in patients with metastatic NSCLC and tumor programmed death-ligand 1 lower than 1%: a pooled analysis. J. Thorac. Oncol. 20, 94–108 (2025).
Durm, G. A. et al. Consolidation nivolumab plus ipilimumab or nivolumab alone following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: BTCRC LUN 16-081. J. Clin. Oncol. 40, 8509 (2022).
Wei, C. X., Althouse, S. K., Mamdani, H., Hanna, N. H. & Durm, G. A. Association of immune-related adverse events with efficacy in consolidation nivolumab plus ipilimumab or nivolumab alone after chemoradiation in patients with unresectable stage III nonsmall cell lung cancer: an exploratory analysis from the Big 10 Cancer Research Consortium Study BTCRC LUN 16-081. Clin. Lung Cancer 26, e154–e162 (2024).
Shahani, S., Althouse, S. K., Hanna, N. H. & Durm, G. A. Updated safety and efficacy analysis comparing elderly vs nonelderly patients treated with consolidation nivolumab or nivolumab plus ipilimumab after chemoradiation for unresectable stage III NSCLC from the BTCRC LUN 16-081 clinical trial. J. Clin. Oncol. 42, e13770 (2024).
Inoue, Y. et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget 8, 8738–8751 (2017).
André, P. et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175, 1731–1743.e13 (2018).
Herbst, R. S. et al. COAST: an open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non-small-cell lung cancer. J. Clin. Oncol. 40, 3383–3393 (2022).
Aggarwal, C. et al. Durvalumab alone or combined with novel agents for unresectable stage III non-small cell lung cancer: update from the COAST randomized clinical trial. JAMA Netw. Open https://doi.org/10.1001/jamanetworkopen.2025 (2025).
Barlesi, F. et al. Phase 3 study of durvalumab combined with oleclumab or monalizumab in patients with unresectable stage III NSCLC (PACIFIC-9). J. Clin. Oncol. 41, TPS8610 (2023).
Harjunpää, H. & Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 200, 108–119 (2020).
Johnson, M. L. et al. ARC-7: randomized phase 2 study of domvanalimab + zimberelimab ± etrumadenant versus zimberelimab in first-line, metastatic, PD-L1-high non-small cell lung cancer (NSCLC). J. Clin. Oncol. 40, 397600 (2022).
Cho, B. C. et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 23, 781–792 (2022).
Spigel, D. R. et al. LBA52 interim analysis of GALAXIES lung-201: phase II, randomized, open-label platform study of belrestotug plus dostarlimab in patients (pts) with previously untreated locally advanced/metastatic (LA/M) PD-L1 high (TPS ≥50%) non-small cell lung cancer (NSCLC). Ann. Oncol. 35, S1242–S1243 (2024).
Özgüroğlu, M. et al. Phase 3 trial of durvalumab combined with domvanalimab following concurrent chemoradiotherapy (cCRT) in patients with unresectable stage III NSCLC (PACIFIC-8). J. Clin. Oncol. 41, TPS8609 (2023).
Dziadziuszko, R. et al. 1190TiP SKYSCRAPER-03: phase III, open-label randomised study of atezolizumab + tiragolumab vs durvalumab in patients with locally advanced, unresectable, stage III non-small cell lung cancer (NSCLC) who have not progressed after platinum-based concurrent chemoradiation (cCRT). Ann. Oncol. 32, S947–S948 (2021).
Jabbour, S. et al. 969TiP randomized, phase III study of MK-7684A plus concurrent chemoradiotherapy (cCRT) followed by MK-7684A vs cCRT followed by durvalumab for unresectable, locally advanced, stage III non-small cell lung cancer (NSCLC): KEYVIBE-006. Ann. Oncol. 33, S990 (2022).
Peters, S. et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer — The ETOP NICOLAS trial. Lung Cancer Amst. Neth. 133, 83–87 (2019).
Peters, S. et al. Progression-free and overall survival for concurrent nivolumab with standard concurrent chemoradiotherapy in locally advanced stage IIIA-B NSCLC: results from the European Thoracic Oncology Platform NICOLAS Phase II Trial (European Thoracic Oncology Platform 6-14). J. Thorac. Oncol. 16, 278–288 (2021).
Jabbour, S. K. et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 7, 1–9 (2021).
Reck, M. et al. 1860: Pembrolizumab (pembro) plus concurrent chemoradiation therapy (cCRT) in unresectable locally advanced non-small cell lung cancer (NSCLC): final analysis of KEYNOTE-799. J. Thorac. Oncol. 20, S124–S125 (2025).
