Abstract
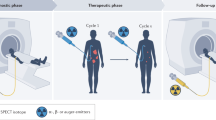
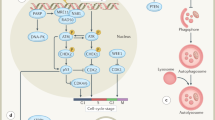
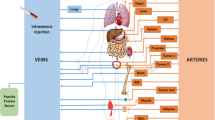
Radiopharmaceutical therapy (RPT) is emerging as a safe and effective targeted approach to treating many types of cancer. In RPT, radiation is systemically or locally delivered using pharmaceuticals that either bind preferentially to cancer cells or accumulate by physiological mechanisms. Almost all radionuclides used in RPT emit photons that can be imaged, enabling non-invasive visualization of the biodistribution of the therapeutic agent. Compared with almost all other systemic cancer treatment options, RPT has shown efficacy with minimal toxicity. With the recent FDA approval of several RPT agents, the remarkable potential of this treatment is now being recognized. This Review covers the fundamental properties, clinical development and associated challenges of RPT.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
07 September 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41573-020-0085-5
References
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286 (2018).
Lin, A. et al. Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl Med. 11, eaaw8412 (2019).
Gill, M. R., Falzone, N., Du, Y. & Vallis, K. A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 18, e414–e423 (2017).
Dolgin, E. Radioactive drugs emerge from the shadows to storm the market. Nat. Biotechnol. 36, 1125–1127 (2018).
Knut, L. Radiosynovectomy in the therapeutic management of arthritis. World J. Nucl. Med. 14, 10–15 (2015).
Kresnik, E. In: Local Treatment of Inflammatory Joint Diseases: Benefits and Risks (eds Kampen, W. U., & Fischer, M.) 81–93 (Springer International Publishing, 2015).
Bentzen, S. M. et al. Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int. J. Radiat. Oncol. Biol. Phys. 76, S3–S9 (2010). Summary of radiation dose versus response data from radiotherapy experience.
Dale, R. & Carabe-Fernandez, A. The radiobiology of conventional radiotherapy and its application to radionuclide therapy. Cancer Biother. Radiopharm. 20, 47–51 (2005).
Amro, H., Wilderman, S. J., Dewaraja, Y. K. & Roberson, P. L. Methodology to incorporate biologically effective dose and equivalent uniform dose in patient-specific 3-dimensional dosimetry for non-Hodgkin lymphoma patients targeted with 131I-tositumomab therapy. J. Nucl. Med. 51, 654–659 (2010).
Fowler, J. F. Radiobiological aspects of low-dose rates in radioimmunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 18, 1261–1269 (1990). Radiobiological treatment of RPT.
McDevitt, M. R. et al. Radioimmunotherapy with alpha-emitting nuclides. Eur. J. Nucl. Med. 25, 1341–1351 (1998).
Wessels, B. W. & Rogus, R. D. Radionuclide selection and model absorbed dose calculations for radiolabeled tumor associated antibodies. Med. Phys. 11, 638–645 (1984).
Bloomer, W. D., McLaughlin, W. H., Adelstein, S. J. & Wolf, A. P. Therapeutic applications of Auger and alpha emitting radionuclides. Strahlentherapie 160, 755–757 (1984).
O’Donoghue, J. A., Bardies, M. & Wheldon, T. E. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J. Nucl. Med. 36, 1902–1909 (1995). Demonstrates that, in contrast to external-beam radiotherapy, in RPT fewer cells do not lead to greater tumour control probability.
Cherry, S. R., Sorenson, J. A., & Phelps, M. E. Physics in Nuclear Medicine e-Book (Elsevier Health Sciences, 2012).
Behr, T. M. et al. Therapeutic advantages of Auger electron- over beta-emitting radiometals or radioiodine when conjugated to internalizing antibodies. Eur. J. Nucl. Med. 27, 753–765 (2000).
Bodei, L., Kassis, A. I., Adelstein, S. J. & Mariani, G. Radionuclide therapy with iodine-125 and other Auger-electron-emitting radionuclides: experimental models and clinical applications. Cancer Biother. Radiopharm. 18, 861–877 (2003).
Howell, R. W. et al. in Biophysical Aspects of Auger Processes (Howell, R. W., Narra, V. R., Sastry, K. S. R. & Rao, D. V. Eds). 290–318 (Medical Physics, 1991).
Kassis, A. I., Adelstein, S. J. & Mariani, G. Radiolabeled nucleoside analogs in cancer diagnosis and therapy. Q. J. Nucl. Med. 40, 301–319 (1996).
Kiess, A. P. et al. Auger radiopharmaceutical therapy targeting prostate-specific membrane antigen. J. Nucl. Med. 56, 1401–1407 (2015).
Macapinlac, H. A. et al. Pilot clinical trial of 5-[125I]iodo-2’-deoxyuridine in the treatment of colorectal cancer metastatic to the liver. J. Nucl. Med. 37 (Suppl. 4), 25–29 (1996).
Daghighian, F. et al. Pharmacokinetics and dosimetry of iodine-125-IUdR in the treatment of colorectal cancer metastatic to liver. J. Nucl. Med. 37 (Suppl. 4), 29–32 (1996).
Sgouros, G. et al. Mathematical model of 5-[125I]iodo-2’-deoxyuridine treatment: continuous infusion regimens for hepatic metastases. Int. J. Radiat. Oncol. Biol. Phys. 41, 1177–1183 (1998).
Rebischung, C. et al. First human treatment of resistant neoplastic meningitis by intrathecal administration of MTX plus 125IUdR. Int. J. Radiat. Biol. 84, 1123–1129 (2008).
Behr, T. M. et al. Therapeutic efficacy and dose-limiting toxicity of Auger-electron vs. beta emitters in radioimmunotherapy with internalizing antibodies: evaluation of 125I- vs. 131I-labeled CO17-1A in a human colorectal cancer model. Int. J. Cancer. 76, 738–748 (1998).
Ku, A., Facca, V. J., Cai, Z. & Reilly, R. M. Auger electrons for cancer therapy - a review. EJNMMI Radiopharm. Chem. 4, 27 (2019).
Brooks, R. C. et al. Metal complexes of bleomycin: evaluation of [Rh-105]-bleomycin for use in targeted radiotherapy. Nucl. Med. Biol. 26, 421–430 (1999).
Miao, Y., Owen, N. K., Fisher, D. R., Hoffman, T. J. & Quinn, T. P. Therapeutic efficacy of a 188Re-labeled alpha-melanocyte-stimulating hormone peptide analog in murine and human melanoma-bearing mouse models. J. Nucl. Med. 46, 121–129 (2005).
Champion, C., Quinto, M. A., Morgat, C., Zanotti-Fregonara, P. & Hindié, E. Comparison between three promising ß-emitting radionuclides, 67Cu, 47Sc and 161Tb, with emphasis on doses delivered to minimal residual disease. Theranostics 6, 1611–1618 (2016).
Howell, R. W., Goddu, S. M. & Rao, D. V. Application of the linear-quadratic model to radioimmunotherapy: further support for the advantage of longer-lived radionuclides. J. Nucl. Med. 35, 1861–1869 (1994).
Watson, E. E., Stabin, M. G., Davis, J. L. & Eckerman, K. F. A model of the peritoneal cavity for use in internal dosimetry. J. Nucl. Med. 30, 2002–2011 (1989).
Zimmermann, R. G. Why are investors not interested in my radiotracer? The industrial and regulatory constraints in the development of radiopharmaceuticals. Nucl. Med. Biol. 40, 155–166 (2013).
Mikheev, N. B. Radioactive colloidal solutions and suspensions for medical use. At. Energy Rev. 14, 3–36 (1976).
Bayly, R. J., Peacegood, J. A. & Peake, S. C. 90Y ferric hydroxide colloid. Ann. Rheum. Dis. 32 (Suppl.), 10 (1973).
Washburn, L. C. et al. 90Y-labeled monoclonal antibodies for cancer therapy. Int. J. Radiat. Appl. Instrum. Part B Nucl. Med. Biol. 13, 453–456 (1986).
Kozak, R. W. et al. Nature of the bifunctional chelating agent used for radioimmunotherapy with yttrium-90 monoclonal antibodies: critical factors in determining in vivo survival and organ toxicity. Cancer Res. 49, 2639–2644 (1989).
Brechbiel, M. W. & Gansow, O. A. Backbone-substituted DTPA ligands for 90Y radioimmunotherapy. Bioconjug. Chem. 2, 187–194 (1991). Introduces a novel DTPA chelate for radioimmunotherapy with metallic radionuclides.
Stewart, J. S. et al. Intraperitoneal radioimmunotherapy for ovarian cancer: pharmacokinetics, toxicity, and efficacy of I-131 labeled monoclonal antibodies. Int. J. Radiat. Oncol. Biol. Phys. 16, 405–413 (1989).
Oei, A. L. et al. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int. J. Cancer 120, 2710–2714 (2007).
Waldmann, T. A. et al. Radioimmunotherapy of interleukin-2R alpha-expressing adult T-cell leukemia with yttrium-90-labeled anti-Tac. Blood 86, 4063–4075 (1995).
Foss, F. M. et al. Phase I study of the pharmacokinetics of a radioimmunoconjugate, 90Y-T101, in patients with CD5-expressing leukemia and lymphoma. Clin. Cancer Res. 4, 2691–2700 (1998).
Witzig, T. E. et al. Phase I/II trial of IDEC-Y2B8 radioimmunotherapy for treatment of relapsed or refractory CD20+ B-cell non-Hodgkin’s lymphoma. J. Clin. Oncol. 17, 3793–3803 (1999).
Nisa, L., Savelli, G. & Giubbini, R. Yttrium-90 DOTATOC therapy in GEP-NET and other SST2 expressing tumors: a selected review. Ann. Nucl. Med. 25, 75–85 (2011).
Salem, R. & Thurston, K. G. Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J. Vasc. Interv. Radiol. 17, 1251–1278 (2006).
Lau, W. Y. et al. Selective internal radiation therapy for nonresectable hepatocellular carcinoma with intraarterial infusion of 90yttrium microspheres. Int. J. Radiat. Oncol. Biol. Phys. 40, 583–592 (1998). Early report of microsphere therapy for hepatic artery infusion.
Popperl, G. et al. Selective internal radiation therapy with SIR-Spheres in patients with nonresectable liver tumors. Cancer Biother. Radiopharm. 20, 200–208 (2005).
Mancini, R. et al. A multicentric phase II clinical trial on intra-arterial hepatic radiotherapy with 90yttrium SIR-spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy: preliminary results on toxicity and response rates. In Vivo 20, 711–714 (2006).
Maleux, G. et al. Yttrium-90 radioembolization for the treatment of chemorefractory colorectal liver metastases: technical results, clinical outcome and factors potentially influencing survival. Acta Oncol. 55, 486–495 (2016).
Yue, J. et al. Comparison of quantitative Y-90 SPECT and non-time-of-flight PET imaging in post-therapy radioembolization of liver cancer. Med. Phys. 43, 5779 (2016).
Das, T. & Banerjee, S. Theranostic applications of lutetium-177 in radionuclide therapy. Curr. Radiopharm. 9, 94–101 (2016).
Tarasov, V. A., Andreev, O. I., Romanov, E. G., Kuznetsov, R. A., Kupriyanov, V. V. & Tselishchev, I. V. Production of no-carrier added lutetium-177 by irradiation of enriched ytterbium-176. Curr. Radiopharm. 8, 95–106 (2015).
Banerjee, S., Pillai, M. R. A. & Knapp, F. F. Lutetium-177 therapeutic radiopharmaceuticals: linking chemistry, radiochemistry, and practical applications. Chem. Rev. 115, 2934–2974 (2015).
Sgouros, G. et al. MIRD Monograph: Radiobiology and Dosimetry for Radiopahrmaceutical Therapy with Alpha-Particle Emitters (ed. Sgouros, G.). (SNMMI, 2015). Comprehensive review of radiobiology and dosimetry for α-emitter RPT.
Sgouros, G. et al. MIRD pamphlet no. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 51, 311–328 (2010).
Parker, C. et al. Targeted alpha therapy, an emerging class of cancer agents a review. JAMA Oncol. 4, 1765–1772 (2018).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013). ASYMPCA trial report that led to approval of radium-223 for treatment of patients with prostate cancer with bone metastases.
Kratochwil, C. et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 57, 1941–1944 (2016). Striking imaging-based demonstration of the remarkable response that may be obtained with α-emitter RPT.
Kratochwil, C., Haberkorn, U. & Giesel, F. L. Radionuclide therapy of metastatic prostate cancer. Semin. Nucl. Med. 49, 313–325 (2019).
Parker, C., Heidenreich, A., Nilsson, S. & Shore, N. Current approaches to incorporation of radium-223 in clinical practice. Prostate Cancer Prostatic Dis. 21, 37–47 (2018).
Subbiah, V. et al. Alpha particle radium 223 dichloride in high-risk osteosarcoma: a phase I dose escalation trial. Clin. Cancer Res. 25, 3802–3810 (2019).
Geva, R. et al. Radium-223 in combination with paclitaxel in cancer patients with bone metastases: safety results from an open-label, multicenter phase Ib study. Eur. J. Nucl. Med. Mol. Imaging 46, 1092–1101 (2019).
Takalkar, A., Paryani, B., Adams, S. & Subbiah, V. Radium-223 dichloride therapy in breast cancer with osseous metastases. BMJ Case Rep. 2015, bcr2015211152 (2015).
Eckerman, K. F. & Enzo, A. MIRD: Radionuclide Data and Decay Schemes, 2nd edn. (Society of Nuclear Medicine, 2008).
Hellman, S., Devita V. T. and Rosenberg, S. A. Cancer: Principles & Practice of Oncology. 265–288 (Lippincott Williams & Wilkins, 2001).
Longcor, J. & Oliver, K. Phase 1, open-label, dose escalation study of I-131-CLR1404 (CLR 131) in patients with relapsed or refractory multiple myeloma. Blood 134, 1864 (2019).
Ciernik, I. F. et al. Proton-based radiotherapy for unresectable or incompletely resected osteosarcoma. Cancer 117, 4522–4530 (2011).
Oertel, S. et al. Radiotherapy in the treatment of primary osteosarcoma-a single center experience. Tumori 96, 582–588 (2010).
Senthamizhchelvan, S. et al. Tumor dosimetry and response for 153Sm-ethylenediamine tetramethylene phosphonic acid therapy of high-risk osteosarcoma. J. Nucl. Med. 53, 215–224 (2012).
Hobbs, R. F. et al. A treatment planning method for sequentially combining radiopharmaceutical therapy and external radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 80, 1256–1262 (2011).
Wessels, B. W. et al. MIRD pamphlet no. 20: the effect of model assumptions on kidney dosimetry and response-implications for radionuclide therapy. J. Nucl. Med. 49, 1884–1899 (2008).
Kiess, A. P. et al. (2S)-2-(3-(1-Carboxy-5-(4-211At-astatobenzamido)pentyl)ureido)-pentanedioic acid for PSMA-targeted alpha-particle radiopharmaceutical therapy. J. Nucl. Med. 57, 1569–1575 (2016).
Jentzen, W. et al. Pre-therapeutic I-124 PET(/CT) dosimetry confirms low average absorbed doses per administered I-131 activity to the salivary glands in radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 37, 884–895 (2010).
Hobbs, R. F., Jentzen, W., Bockisch, A. & Sgouros, G. Monte Carlo-based 3-dimensional dosimetry of salivary glands in radioiodine treatment of differentiated thyroid cancer estimated using 124I PET. Q. J. Nucl. Med. Mol. Imaging 57, 79–91 (2013).
Hobbs, R. F. et al. A bone marrow toxicity model for 223Ra alpha-emitter radiopharmaceutical therapy. Phys. Med. Biol. 57, 3207–3222 (2012).
Back, T. & Jacobsson, L. The alpha-camera: a quantitative digital autoradiography technique using a charge-coupled device for ex vivo high-resolution bioimaging of alpha-particles. J. Nucl. Med. 51, 1616–1623 (2010). α-Camera imaging technique to assess distribution of α-particles in tissues.
Miller, B. W. et al. Quantitative single-particle digital autoradiography with alpha-particle emitters for targeted radionuclide therapy using the iQID camera. Med. Phys. 42, 4094–4105 (2015).
Miller, B. W. Radiation imagers for quantitative, single-particle digital autoradiography of alpha- and beta-particle emitters. Semin. Nucl. Med. 48, 367–376 (2018).
Ljungberg, M. & Gleisner, K. S. 3-D image-based dosimetry in radionuclide therapy. IEEE Trans. Radiat. Plasma Med. Sci. 2, 527–540 (2018).
Sgouros, G. & Hobbs, R. F. Dosimetry for radiopharmaceutical therapy. Semin. Nucl. Med. 44, 172–178 (2014).
Dewaraja, Y. K., Ljungberg, M., Green, A. J., Zanzonico, P. B. & Frey, E. C. MIRD pamphlet no. 24: guidelines for quantitative 131I SPECT in dosimetry applications. J. Nucl. Med. 54, 122390 (2013).