Liu, Y. et al. Final efficacy outcomes of atezolizumab with chemoradiation for unresectable NSCLC: the phase II DETERRED trial. Lung Cancer Amst. Neth. 174, 112–117 (2022).
Bradley, J. D. et al. LBA1 durvalumab in combination with chemoradiotherapy for patients with unresectable stage III NSCLC: final results from PACIFIC-2. ESMO Open 9 102986 (2024).
Higgins, K. et al. Concurrent chemoradiation ± atezolizumab (atezo) in limited-stage small cell lung cancer (LS-SCLC): results of NRG Oncology/Alliance LU005. Int. J. Radiat. Oncol. 120, S2 (2024).
Varlotto, J. M. et al. ECOG-ACRIN EA5181: phase 3 trial of concurrent and consolidative durvalumab vs consolidation durvalumab alone for unresectable stage III NSCLC. In 2025 World Conference on Lung Cancer, Abstract PL-3.04. (Barcelona, Spain, 6–9 September 2025).
Hochmair, M. et al. Pembrolizumab with or without maintenance olaparib for metastatic squamous NSCLC that responded to first-line pembrolizumab plus chemotherapy. J. Thorac. Oncol. 20, 203–218 (2025).
Gray, J. E. et al. The phase 3 KEYLYNK-006 study of pembrolizumab plus olaparib versus pembrolizumab plus pemetrexed as maintenance therapy for metastatic nonsquamous NSCLC. J. Thorac. Oncol. 20, 219–232 (2025).
Shaverdian, N. et al. MA01.06 phase I/II trial of canakinumab with chemoradiation and durvalumab in stage III non-small cell lung cancer (CHORUS). https://wclc.iaslc.org/wp-content/uploads/2025/09/WCLC-2025-Abstract-Book.pdf (2025).
Wakelee, H. et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N. Engl. J. Med. 389, 491–503 (2023).
Forde, P. M. et al. Overall survival with neoadjuvant nivolumab plus chemotherapy in lung cancer. N. Engl. J. Med. 393, 741–752 (2025).
Versluis, J. M., Long, G. V. & Blank, C. U. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat. Med. 26, 475–484 (2020).
Ross, H. J. et al. Atezolizumab before and after chemoradiation for unresectable stage III non-small cell lung cancer: a phase II nonrandomized controlled trial. JAMA Oncol. 10, 1212–1219 (2024).
Provencio, M. et al. OA12.05 APOLO: phase II trial of induction chemo-immunotherapy plus chemoradiotherapy and maintenance immunotherapy in stage III NSCLC. J. Thorac. Oncol. 19, S37 (2024).
William, W. N. Jr et al. OA12.06 intensified chemo-immuno-radiotherapy with durvalumab for stage III NSCLCs: a single arm phase II study — PACIFIC-BRAZIL (LACOG 2218). J. Thorac. Oncol. 19, S37–S38 (2024).
Ohri, N. et al. Selective personalized radioimmunotherapy for locally advanced non-small-cell lung cancer trial (SPRINT). J. Clin. Oncol. 42, 562–570 (2024).
Filippi, A. R. et al. 125P preliminary analysis on safety of the DEDALUS phase II trial: induction chemo-durvalumab followed by reduced-dose radiotherapy and maintenance durvalumab for patients with unresectable stage III NSCLC. ESMO Open 9 102704 (2024).
Saddi, J. et al. 198TiP DEDALUS trial: a single-arm, phase II, multi-center study of chemo-immunotherapy followed by hypo-fractionated RT and maintenance immunotherapy in patients with unresectable stage III NSCLC. Immuno-Oncol. Technol. 16 100310 (2022).
Smeenk, M. M. et al. Tremelimumab plus durvalumab prior to chemoradiotherapy in unresectable, locally advanced non-small cell lung cancer: the induction trial. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-24-3476 (2025).
Liu, H. et al. A phase II randomized trial evaluating consolidative nivolumab in locally advanced non-small cell lung cancer post neoadjuvant chemotherapy plus nivolumab and concurrent chemoradiotherapy (GASTO-1091). J. Clin. Oncol. 42, 8008 (2024).
Zhang, J., Wu, Q., Wu, H., Zhao, W. & Shi, M. 1242MO. The addition of belomethasone propionate inhalation to radical radiotherapy for patients with locally advanced non-small cell lung cancer: a randomized controlled, open-label phase II study. Ann. Oncol. 35, S795 (2024).
Hondelink, L. M. et al. Prevalence, clinical and molecular characteristics of early stage EGFR-mutated lung cancer in a real-life West-European cohort: implications for adjuvant therapy. Eur. J. Cancer 181, 53–61 (2023).
Soo, R. A. et al. Prevalence of EGFR mutations in patients with resected stages I to III NSCLC: results from the EARLY-EGFR study. J. Thorac. Oncol. 19, 1449–1459 (2024).
Nakamura, M. et al. Impact of EGFR mutation and ALK translocation on recurrence pattern after definitive chemoradiotherapy for inoperable stage III non-squamous non-small-cell lung cancer. Clin. Lung Cancer 20, e256–e264 (2019).
Yagishita, S. et al. Epidermal growth factor receptor mutation is associated with longer local control after definitive chemoradiotherapy in patients with stage III nonsquamous non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 91, 140–148 (2015).
Kim, H. et al. EGFR mutation-positive unresectable stage III non-squamous lung cancer is associated with a high incidence of brain metastasis. Cancer Res. Treat. 55, 498–505 (2023).
Tanaka, K. et al. EGFR mutation impact on definitive concurrent chemoradiation therapy for inoperable stage III adenocarcinoma. J. Thorac. Oncol. 10, 1720–1725 (2015).
Park, S. E. et al. EGFR mutation is associated with short progression-free survival in patients with stage III non-squamous cell lung cancer treated with concurrent chemoradiotherapy. Cancer Res. Treat. 51, 493–501 (2019).
Aredo, J. V. et al. Durvalumab for stage III EGFR-mutated NSCLC after definitive chemoradiotherapy. J. Thorac. Oncol. 16, 1030–1041 (2021).
Nassar, A. H. et al. Consolidation osimertinib versus durvalumab versus observation after concurrent chemoradiation in unresectable EGFR-mutant NSCLC: a multicenter retrospective cohort study. J. Thorac. Oncol. 19, 928–940 (2024).
Naidoo, J. et al. Brief report: durvalumab after chemoradiotherapy in unresectable stage III EGFR-mutant NSCLC: a post hoc subgroup analysis from PACIFIC. J. Thorac. Oncol. 18, 657–663 (2023).
Choi, D.-H. et al. Clinical outcomes of maintenance durvalumab after definitive concurrent chemoradiotherapy in unresectable locally advanced stage III NSCLC according to EGFR and ALK status: Korean Cancer Study Group LU-22-18. JTO Clin. Res. Rep. 5, 100734 (2024).
Saw, S. P. L. et al. PD-L1 score as a prognostic biomarker in Asian early-stage epidermal growth factor receptor-mutated lung cancer. Eur. J. Cancer 178, 139–149 (2023).
Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50 (2020).
Tsuboi, M. et al. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N. Engl. J. Med. 389, 137–147 (2023).
Lu, S. et al. Osimertinib after definitive chemoradiotherapy in unresectable stage III epidermal growth factor receptor-mutated non-small-cell lung cancer: analyses of central nervous system efficacy and distant progression from the phase III LAURA study. Ann. Oncol. 35, 1116–1125 (2024).
Chang, A. E. B. et al. The ASCENT trial: a phase 2 study of induction and consolidation afatinib and chemoradiation with or without surgery in stage III EGFR-mutant NSCLC. Oncologist 29, 609–618 (2024).
Negrao, M. V. et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J. Immunother. Cancer 9, e002891 (2021).
Nassar, A. H. et al. Consolidation ALK tyrosine kinase inhibitors versus durvalumab or observation after chemoradiation in unresectable stage III ALK-positive NSCLC. J. Thorac. Oncol. 20, 109–118 (2024).
Riudavets, M. et al. Durvalumab consolidation in patients with unresectable stage III non-small cell lung cancer with driver genomic alterations. Eur. J. Cancer 167, 142–148 (2022).
Cortiula, F. et al. Adjuvant durvalumab after concurrent chemoradiotherapy for patients with unresectable stage III NSCLC harbouring uncommon genomic alterations. Eur. J. Cancer 184, 172–178 (2023).
Shaverdian, N. et al. The impact of durvalumab on local-regional control in stage III NSCLCs treated with chemoradiation and on KEAP1-NFE2L2-mutant tumors. J. Thorac. Oncol. 16, 1392–1402 (2021).