Bolch, W. E., Eckerman, K. F., Sgouros, G. & Thomas, S. R. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry-standardization of nomenclature. J. Nucl. Med. 50, 477–484 (2009). Mathematical formalism for radiopharmaceutical dosimetry.
Vaziri, B., Wu, H., Dhawan, A. P., Du, P. & Howell, R. W. MIRD pamphlet no. 25: MIRDcell V2.0 software tool for dosimetric analysis of biologic response of multicellular populations. J. Nucl. Med. 55, 1557–1564 (2014).
Dewaraja, Y. K. et al. MIRD pamphlet no. 23: quantitative SPECT for patient-specific 3-dimensional dosimetry in internal radionuclide therapy. J. Nucl. Med. 53, 1310–1325 (2012).
Bolch, W. E. et al. MIRD pamphlet no. 17: the dosimetry of nonuniform activity distributions-radionuclide S values at the voxel level. Medical Internal Radiation Dose Committee. J. Nucl. Med. 40, 11S–36S (1999).
Furhang, E. E., Chui, C. S., Kolbert, K. S., Larson, S. M. & Sgouros, G. Implementation of a Monte Carlo dosimetry method for patient-specific internal emitter therapy. Med. Phys. 24, 1163–1172 (1997).
Kolbert, K. S. et al. Implementation and evaluation of patient-specific three-dimensional internal dosimetry. J. Nucl. Med. 38, 301–308 (1997).
Cremonesi, M. et al. Correlation of dose with toxicity and tumour response to Y-90- and Lu-177-PRRT provides the basis for optimization through individualized treatment planning. Eur. J. Nucl. Med. Mol. Imaging 45, 2426–2441 (2018).
Pacilio, M. et al. A case report of image-based dosimetry of bone metastases with Alpharadin (Ra-223-dichloride) therapy: inter-fraction variability of absorbed dose and follow-up. Ann. Nucl. Med. 30, 163–168 (2016).
Stokke, C. et al. Dosimetry-based treatment planning for molecular radiotherapy: a summary of the 2017 report from the Internal Dosimetry Task Force. EJNMMI Phys. 4, 27 (2017).
Dewaraja, Y. K. et al. Tumor-absorbed dose predicts progression-free survival following 131I-tositumomab radioimmunotherapy. J. Nucl. Med. 55, 1047–1053 (2014).
O’Donoghue, J. A. et al. Hematologic toxicity in radioimmunotherapy: dose-response relationships for I-131 labeled antibody therapy. Cancer Biother. Radiopharm. 17, 435–443 (2002).
Sgouros, G. & Goldenberg, D. M. Radiopharmaceutical therapy in the era of precision medicine. Eur. J. Cancer 50, 2360–2363 (2014).
Hertz, S. & Roberts, A. Radioactive iodine in the study of thyroid physiology. 7. The use of radioactive iodine therapy in hyperthyroidism. JAMA 131, 81–86 (1946). Potential of radioiodine to treat thyroid disease.
Campbell, J. E., Robajdek, E. S. & Anthony, D. S. The metabolism of Ac-227 and its daughters Th-227 and Ra-223 by rats. Radiat. Res. 4, 294–302 (1956).
Joshi, D. P., Seery, W. H., Goldberg, L. G. & Goldman, L. Evaluation of phosphorus 32 for intractable pain secondary to prostatic carcinoma metastasES. JAMA 193, 621–623 (1965).
Harrison, G. E., Carr, T. E., Sutton, A. & Rundo, J. Plasma concentration and excretion of calcium-47, strontium-85, barium-133 and radium-223 following successive intravenous doses to a healthy man. Nature 209, 526–527 (1966).
Foss, C. A. et al. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin. Cancer Res. 11, 4022–4028 (2005).
Zechmann, C. M. et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 41, 1280–1292 (2014).
Kong, G. & Hicks, R. J. Peptide receptor radiotherapy: current approaches and future directions. Curr. Treat. Options Oncol. 20, 77 (2019).
Muller, C., Vlahov, I. R., Santhapuram, H. K. R., Leamon, C. P. & Schibli, R. Tumor targeting using Ga-67-DOTA-Bz-folate - investigations of methods to improve the tissue distribution of radiofolates. Nucl. Med. Biol. 38, 715–723 (2011).
Muller, C., Struthers, H., Winiger, C., Zhernosekov, K. & Schibli, R. DOTA conjugate with an albumin-binding entity enables the first folic acid-targeted 177Lu-radionuclide tumor therapy in mice. J. Nucl. Med. 54, 124–131 (2013).
Ambrosini, V., Fani, M., Fanti, S., Forrer, F. & Maecke, H. R. Radiopeptide imaging and therapy in Europe. J. Nucl. Med. 52, 42S–55S (2011).
Song, H. & Sgouros, G. Radioimmunotherapy of solid tumors: searching for the right target. Curr. Drug Deliv. 8, 26–44 (2011).
Leaman Alcibar, O. et al. Time for radioimmunotherapy: an overview to bring improvements in clinical practice. Clin. Transl Oncol. 21, 992–1004 (2019).
Larson, S. M., Carrasquillo, J. A. & Reynolds, J. C. Radioimmunodetection and radioimmunotherapy. Cancer Invest. 2, 363–381 (1984).
Goldenberg, D. M. Targeting of cancer with radiolabeled antibodies. Prospects for imaging and therapy. Arch. Pathol. Lab. Med. 112, 580–587 (1988).
Jeon, J. Review of therapeutic applications of radiolabeled functional nanomaterials. Int. J. Mol. Sci 20, 2323 (2019).
Sofou, S. & Sgouros, G. Antibody-targeted liposomes in cancer therapy and imaging. Expert. Opin. Drug Deliv 5, 189–204 (2008).
Lee, E. J., Chung, H. W., Jo, J. H. & So, Y. Radioembolization for the treatment of primary and metastatic liver cancers. Nucl. Med. Mol. Imaging 53, 367–373 (2019).
Wu, A. M. & Senter, P. D. Arming antibodies: prospects and challenges for immunoconjugates. Nat. Biotech. 23, 1137–1146 (2005).
Hu, S. Z. et al. Minibody: a novel engineered anti-carcinoembryonic antigen antibody fragment (single-chain Fv-CH3) which exhibits rapid, high-level targeting of xenografts. Cancer Res. 56, 3055–3061 (1996).
Wu, A. M. et al. Tumor localization of anti-CEA single-chain Fvs: improved targeting by non-covalent dimers. Immunotechnology 2, 21–36 (1996).
Cheung, N. K. V. et al. Single-chain Fv-streptavidin substantially improved therapeutic index in multistep targeting directed at disialoganglioside GD2. J. Nucl. Med. 45, 867–877 (2004).
Boerman, O. C. et al. Pretargeting of renal cell carcinoma: improved tumor targeting with a bivalent chelate. Cancer Res. 59, 4400–4405 (1999).
Chang, C. H. et al. Molecular advances in pretargeting radioimunotherapy with bispecific antibodies. Mol. Cancer Ther. 1, 553–563 (2002).
McBride, W. J. et al. Bispecific antibody pretargeting PET (ImmunoPET) with an I-124-labeled hapten-peptide. J. Nucl. Med. 47, 1678–1688 (2006).
Catz, B. et al. Treatment of cancer of the thyroid postoperatively with suppressive thyroid medication, radioactive iodine, and thyroid-stimulating hormone. Cancer 12, 371–383 (1959).
Hamilton, J. G. & Soley, M. H. Studies in iodine metabolism of the thyroid gland in situ by the use of radio-iodine in normal subjects and in patients with various types of goiter. Am. J. Physiol. 131, 0135–0143 (1940).
Benua, R. S., Rawson, R. W., Sonenberg, M. & Cicale, N. R. Relation of radioiodine dosimetry to results and complications in treatment of metastatic thyroid cancer. Am. J. Roentgenol. Radium Ther. Nucl. Med. 87, 171–182 (1962).
Maxon, H. R. et al. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N. Engl. J. Med. 309, 937–941 (1983).
Livingood, J. J. & Seaborg, G. T. Radioactive iodine isotopes. Phys. Rev. 53, 1015 (1938).
Knapp, R. F. F. & Dash, A. Radiopharmaceuticals for Therapy - 2016. (Springer, 2016).
Hertz, S., Roberts, A., Means, J. H. & Evans, R. D. Radioactive iodine as an indicator in thyroid physiology - Iodine collection by normal and hyperplastic thyroids in rabbits. Am. J. Physiol. 128, 565–576 (1940).
Cooper, D. S. et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19, 1167–1214 (2009).
Fagin, J. A. & Wells, S. A. Jr. Biologic and clinical perspectives on thyroid cancer. N. Engl. J. Med. 375, 1054–1067 (2016).
Gao, W. et al. Internal radiotherapy using 32P colloid or microsphere for refractory solid tumors. Ann. Nucl. Med. 22, 653–660 (2008).
Morris, M. J. et al. Radium-223 mechanism of action: implications for use in treatment combinations. Nat. Rev. Urol. 16, 745–756 (2019).
Leung, C. N. et al. Dose-dependent growth delay of breast cancer xenografts in the bone marrow of mice treated with 223Ra: the role of bystander effects and their potential for therapy. J. Nucl. Med. 61, 89–95 (2020).
Tombal, B. F. et al. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: an interim safety analysis. J. Clin. Oncol. 37, 5007 (2019).
Smith, M. R. et al. ERA 223: a phase 3 trial of radium-223 dichloride (Ra-223) in combination with abiraterone acetate (abiraterone) and prednisone in the treatment of asymptomatic or mildly symptomatic chemotherapy-naïve patients (pts) with bone predominant metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 33, TPS5082 (2015).
Dalla Volta, A., Formenti, A. M. & Berruti, A. Higher risk of fragility fractures in prostate cancer patients treated with combined radium-223 and abiraterone: prednisone may be the culprit. Eur. Urol. 75, 894–895 (2019).
Sartor, O. Overview of samarium Sm 153 lexidronam in the treatment of painful metastatic bone disease. Rev. Urol. 6, S3–S12 (2004).
Anderson, P. M., Subbiah, V. & Rohren, E. In: Current Advances in Osteosarcoma. 291–304 (Springer International Publishing, 2014).
Longo, J., Lutz, S. & Johnstone, C. Samarium-153-ethylene diamine tetramethylene phosphonate, a beta-emitting bone-targeted radiopharmaceutical, useful for patients with osteoblastic bone metastases. Cancer Manag. Res. 5, 235–242 (2013).
Chirby, D., Franck, S. & Troutner, D. E. Adsorption of 153Sm-EDTMP on calcium hydroxyapatite. Int. J. Radiat. Appl. Instrum. Part A Appl. Radiat. Isotopes 39, 495–499 (1988).
Eary, J. F. et al. Samarium-153-EDTMP biodistribution and dosimetry estimation. J. Nucl. Med. 34, 1031–1036 (1993).
Goyal, J. & Antonarakis, E. S. Bone-targeting radiopharmaceuticals for the treatment of prostate cancer with bone metastases. Cancer Lett. 323, 135–146 (2012).
Simon, J. J. et al. Preclinical evaluation of Sm-153-DOTMP as a therapeutic bone-seeking radiopharmaceutical. J. Nucl. Med. 52, 1751 (2011).
Simón, J. et al. A preclinical investigation of the saturation and dosimetry of 153Sm-DOTMP as a bone-seeking radiopharmaceutical. Nucl. Med. Biol. 39, 770–776 (2012).
Schmidt, M., Hero, B. & Simon, T. I-131-mIBG therapy in neuroblastoma: established role and prospective applications. Clin. Transl Imaging 4, 87–101 (2016).
Schoot, R. A. et al. The role of 131I-metaiodobenzylguanidine (MIBG) therapy in unresectable and compromising localised neuroblastoma. Eur. J. Nucl. Med. Mol. Imaging 40, 1516–1522 (2013).
George, S. L. et al. Individualized I-131-mIBG therapy in the management of refractory and relapsed neuroblastoma. Nucl. Med. Commun. 37, 466–472 (2016).
Eisenhut, M. M. W. in Handbook of Nuclear Chemistry. (eds Vértes, A. N. S., Klencsár, Z., Lovas, R. G., Rösch, F.) (Springer, 2011).
Inaki, A. et al. A phase I clinical trial for [131I]meta-iodobenzylguanidine therapy in patients with refractory pheochromocytoma and paraganglioma: a study protocol. J. Med. Invest. 64, 205–209 (2017).
Modak, S. et al. Arsenic trioxide as a radiation sensitizer for 131I-metaiodobenzylguanidine therapy: results of a phase II study. J. Nucl. Med. 57, 231–237 (2016).
Shilkrut, M., Bar-Deroma, R., Bar-Sela, G., Berniger, A. & Kuten, A. Low-dose iodine-131 metaiodobenzylguanidine therapy for patients with malignant pheochromocytoma and paraganglioma: single center experience. Am. J. Clin. Oncol. 33, 79–82 (2010).
Coleman, R. E. et al. Radiation dosimetry, pharmacokinetics, and safety of ultratrace Iobenguane I-131 in patients with malignant pheochromocytoma/paraganglioma or metastatic carcinoid. Cancer Biother. Radiopharm 24, 469–475 (2009).
Gonias, S. et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J. Clin. Oncol. 27, 4162–4168 (2009).
Fitzgerald, P. A. et al. Malignant pheochromocytomas and paragangliomas: a phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG). Ann. NY Acad. Sci. 1073, 465–490 (2006).
Sisson, J. C. et al. Treatment of malignant pheochromocytomas with 131-I metaiodobenzylguanidine and chemotherapy. Am. J. Clin. Oncol. 22, 364–370 (1999).
Mundschenk, J., Kopf, D. & Lehnert, H. Therapy of malignant pheochromocytoma. Invitation to participate in a randomized multicenter study. Dtsch. medizinische Wochenschr. 123, 32–33 (1998).
Noto, R. B. et al. Phase 1 study of high-specific-activity I-131 MIBG for metastatic and/or recurrent pheochromocytoma or paraganglioma. J. Clin. Endocrinol. Metab. 103, 213–220 (2017).
Pinto, J. T. et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin. Cancer Res. 2, 1445–1451 (1996).
Heston, W. D. Characterization and glutamyl preferring carboxypeptidase function of prostate specific membrane antigen: a novel folate hydrolase. Urology 49 (Suppl. 3A), 104–112 (1997).
Murphy, D. G., Sathianathen, N., Hofman, M. S., Azad, A. & Lawrentschuk, N. Where to next for theranostics in prostate cancer? Eur. Urol. Oncol. 2, 163–165 (2019).
Tateishi, U. Prostate-specific membrane antigen (PSMA)–ligand positron emission tomography and radioligand therapy (RLT) of prostate cancer. Jap. J. Clin. Oncol. 50, 349–356 (2020).
Novakova, Z. et al. Design of composite inhibitors targeting glutamate carboxypeptidase II: the importance of effector functionalities. FEBS J. 283, 130–143 (2016).
Banerjee, S. R. et al. 68Ga-labeled inhibitors of prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J. Med. Chem. 53, 5333–5341 (2010).
Barinka, C. et al. Structural insight into the pharmacophore pocket of human glutamate carboxypeptidase II. J. Med. Chem. 50, 3267–3273 (2007).
Bařinka, C., Rojas, C., Slusher, B. & Pomper, M. Glutamate carboxypeptidase II in diagnosis and treatment of neurologic disorders and prostate cancer. Curr. Med. Chem. 19, 856–870 (2012).
Kozikowski, A. P. et al. Synthesis of urea-based inhibitors as active site probes of glutamate carboxypeptidase II: efficacy as analgesic agents. J. Med. Chem. 47, 1729–1738 (2004). Early report on small-molecule PSMA inhibitors.
Zhou, J., Neale, J. H., Pomper, M. G. & Kozikowski, A. P. NAAG peptidase inhibitors and their potential for diagnosis and therapy. Nat. Rev. Drug Discov. 4, 1015–1026 (2005).
Wu, L. Y. et al. The molecular pruning of a phosphoramidate peptidomimetic inhibitor of prostate-specific membrane antigen. Bioorg. Med. Chem. 15, 7434–7443 (2007).
Liu, T., Toriyabe, Y., Kazak, M. & Berkman, C. E. Pseudoirreversible inhibition of prostate-specific membrane antigen by phosphoramidate peptidomimetics. Biochemistry 47, 12658–12660 (2008).
Choy, C. J. et al. Lu-177-labeled phosphoramidate-based PSMA inhibitors: the effect of an albumin binder on biodistribution and therapeutic efficacy in prostate tumor-bearing mice. Theranostics 7, 1928–1939 (2017).
Hofman, M. S. et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 19, 825–833 (2018). Efficacy and toxicity of anti-PSMA therapy in prostate cancer using lutetium-177.