Peters, S. et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann. Oncol. 30, 161–165 (2019).
Normanno, N. et al. Circulating tumour DNA in early stage and locally advanced NSCLC: ready for clinical implementation? Nat. Rev. Clin. Oncol. 22, 215–231 (2025).
Jun, S. et al. Analysis of circulating tumor DNA predicts outcomes of short-course consolidation immunotherapy in unresectable stage III NSCLC. J. Thorac. Oncol. 19, 1427–1437 (2024).
Wang, Y. et al. Longitudinal circulating tumour DNA dynamics predict failure patterns and efficacy of consolidation immunotherapy after chemoradiotherapy in locally advanced non-small-cell lung cancer. Clin. Transl. Med. 14, e1619 (2024).
Moding, E. J. et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat. Cancer 1, 176–183 (2020).
Newman, A. M. et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat. Med. 20, 548–554 (2014).
Chetan, M. R. & Gleeson, F. V. Radiomics in predicting treatment response in non-small-cell lung cancer: current status, challenges and future perspectives. Eur. Radiol. 31, 1049–1058 (2021).
Jazieh, K. et al. Novel imaging biomarkers predict progression-free survival in stage 3 NSCLC treated with chemoradiation and durvalumab. J. Clin. Oncol. 39, 3054 (2021).
Ramella, S. et al. A radiomic approach for adaptive radiotherapy in non-small cell lung cancer patients. PLoS ONE 13, e0207455 (2018).
Lee, H. I. et al. Predictive value of primary tumor volume change during concurrent chemoradiotherapy in patients with unresectable stage III non-small cell lung cancer. Radiother. Oncol. 198, 110383 (2024).
Liu, S. et al. Radiotherapy remodels the tumor microenvironment for enhancing immunotherapeutic sensitivity. Cell Death Dis. 14, 1–19 (2023).
Raben, D. et al. Patterns of disease progression with durvalumab in stage III non-small-cell lung cancer (PACIFIC). Int. J. Rad. Oncol. Biol. Phys. 105, 683 (2019).
Rheinheimer, S. et al. Oligoprogressive non-small-cell lung cancer under treatment with PD-(L)1 inhibitors. Cancers 12, E1046 (2020).
Schoenfeld, A. J. et al. Systemic and oligo-acquired resistance to PD-(L)1 blockade in lung cancer. Clin. Cancer Res. 28, 3797–3803 (2022).
Tsai, C. J. et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of radiotherapy to block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet Lond. Engl. 403, 171–182 (2024).
Ciammella, P. et al. Locally advanced NSCLC: overview of real-world pattern of recurrence in durvalumab era (LEOPARD trial). Lung Cancer https://doi.org/10.1016/j.lungcan.2025.108718 (2025).
Hasegawa, T. et al. Subsequent treatment for locally advanced non-small-cell lung cancer that progressed after definitive chemoradiotherapy and consolidation therapy with durvalumab: a multicenter retrospective analysis (TOPGAN 2021-02). Cancer Chemother. Pharmacol. 92, 29–37 (2023).
Kawachi, H. et al. Real-world outcomes of subsequent chemotherapy after progression following chemoradiation and consolidative durvalumab therapy in locally advanced non-small cell lung cancer: an exploratory analysis from the CRIMSON study (HOPE-005). Clin. Lung Cancer 25, 643–652.e4 (2024).
Crespi, V. et al. Real-world outcomes of subsequent treatment strategies after durvalumab consolidation in stage III unresectable non-small cell lung cancer. Lung Cancer Amst. Neth. 204, 108576 (2025).
Cortiula, F. et al. Comparative efficacy of immunotherapy-based treatment versus chemotherapy-only in patients with unresectable NSCLC with disease progression post chemoradiation and durvalumab. Eur. J. Cancer 219, 115302 (2025).
Kutiel, T. S. et al. P2.07-09 stage 3 NSCLC patients progressing after concurrent chemo-radiotherapy and durvalumab — is there a role for another IO? J. Thorac. Oncol. 18, S327 (2023).
Schoenfeld, A. J. et al. Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Ann. Oncol. 32, 1597–1607 (2021).
Bryant, A. K. et al. De-escalating adjuvant durvalumab treatment duration in stage III non-small cell lung cancer. Eur. J. Cancer 171, 55–63 (2022).
Remon, J. et al. De-escalation strategies with immune checkpoint blockers in non-small cell lung cancer: do we already have enough evidence? J. Clin. Oncol. 43, 1148–1156 (2025).