Derlin, T. & Schmuck, S. [177Lu]-PSMA-617 radionuclide therapy in patients with metastatic castration-resistant prostate cancer. Lancet Oncol. 19, e372 (2018).
Rahbar, K., Ahmadzadehfar, H., Seifert, R. & Boegemann, M. [177Lu]-PSMA-617 radionuclide therapy in patients with metastatic castration-resistant prostate cancer. Lancet Oncol. 19, e371 (2018).
Hofman, M. S., Violet, J., Hicks, R. J. & Sandhu, S. [177Lu]-PSMA-617 radionuclide therapy in patients with metastatic castration-resistant prostate cancer - Author’s reply. Lancet Oncol. 19, e373 (2018).
Muzio, V. et al. Assessment of in vivo biodistribution and treatment efficacy of Lu-177 PSMA-R2 and Lu-177-PSMA-617 on mice bearing prostate cancer tumors. Eur. J. Nucl. Med. Mol. Imaging 46 (Suppl. 1), 17 (2019).
Nedrow-Byers, J. R. et al. A phosphoramidate-based prostate-specific membrane antigen-targeted SPECT agent. Prostate 72, 904–912 (2012).
Choy, C. J. et al. 177Lu-labeled phosphoramidate-based PSMA inhibitors: the effect of an Albumin Binder on biodistribution and therapeutic efficacy in prostate tumor-bearing mice. Theranostics 7, 1928–1939 (2017).
Gourni, E. & Henriksen, G. Metal-based PSMA radioligands. Molecules 22, 523 (2017).
Wester, H.-J. & Schottelius, M. PSMA-targeted radiopharmaceuticals for imaging and therapy. Semin. Nucl. Med. 49, 302–312 (2019).
Ross, J. F., Chaudhuri, P. K. & Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and Clinical Implications. Cancer 73, 2432–2443 (1994).
Cheung, A. et al. Targeting folate receptor alpha for cancer treatment. Oncotarget. 7, 52553–52574 (2016).
Kettenbach, K. et al. Comparison study of two differently clicked 18F-folates-lipophilicity plays a key role. Pharmaceuticals 11, 30 (2018).
Siwowska, K. & Müller, C. Preclinical development of small-molecular-weight folate-based radioconjugates: a pharmacological perspective. Q. J. Nucl. Med. Mol. Imaging 59, 269–286 (2015).
Siwowska, K. et al. Therapeutic potential of 47Sc in comparison to 177Lu and 90Y: preclinical investigations. Pharmaceutics 11, 424 (2019).
Gupta, A. et al. Voxel-based dosimetry of iron oxide nanoparticle-conjugated 177Lu-labeled folic acid using SPECT/CT imaging of mice. Mol. Pharmaceutics. 16, 1498–1506 (2019).
Müller, C. et al. Direct in vitro and in vivo comparison of 161Tb and 177Lu using a tumour-targeting folate conjugate. Eur. J. Nucl. Med. Mol. Imaging 41, 476–485 (2014).
Snyder, F. & Wood, R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 29, 251–257 (1969).
Snyder, F., Blank, M. L. & Morris, H. P. Occurrence and nature of O-alkyl and O-alk-I-enyl moieties of glycerol in lipids of Morris transplanted hepatomas and normal rat liver. Biochim. Biophys. Acta 176, 502–510 (1969).
Counsell, R. E., Schwendner, S. W., Meyer, K. L., Haradahira, T. & Gross, M. D. Tumor visualization with a radioiodinated phospholipid ether. J. Nucl. Med. 31, 332–336 (1990).
Meyer, K. L., Schwendner, S. W. & Counsell, R. E. Potential tumor or organ-imaging agents. 30. Radioiodinated phospholipid ethers. J. Med. Chem. 32, 2142–2147 (1989).
Pinchuk, A. N. et al. Synthesis and structure−activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. J. Med. Chem. 49, 2155–2165 (2006).
Weichert, J. P. et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Sci. Transl Med. 6, 240ra75 (2014). Phosphocholine-based RPT.
Baiu, D. C. et al. Targeted molecular radiotherapy of pediatric solid tumors using a radioiodinated alkyl-phospholipid ether analog. J. Nucl. Med. 59, 244–250 (2018).
Hall, L. T. et al. PET/CT imaging of the diapeutic alkylphosphocholine analog I-124-CLR1404 in high and low-grade brain tumors. Am. J. Nucl. Med. Mol. Imaging 7, 157–166 (2017).
Hall, L. T. et al. I-124 CLR1404 PET/CT in high-grade primary and metastatic brain tumors. Mol. Imaging Biol. 22, 434–443 (2020).
Morris, Z. S. et al. Therapeutic combination of radiolabeled CLR1404 with external beam radiation in head and neck cancer model systems. Radiother. Oncol. 116, 504–509 (2015).
Bodei, L., Kwekkeboom, D. J., Kidd, M., Modlin, I. M. & Krenning, E. P. Radiolabeled somatostatin analogue therapy of gastroenteropancreatic cancer. Semin. Nucl. Med. 46, 225–238 (2016).
Fani, M., Nicolas, G. P. & Wild, D. Somatostatin receptor antagonists for imaging and therapy. J. Nucl. Med. 58, 61S–66S (2017). Trial that demonstrates the greater tumour uptake and absorbed dose with somatostatin receptor antagonists compared with agonists.
Strosberg, J. et al. Phase 3 trial of Lu-177-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Strosberg, J. et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the phase III NETTER-1 trial. J. Clin. Oncol. 36, 2578–2584 (2018). Trial that led to FDA approval of 177Lu-DOTATATE.
Kwekkeboom, D. J. et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J. Clin. Oncol. 26, 2124–2130 (2008).
Atcher, R. W., Friedman, A. M. & Hines, J. J. An improved generator for the production of Pb-212 and Bi-212 from Ra-224. Appl. Radiat. Isot. 39, 283–286 (1988).
Delpassand, E. et al. First clinical experience using targeted alpha-emitter therapy with Pb-212-DOTAMTATE (AlphaMedix TM) in patients with SSTR(+) neuroendocrine tumors. J. Nucl. Med. 60, (2019).
Stallons, T. A. R., Saidi, A., Tworowska, I., Delpassand, E. S. & Torgue, J. J. Preclinical Investigation of Pb-212-DOTAMTATE for peptide receptor radionuclide therapy in a neuroendocrine tumor model. Mol. Cancer Ther 18, 1012–1021 (2019).
Bodei, L. et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 42, 5–19 (2015). Study that demonstrates the toxicity profile of PRRT with 90Y, 177Lu or their combination in a large series of patients.
Reidy-Lagunes, D. et al. Phase I trial of well-differentiated neuroendocrine tumors (NETs) with radiolabeled somatostatin antagonist 177Lu-satoreotide tetraxetan. Clin. Cancer Res. 25, 6939–6947 (2019).
Jensen, R. T., Battey, J. F., Spindel, E. R. & Benya, R. V. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 60, 1–42 (2008).
Baratto, L., Jadvar, H. & Iagaru, A. Prostate cancer theranostics targeting gastrin-releasing peptide receptors. Mol. Imaging Biol. 20, 501–509 (2018).
Pooja, D., Gunukula, A., Gupta, N., Adams, D. J. & Kulhari, H. Bombesin receptors as potential targets for anticancer drug delivery and imaging. Int. J. Biochem. Cell Biol. 114, 105567 (2019).
Morgat, C. et al. Comparison of the binding of the gastrin-releasing peptide receptor (GRP-R) antagonist 68Ga-RM2 and 18F-FDG in breast cancer samples. PLoS ONE 14, e0210905 (2019).
Abouzayed, A. et al. Synthesis and preclinical evaluation of radio-iodinated GRPR/PSMA bispecific heterodimers for the theranostics application in prostate cancer. Pharmaceutics 11, 358 (2019).
Liolios, C., Sachpekidis, C., Schäfer, M. & Kopka, K. Bispecific radioligands targeting prostate-specific membrane antigen and gastrin-releasing peptide receptors on the surface of prostate cancer cells. J. Label. Comp. Radiopharm. 62, 510–522 (2019).
Baratto, L., Duan, H., Maecke, H. R. & Iagaru, A. Imaging the distribution of gastrin releasing peptide receptors in cancer. J. Nucl. Med 61, 792–798 (2020).
Bodei, L. et al. Lu-177-AMBA bombesin analogue in hormone refractory prostate cancer patients: a phase I escalation study with single-cycle administrations. Eur. J. Nucl. Med. Mol. Imaging 34, S221 (2007).
Cescato, R. et al. Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J. Nucl. Med. 49, 318–326 (2008). Comparative in vitro/in vivo study indicating that GRPR antagonists may be superior targeting agents to GRPR agonists.
Dalm, S. U. et al. 68Ga/177Lu-NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology. J. Nucl. Med. 58, 293–299 (2017).
Kurth, J. et al. First-in-human dosimetry of gastrin-releasing peptide receptor antagonist [177Lu]Lu-RM2: a radiopharmaceutical for the treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 47, 123–135 (2020).
Thomas, V. A. & Balthasar, J. P. Understanding inter-individual variability in monoclonal antibody disposition. Antibodies 8, E56 (2019).
Wohlrab, J. Pharmacokinetic characteristics of therapeutic antibodies. JDDG 13, 530–534 (2015).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975). Nobel Prize-winning work to produce antibodies from a single clone, an essential precursor to antibody-based RPT.
Larson, S. M. et al. Imaging of melanoma with I-131-labeled monoclonal antibodies. J. Nucl. Med. 24, 123–129 (1983). Among the earliest reports of antibody-based cancer imaging; precursor to antibody-based RPT.
Mach, J. P. et al. Tumor localization in patients by radiolabeled monoclonal antibodies against colon carcinoma. Cancer Res. 43, 5593–5600 (1983). Among the earliest reports of antibody-based cancer imaging; precursor to antibody-based RPT.
Colcher, D. et al. Radiolabeled monoclonal-antibody B72.3 localization in metastatic lesions of colorectal-cancer patients. Nucl. Med. Biol. 14, 251 (1987).
Goldenberg, D. M. et al. Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. N. Engl. J. Med. 298, 1384–1386 (1978). Among the earliest reports of antibody-based cancer imaging; precursor to antibody-based RPT.
Mahe, M. A. et al. A phase II study of intraperitoneal radioimmunotherapy with iodine-131-labeled monoclonal antibody OC-125 in patients with residual ovarian carcinoma. Clin. Cancer Res. 5 (Suppl. 10), 3249–3253 (1999).
Cassaday, R. D. et al. Phase I study of a CD45-targeted antibody-radionuclide conjugate for high-risk lymphoma. Clin. Cancer Res. 25, 6932–6938 (2019).
Tomlinson, B. K. et al. Rapid reduction of peripheral blasts in older patients with refractory acute myeloid leukemia (AML) using re-induction with single agent anti-CD45 targeted iodine (I-131) apamistamab Iomab-B radioimmunotherapy in the phase III SIERRA trial. Clin. Lymphoma Myeloma Leukemia 19, S232 (2019).
Pagel, J. M. et al. Allogeneic hematopoietic cell transplantation after conditioning with I-131-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 114, 5444–5453 (2009).
Gopal, A. K., Pagel, J. M., Fromm, J. R., Wilbur, S. & Press, O. W. I-131 anti-CD45 radioimmunotherapy effectively targets and treats T-cell non-Hodgkin lymphoma. Blood 113, 5905–5910 (2009).
Mawad, R. et al. Radiolabeled anti-CD45 antibody with reduced-intensity conditioning and allogeneic transplantation for younger patients with advanced acute myeloid leukemia or myelodysplastic syndrome. Biol. Blood Marrow Transplant. 20, 1363–1368 (2014).
Tomlinson, B. et al Rapid reduction of peripheral blasts in older patients with refractory acute myeloid leukemia (AML) using reinduction with single agent anti-CD45 targeted iodine (I-131) apamistamab Iomab-B radioimmunotherapy in the phase III SIERRA trial. J. Clin. Oncol. 37 (Suppl. 15), 7048 (2019).
Griffeth, L. et al. Personalized dosimetry using I-131-anti-CD45-apamistamab (Iomab-B) prior to high-dose myeloablative radioimmunotherapy for hematopoietic stem cell transplant (HCT) in active, relapsed, or refractory acute myelogenous leukemia: novel re-induction and targeted conditioning feasibility and engraftment results from the SIERRA trial. J. Nucl. Med. 60 (Suppl. 1), 434 (2019).
Pandit-Taskar, N. et al. Optimizing dosimetry imaging in high-dose radioimmunotherapy using the novel, anti-CD45 re-induction and targeted conditioning agent iodine (I-131) apamistamab Iomab-B in patients 55 years or older with active, relapsed or refractory acute myeloid leukemia (SIE phase III trial). J. Nucl. Med. 60 (Suppl. 1), 433 (2019).
Schwartz, M. A. et al. Dose-escalation trial of M195 labeled with I-131 for cytoreduction and marrow ablation in relapsed or refractory myeloid leukemias. J. Clin. Oncol. 11, 294–303 (1993).
Pagel, J. M. et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood 114, 5444–5453 (2009).
Press, O. W. et al. Phase II trial of 131I-B1 (anti-CD20) antibody therapy with autologous stem cell transplantation for relapsed B cell lymphomas. Lancet 346, 336–340 (1995). Use of RPT for bone marrow ablation in preparation for transplantation.
Orozco, J. J. et al. Anti-CD45 radioimmunotherapy using At-211 with bone marrow transplantation prolongs survival in a disseminated murine leukemia model. Blood 121, 3759–3767 (2013).
Li, Y. W. et al. cGMP production of astatine-211-labeled anti-CD45 antibodies for use in allogeneic hematopoietic cell transplantation for treatment of advanced hematopoietic malignancies. Plos ONE 13, e0205135 (2018).
Scheinberg, D. A. et al. A phase I trial of monoclonal antibody M195 in acute myelogenous leukemia: specific bone marrow targeting and internalization of radionuclide. J. Clin. Oncol. 9, 478–490 (1991).
Sgouros, G. et al. Pharmacokinetics and dosimetry of an alpha-particle emitter labeled antibody: 213Bi-HuM195 (anti-CD33) in patients with leukemia. J. Nucl. Med. 40, 1935–1946 (1999). Dosimetry for α-emitter RPT in humans; one of the first such reports.
McDevitt, M. R., Finn, R. D., Sgouros, G., Ma, D. & Scheinberg, D. A. An 225Ac/213Bi generator system for therapeutic clinical applications: construction and operation. Appl. Radiat. Isot. 50, 895–904 (1999).
McDevitt, M. R., Finn, R. D., Ma, D., Larson, S. M. & Scheinberg, D. A. Preparation of alpha-emitting 213Bi-labeled antibody constructs for clinical use. J. Nucl. Med. 40, 1722–1727 (1999).
Kolbert, K. S. et al. Parametric images of antibody pharmacokinetics in Bi213-HuM195 therapy of leukemia. J. Nucl. Med. 42, 27–32 (2001).
Rosenblat, T. L. et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin. Cancer Res. 16, 5303–5311 (2010). Clinical α-particle therapy.
Jurcic, J. G. Targeted alpha-particle therapy for hematologic malignancies. J. Med. Imaging Radiat. Sci 50, S53–S57 (2019).
Jurcic, J. G. et al. Phase I trial of the targeted alpha-particle nano-generator actinium-225 (225Ac-lintuzumab) (anti-CD33; HuM195) in acute myeloid leukemia (AML). J. Clin. Oncol. 29, 6516 (2011). Clinical α-particle therapy.
Jurcic, J. G. et al. Phase I trial of the targeted alpha-particle nano-generator actinium-225 (225Ac)-lintuzumab (anti-CD33) in combination with low-dose cytarabine (LDAC) for older patients with untreated acute myeloid leukemia (AML). Blood 122, 1460 (2013).
Jurcic, J. G. Targeted alpha-particle therapy for hematologic malignancies. Semin. Nucl. Med. 50, 152–161 (2020).
Berger, M. S. et al. Actinium labeled daratumumab demonstrates enhanced killing of multiple myeloma cells over naked daratumumab. Blood 130, (2017).
Dadachova, E. et al. Ac-225-CD38 antibody targeting is effective and well tolerated in experimental models of lymphoma and multiple myeloma. J. Nucl. Med. 60 (Suppl. 1), 1410 (2019).
Juergens, R. A. et al. A phase I study of Ac-225 -FPI-1434 radioimmunotherapy in patients with IGF-1R expressing solid tumors (Poster). J. Clin. Oncol. 37, TPS3152 (2019).
Hagemann, U. B. et al. Advances in precision oncology: targeted thorium-227 conjugates as a new modality in targeted alpha therapy. Cancer Biother. Radiopharm https://doi.org/10.1089/cbr.2020.3568 (2020).
Hagemann, U. B. et al. A novel high energy alpha pharmaceutical: in vitro and in vivo potency of a mesothelin-targeted thorium-227 conjugate (TTC) in a model of bone disease. Cancer Res. 76, 591 (2016).