Moding, E. J. et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small cell lung cancer. Nat. Cancer 1, 176–183 (2020).
Rousseau, A. et al. Impact of pembrolizumab treatment duration on overall survival and prognostic factors in advanced non-small cell lung cancer: a nationwide retrospective cohort study. Lancet Reg. Health Eur. 43, 100970 (2024).
Socinski, M. A. et al. Durvalumab after concurrent chemoradiotherapy in elderly patients with unresectable stage III non-small-cell lung cancer (PACIFIC). Clin. Lung Cancer 22, 549–561 (2021).
Mouri, A. et al. A phase II study of daily carboplatin plus irradiation followed by durvalumab therapy for older adults (≥75 years) with unresectable III non-small-cell lung cancer and performance status of 2: NEJ039A. ESMO Open 9, 103939 (2024).
Tachihara, M. et al. Durvalumab plus concurrent radiotherapy for treatment of locally advanced non-small cell lung cancer: the DOLPHIN phase 2 nonrandomized controlled trial. JAMA Oncol. 9, 1505–1513 (2023).
Zhang, Y. et al. Concerning safety and efficacy of concurrent and consolidative durvalumab with thoracic radiation therapy in PDL1-unselected stage III non-small cell lung cancer: brief report. Int. J. Radiat. Oncol. Biol. Phys. 121, 68–74 (2025).
Filippi, A. R. et al. Durvalumab after radiotherapy in patients with unresectable stage III non-small-cell lung cancer ineligible for chemotherapy: the DUART phase II nonrandomized controlled study. ESMO Open 10, 105560 (2025).
Yamada, T. et al. A phase 2 trial of durvalumab treatment following radiation monotherapy in patients with non-small cell lung cancer ineligible for stage III chemoradiotherapy: the SPIRAL-RT study. Eur. J. Cancer 195, 113373 (2023).
Walls, G. M. et al. CONCORDE: a phase I platform study of novel agents in combination with conventional radiotherapy in non-small-cell lung cancer. Clin. Transl. Radiat. Oncol. 25, 61–66 (2020).
Chun, S. G. et al. Long-term prospective outcomes of intensity modulated radiotherapy for locally advanced lung cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 10, 1111–1115 (2024).
Steinfort, D. P. et al. Systematic endoscopic staging of mediastinum to guide radiotherapy planning in patients with locally advanced non-small-cell lung cancer (SEISMIC): an international, multicentre, single-arm, clinical trial. Lancet Respir. Med. 12, 467–475 (2024).
Nestle, U. et al. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. Lancet Oncol. 21, 581–592 (2020).
Price, G. et al. Can real-world data and rapid learning drive improvements in lung cancer survival? the RAPID-RT study. Clin. Oncol. R. Coll. Radiol. 34, 407–410 (2022).
Kong, F.-M. S. et al. Primary results of NRG-RTOG1106/ECOG-ACRIN 6697: a randomized phase II trial of individualized adaptive (chemo)radiotherapy using midtreatment 18F-fluorodeoxyglucose positron emission tomography/computed tomography in stage III non-small cell lung cancer. J. Clin. Oncol. 42, 3935–3946 (2024).
Vera, P. et al. Adaptive radiotherapy (up to 74 Gy) or standard radiotherapy (66 Gy) for patients with stage III non-small-cell lung cancer, according to [18F]FDG-PET tumour residual uptake at 42 Gy (RTEP7-IFCT-1402): a multicentre, randomised, controlled phase 2 trial. Lancet Oncol. 25, 1176–1187 (2024).
Wu, T. C. et al. Accelerated hypofractionated chemoradiation followed by stereotactic ablative radiotherapy boost for locally advanced, unresectable non-small cell lung cancer: a nonrandomized controlled trial. JAMA Oncol. 10, 352–359 (2024).
Heinzerling, J. H. et al. Primary lung tumour stereotactic body radiotherapy followed by concurrent mediastinal chemoradiotherapy and adjuvant immunotherapy for locally advanced non-small-cell lung cancer: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 26, 85–97 (2025).
Jin, J.-Y. et al. Higher radiation dose to the immune cells correlates with worse tumor control and overall survival in patients with stage III NSCLC: a secondary analysis of RTOG0617. Cancers 13, 6193 (2021).
Abravan, A., Faivre-Finn, C., Kennedy, J., McWilliam, A. & van Herk, M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J. Thorac. Oncol. 15, 1624–1635 (2020).