Hagemann, U. B. et al. Mesothelin-targeted thorium-227 conjugate (MSLN-TTC): preclinical evaluation of a new targeted alpha therapy for mesothelin-positive cancers. Clin. Cancer Res. 25, 4723–4734 (2019).
Hammer, S. et al. Preclinical pharmacology of the PSMA-targeted thorium-227 conjugate PSMA-TTC: a novel targeted alpha therapeutic for the treatment of prostate cancer. Cancer Res. https://doi.org/10.1158/1538-7445.AM2017-5200 (2017).
Hammer, S. et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted alpha therapy for prostate cancer. Clin. Cancer Res. 26, 1985–1996 (2020).
Grant, D. et al. Pharmacokinetics and dosimetry of BAY 1862864, an alpha-emitting targeted thorium conjugate (CD22-TTC) in the Cynomolgus monkey. Eur. J. Nucl. Med. Mol. Imaging 45, S124 (2018).
Karlsson, J. et al. HER2-targeted thorium-227 conjugate (HER2-TTC): efficacy in a HER2 positive orthotopic bone model. Cancer Res. https://doi.org/10.1158/1538-7445.AM2017-5857 (2017).
Karlsson, J. et al. HER2-targeted thorium-227 conjugate (HER2-TTC): efficacy in preclinical models of trastuzumab and T-DM1 resistance. Cancer Res. https://doi.org/10.1158/1538-7445.AM2017-5859 (2017).
Karlsson, J. et al. In vitro and in vivo activity of a HER2-targeted thorium-227 conjugate (HER2-TTC) in HER2 low expressing and T-DM1/trastuzumab resistant preclinical mouse models. Eur. J. Cancer 103, E122 (2018).
Wickstroem, K. et al. Synergistic effect of a HER2 targeted thorium-227 conjugate in combination with olaparib in a BRCA2 deficient xenograft model. Pharmaceuticals 12, 155 (2019).
Waldmann, T. ABCs of radioisotopes used for radioimmunotherapy: alpha- and beta-emitters. Leuk. Lymphoma. 44, S107–S113 (2003).
Wahl, R. L. et al. Iodine-131 anti-B1 antibody for B-cell lymphoma: an update on the Michigan phase I experience. J. Nucl. Med. 39 (Suppl. 8), 21–27 (1998).
Wahl, R. L. Iodine-131 anti-B1 antibody therapy in non-Hodgkin’s lymphoma: dosimetry and clinical implications. J. Nucl. Med. 39 (Suppl. 8), 1 (1998).
Wahl, R. L., Kroll, S. & Zasadny, K. R. Patient-specific whole-body dosimetry: principles and a simplified method for clinical implementation. J. Nucl. Med. 39 (Suppl. 8), 14–20 (1998).
Witzig, T. E. et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J. Clin. Oncol. 20, 3262–3269 (2002).
Davis, T. A. et al. Results of a randomized study of Bexxar (TM) (tositumomab and iodine I 131 tositumomab) vs. unlabeled tositumomab in patients with relapsed or refractory low-grade or transformed non-Hodgkin’s lymphoma (NHL). Blood 98, 843A (2001).
Wahl, R. L. The clinical importance of dosimetry in radioimmunotherapy with tositumomab and iodine I 131 tositumomab. Semin. Oncol. 30, 31–38 (2003).
Baxter, L. T. & Jain, R. K. Transport of fluid and macromolecules in tumors. IV. A microscopic model of the perivascular distribution. Microvasc. Res. 41, 252–272 (1991).
Jain, R. K. & Baxter, L. T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 48, 7022–7032 (1988).
Jain, R. K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 6, 559–593 (1987).
Saga, T. et al. Targeting cancer micrometastases with monoclonal antibodies: a binding-site barrier. Proc. Natl Acad. Sci. USA 92, 8999–9003 (1995).
van Osdol, W., Fujimori, K. & Weinstein, J. N. An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a “binding site barrier”. Cancer Res. 51, 4776–4784 (1991).
Fujimori, K., Covell, D. G., Fletcher, J. E. & Weinstein, J. N. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J. Nucl. Med. 31, 1191–1198 (1990).
Sgouros, G. Plasmapheresis in radioimmunotherapy of micrometastases: a mathematical modeling and dosimetrical analysis [see comments]. J. Nucl. Med. 33, 2167–2179 (1992).
Jaggi, J. S. et al. Improved tumor imaging and therapy via i.v. IgG-mediated time-sequential modulation of neonatal Fc receptor. J. Clin. Invest 117, 2422–2430 (2007).
Goodwin, D. A., Meares, C. F. & Osen, M. Biological properties of biotin-chelate conjugates for pretargeted diagnosis and therapy with the avidin/biotin system. J. Nucl. Med. 39, 1813–1818 (1998). Initial approach to separating radionuclide delivery from cancer targeting to reduce haematological toxicity.
Verhoeven, M., Seimbille, Y. & Dalm, S. U. Therapeutic applications of pretargeting. Pharmaceutics 11, 434 (2019).
Liapi, E. & Geschwind, J.-F. H. Intra-arterial therapies for hepatocellular carcinoma: where do we stand? Ann. Surg. Oncol. 17, 1234–1246 (2010).
Lewandowski, R. J., Geschwind, J.-F., Liapi, E. & Salem, R. Transcatheter intraarterial therapies: rationale and overview. Radiology 259, 641–657 (2011).
Lewandowski, R. J. & Salem, R. Yttrium-90 radioembolization of hepatocellular carcinoma and metastatic disease to the liver. Semin. Intervent. Radiol. 23, 64–72 (2006).
Riaz, A., Awais, R. & Salem, R. Side effects of yttrium-90 radioembolization. Front. Oncol. 4, 198 (2014).
Van Der Gucht, A. et al. Resin versus glass microspheres for 90Y transarterial radioembolization: comparing survival in unresectable hepatocellular carcinoma using pretreatment partition model dosimetry. J. Nucl. Med. 58, 1334–1340 (2017).
Biederman, D. M. et al. Outcomes of radioembolization in the treatment of hepatocellular carcinoma with portal vein invasion: resin versus glass microspheres. J. Vasc. Intervent. Radiol. 27, 812–21 (2016).
Mumper, R. J., Ryo, U. Y. & Jay, M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres - a potential agent for the internal radiation-therapy of hepatic-tumors. J. Nucl. Med. 32, 2139–2143 (1991).
Smits, M. L. J. et al. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol. 13, 1025–1034 (2012).
Arranja, A. G., Hennink, W. E., Chassagne, C., Denkova, A. G. & Nijsen, J. F. W. Preparation and characterization of inorganic radioactive holmium-166 microspheres for internal radionuclide therapy. Mater. Sci. Eng. C Mater. Biol. Applications. 106, 110244 (2020).
Wang, X.-M. et al. Preventive effect of regional radiotherapy with phosphorus-32 glass microspheres in hepatocellular carcinoma recurrence after hepatectomy. World J. Gastroenterol. 14, 518–523 (2008).
Jeong, J. M. & Chung, J.-K. Therapy with 188Re-labeled radiopharmaceuticals: an overview of promising results from initial clinical trials. Cancer Biother. Radiopharm. 18, 707–717 (2003).
Bretagne, J. F. et al. Hepatic artery injection of I-131-labeled lipiodol. Part II. Preliminary results therapeutic use patients Hepatocellular carcinoma liver metastases. Radiology 168, 547–550 (1988).
Raoul, J. L. et al. Hepatic artery injection of I-131-labeled lipiodol. Part I. Biodistribution study results in patients with hepatocellular carcinoma and liver metastases. Radiology 168, 541–545 (1988).
Raoul, J. I. et al. Internal radiation therapy for hepatocellular carcinoma. Results of a French multicenter phase II trial of transarterial injection of iodine 131-labeled Lipiodol. Cancer 69, 346–352 (1992).
Raoul, J. L. et al. Randomized controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131-iodized oil versus medical support. J. Nucl. Med. 35, 1782–1787 (1994).
Schwarz, L. et al. Adjuvant I-131 Lipiodol after resection or radiofrequency ablation for hepatocellular carcinoma. World J. Surg. 40, 1941–1950 (2016).
Lau, W. Y. et al. Adjuvant intra-arterial lipiodol-iodine-131 for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet 353, 797–801 (1999).
Lau, W. Y., Lai, E. C. H., Leung, T. W. T. & Yu, S. C. H. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: a prospective randomized trial-update on 5-year and 10-year survival. Ann. Surg. 247, 43–48 (2008).
Berenson, A. Market Forces Cited in Lymphoma Drugs’ Disuse. New York Times https://www.nytimes.com/2007/07/14/health/14lymphoma.html (2007).
Mercadante, S. & Fulfaro, F. Management of painful bone metastases. Curr. Opin. Oncol. 19, 308–314 (2007).
John, K. U. S. DOE tri-lab production effort to provide accelerator-produced 225Ac for radiotherapy: 2019 update. J. Nucl. Med. 60, 1612 (2019).
Hoehr, C. et al. Medical isotope production at TRIUMF – from imaging to treatment. Phys. Procedia. 90, 200–208 (2017).
Karimian, A., Ji, N. T., Song, H. & Sgouros, G. Mathematical modeling of pre-clinical alpha-emitter radiopharmaceutical therapy. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-19-2553 (2019).
Eckerman, K. & Endo, A. ICRP publication 107. Nuclear decay data for dosimetric calculations. Ann. ICRP 38, 7–96 (2007).
Papadimitroulas, P., Loudos, G., Nikiforidis, G. C. & Kagadis, G. C. A dose point kernel database using GATE Monte Carlo simulation toolkit for nuclear medicine applications: comparison with other Monte Carlo codes. Med. Phys. 39, 5238–5247 (2012).
Simpkin, D. J. & Mackie, T. R. EGS4 Monte-Carlo determination of the beta-dose kernel in water. Med. Phys. 17, 179–186 (1990).
[No authors listed.] Report 90: Key data for ionizing-radiation dosimetry: measurement standards and applications. J. Int. Comm. Rad. Units Meas. https://doi.org/10.1093/jicru/ndw043 (2016).
Schlom, J. et al. Monoclonal antibody-based therapy of a human tumor xenograft with a 177lutetium-labeled immunoconjugate. Cancer Res. 51, 2889–2896 (1991).
Kopka, K., Benešová, M., Barinka, C., Haberkorn, U. & Babich, J. Glu-ureido–based inhibitors of prostate-specific membrane antigen: lessons learned during the development of a novel class of low-molecular-weight theranostic radiotracers. J. Nucl. Med. 58, 17S–26S (2017).
Maurer, T., Eiber, M., Schwaiger, M. & Gschwend, J. E. Current use of PSMA–PET in prostate cancer management. Nat. Rev. Urol. 13, 226–235 (2016).
Muller, C. & Schibli, R. Folic acid conjugates for nuclear imaging of folate receptor-positive cancer. J. Nucl. Med. 52, 1–4 (2011).
Larson, S. M., Carrasquillo, J. A., Cheung, N. K. & Press, O. W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 15, 347–360 (2015).
Loevinger, R. & Berman, M. A schema for calculating the absorbed dose from biologically distributed radionuclides. MIRD pamphlet no. 1. J. Nucl. Med. 9, 5 (1968).
Ljungberg, M. et al. MIRD pamphlet no. 26: joint EANM/MIRD guidelines for quantitative 177Lu SPECT applied for dosimetry of radiopharmaceutical therapy. J. Nucl. Med. 57, 151–162 (2016).
Prideaux, A. R. et al. Three-dimensional radiobiologic dosimetry: application of radiobiologic modeling to patient-specific 3-dimensional imaging-based internal dosimetry. J. Nucl. Med. 48, 1008–1016 (2007).
Sgouros, G. et al. Three-dimensional imaging-based radiobiological dosimetry. Semin. Nucl. Med. 38, 321–334 (2008).
Sgouros, G. et al. Treatment planning for internal radionuclide therapy: three-dimensional dosimetry for nonuniformly distributed radionuclides. J. Nucl. Med. 31, 1884–1891 (1990). Imaging-based patient specific dosimetry for RPT treatment planning.
Acknowledgements
G.S. acknowledges NIH grants R01CA116477 and R01CA187037.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.S. is a founder of and holds equity in Radiopharmaceutical Imaging and Dosimetry LLC (Rapid). He serves as a member of Rapid’s Board of Directors; this arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. M.M. was a consultant for Actinium Pharmaceuticals, Regeneron, Progenics, Bridge Medicine and General Electric. Memorial Sloan Kettering has filed for IP protection for inventions of M.M. related to alpha particle technology. L.B. was a non-paid consultant for Advanced Accelerator Applications (AAA), Ipsen, Clovis, ITM and Curium, and receives research support from AAA. Johns Hopkins University has filed for IP protection for inventions of J.N. related to alpha particle technology.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Radionuclides
-
Interchangeable with ‘radioactive atoms’, ‘radioactive elements’ and ‘radioactive isotopes’. Although these are all correct, ‘radionuclide’ (not ‘radionucleotide’) is the preferred term in the context of nuclear medicine, in general, and radiopharmaceutical therapy, in particular.
- β-Particles
-
Light, energetic electrons that are either positively or negatively charged and emitted spontaneously from atomic nuclei during a nuclear transformation of many radionuclides. These particles are characterized by a spectrum of energies and associated ranges, typically characterized as the maximum or end-point energy or range.
- α-Particles
-
Positively charged, heavy particles ejected spontaneously from the nucleus of some radionuclides that are identical to a helium nucleus (mass number of 4 and electrical charge of +2). The DNA damage resulting from these short-range particles per unit of energy deposition is greater and more complex than that associated with β-particles.
- Absorbed dose
-
The energy absorbed per unit mass of tissue.
- Gy
-
Abbreviation for the SI unit (gray) for radiation dose (1 Gy= 1 J kg−1). It is the absorbed dose per unit mass of tissue. The corresponding CGS unit is the rad (100 rad = 1 Gy). The rad is not recommended for use in the scientific literature.
- Photons
-
A photon is a quantum of high-frequency electromagnetic radiation that is emitted spontaneously, either during a nuclear transformation (γ-rays) or as a result of orbital electron transitions (X-rays). In the context of radiation delivery, the energy or frequency of photons is sufficient to ionize atoms and lead to potential DNA damage.
- X-rays
-
Photons emitted as a result of orbital electron transitions.
- γ-Rays
-
Photons emitted during a nuclear transformation of a radionuclide.
- keV
-
The abbreviation for a unit of energy (kiloelectronvolts) typically used to represent the energy associated with radionuclide emissions.
- Auger electrons
-
Electrons ejected during suborbital transitions of an atom, typically following spontaneous nuclear transitions (radioactive decay).
- Activities
-
A measure of radioactivity, typically in the unit of becquerel (Bq) or millicurie (mCi; the CGS unit). Radioactivity corresponds to the number of radionuclide transformations per unit time.
- Theranostic
-
The general concept of using a radionuclide-labelled agent that may be imaged to guide radiopharmaceutical therapy; a radionuclide that may be used for both imaging and therapy.
- Time-versus-activity curves
-
The amount of radioactivity in a particular region of the body as a function of time. Time-versus-activity curves are used in absorbed dose calculations; they may be obtained from imaging or direct sampling (for example, urine or serial biopsy or from animal studies).
- Radionuclide transformations
-
The nuclear or atomic transformations associated with radioactive decay; the term ‘radionuclide disintegrations’ is also used.
- Radiohalogen
-
Radioactive element in group 17 of the periodic table.
- Radiosynovectomy
-
A treatment for arthritis or, more generally, inflammation that involves injection of radioactivity, typically as colloidal particles into the synovial cavity.
Rights and permissions
About this article
Cite this article
Sgouros, G., Bodei, L., McDevitt, M.R. et al. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov 19, 589–608 (2020). https://doi.org/10.1038/s41573-020-0073-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-020-0073-9
This article is cited by
-
191Pt-labeled trithiol-Hoechst-PSMA: preliminary evaluation of conjugates designed for delivery to genomic DNA of PSMA-positive cancers
EJNMMI Radiopharmacy and Chemistry (2026)
-
Evaluation of end-to-end 3D absorbed dose distribution in 90Y-SIRT and SBRT combination therapy using MAGIC-f polymer gel dosimeter
European Journal of Nuclear Medicine and Molecular Imaging (2026)
-
Epigallocatechin-3-gallate Restores X-irradiation-Induced Impairments in Cognitive Function and Hippocampal Neurogenesis by Suppressing the TLR4-NOX3/4 and ROS-NF-κB Pathways in Microglia
Molecular Neurobiology (2026)
-
Bacteria outer membrane-based oxygen gels alleviate tumor hypoxia for enhanced systemic immune response to radiotherapy
Science China Materials (2026)
-
153Sm-EDTMP Therapy as a Therapeutic Option in Mixed Skeletal Metastases from Renal Cell Carcinoma with Disease Progression Post 2nd Line Tyrosine Kinase Therapy
Nuclear Medicine and Molecular Imaging (2026)