Lavaud, P. et al. Early-stage non-small cell lung cancer: new challenges with immune checkpoint blockers and targeted therapies. Cancers 16, 2779 (2024).
Sorin, M. et al. Neoadjuvant chemoimmunotherapy for NSCLC: a systematic review and meta-analysis. JAMA Oncol. 10, 621–633 (2024).
Wang, M. et al. Conversion surgery for initially unresectable stage III non-small cell lung cancer after induction treatment of immunochemotherapy: a multicenter study. Clin. Lung Cancer 26, e131–e140.e1 (2025).
Ricciuti, B. et al. Neoadjuvant PD-1 and PD-L1 blockade with chemotherapy for borderline resectable and unresectable stage III non-small cell lung cancer. JAMA Oncol. 11, 735–741 (2025).
Zhao, Z. et al. The safety and efficacy of induction chemoimmunotherapy in initially unresectable stage III non-small cell lung cancer. J. Clin. Oncol. 42, 8058–8058 (2024).
Zhou, Q. et al. Neoadjuvant SHR-1701 with or without chemotherapy in unresectable stage III non-small-cell lung cancer: a proof-of-concept, phase 2 trial. Cancer Cell 42, 1258–1267.e2 (2024).
Bian, D. et al. Neoadjuvant aumolertinib for unresectable stage III EGFR-mutant non-small cell lung cancer: a single-arm phase II trial. Nat. Commun. 16, 3143 (2025).
Goss, G. et al. LBA48 CCTG BR.31: a global, double-blind placebo-controlled, randomized phase III study of adjuvant durvalumab in completely resected non-small cell lung cancer (NSCLC). Ann. Oncol. 35, S1238 (2024).
Author information
Authors and Affiliations
Contributions
J.R., R.G. and M.B. researched data for the manuscript, J.R., A.L., R.G., I.M.-L., M.B., L.E.L.H., C.F.-F. and M.P. wrote the manuscript, J.R., C.F.-F., M.R. and M.P. made a substantial contribution to discussions of content and all authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.R. has acted as a consultant and/or adviser of AstraZeneca, Johnson & Johnson and Regeneron, has acted as a speaker for AstraZeneca, Johnson & Johnson, Roche and Sanofi, has received research funding from AstraZeneca, Merck and MSD, has received travel support from MSD and Ose Immunotherapeutics and is the chair of EORTC-LCG and a member of the editorial boards of the Journal of Clinical Oncology, Lung Cancer and Journal of Thoracic Oncology. A.L. has received research funding from AstraZeneca, Beigene, MSD, Pharmamar and Roche. L.E.L.H. has acted as a consultant and/or adviser of Abbvie, Amgen, Anhearth, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi, Gilead, GSK, Janssen, Lilly, Merck, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, Summit Therapeutics and Takeda, has acted as a speaker for AstraZeneca, Bayer, Benecke, GSK, Janssen, high5oncology, Lilly, Medimix, Medtalks, MSD, Pfizer, Sanofi, Takeda and VJOncology, has received research funding from Amgen, AstraZeneca, Boehringer Ingelheim, Gilead, Merck, Novartis, Pfizer and Takeda and has acted as a guideline committee member for the Dutch guidelines on NSCLC, brain metastases and leptomeningeal metastases, the ESMO guidelines on metastatic NSCLC, non-metastatic NSCLC and SCLC, is the former secretary and current chair of the NVALT Studies Foundation, is the former subchair and current secretary of the EORTC Metastatic NSCLC Systemic Therapy Group and the vice-chair of the scientific committee of the Dutch Thoracic Group. M.P. has acted as a consultant and/or adviser of Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Gilead Sciences, GlaxoSmithKline, Ipsen, Janssen Oncology, Lilly, Merck Sharp & Dohme, Novartis, Novocure, Pfizer, Roche/Genentech, Sanofi and Takeda, has received research funding from Takeda, has received travel support from AstraZeneca, Chugai Pharma, Bristol Myers Squibb, Merck Sharp & Dohme, Pfizer, Roche and Takeda and is a member of the editorial board of the Journal of Clinical Oncology. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks T. Cascone, R. Dziadziuszko, S. Ramalingam, and J.C.H. Yang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Remon, J., Levy, A., Gille, R. et al. Unresectable stage III non-small-cell lung cancer: state of the art and challenges. Nat Rev Clin Oncol (2025). https://doi.org/10.1038/s41571-025-01080-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s41571-025-01080-4