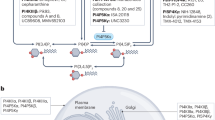
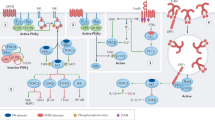
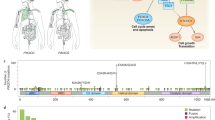
Abstract
Lipid phosphoinositides are master regulators of almost all aspects of a cell’s life and death and are generated by the tightly regulated activity of phosphoinositide kinases. Although extensive efforts have focused on drugging class I phosphoinositide 3-kinases (PI3Ks), recent years have revealed opportunities for targeting almost all phosphoinositide kinases in human diseases, including cancer, immunodeficiencies, viral infection and neurodegenerative disease. This has led to widespread efforts in the clinical development of potent and selective inhibitors of phosphoinositide kinases. This Review summarizes our current understanding of the molecular basis for the involvement of phosphoinositide kinases in disease and assesses the preclinical and clinical development of phosphoinositide kinase inhibitors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Balla, T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137 (2013).
Dickson, E. J. & Hille, B. Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids. Biochem. J. 476, 1–23 (2019).
Schink, K. O., Tan, K.-W. & Stenmark, H. Phosphoinositides in control of membrane dynamics. Annu. Rev. Cell Dev. Biol. 32, 143–171 (2016).
Hasegawa, J., Strunk, B. S. & Weisman, L. S. PI5P and PI(3,5)P2: minor, but essential phosphoinositides. Cell Struct. Funct. 42, 49–60 (2017).
Gozzelino, L., De Santis, M. C., Gulluni, F., Hirsch, E. & Martini, M. PI(3,4)P2 signaling in cancer and metabolism. Front. Oncol. 10, 360 (2020).
Batrouni, A. G. & Baskin, J. M. The chemistry and biology of phosphatidylinositol 4-phosphate at the plasma membrane. Bioorg. Med. Chem. 40, 116190 (2021).
Hammond, G. R. V. & Burke, J. E. Novel roles of phosphoinositides in signaling, lipid transport, and disease. Curr. Opin. Cell Biol. 63, 57–67 (2020).
Baba, T. & Balla, T. Emerging roles of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate as regulators of multiple steps in autophagy. J. Biochem. 168, 329–336 (2020).
Vanhaesebroeck, B., Perry, M. W. D., Brown, J. R., André, F. & Okkenhaug, K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 20, 741–769 (2021).
Vasan, N. & Cantley, L. C. At a crossroads: how to translate the roles of PI3K in oncogenic and metabolic signalling into improvements in cancer therapy. Nat. Rev. Clin. Oncol. 19, 471–485 (2022).
Li, J. et al. PI-273, a substrate-competitive, specific small molecule inhibitor of PI4KIIα, inhibits the growth of breast cancer cells. Cancer Res. 77, 6253–6266 (2017).
Sengupta, N. et al. A large scale high-throughput screen identifies chemical inhibitors of phosphatidylinositol 4-kinase type II alpha. J. Lipid Res. 60, 683–693 (2019).
Zolov, S. N. et al. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc. Natl Acad. Sci. USA 109, 17472–17477 (2012).
Giridharan, S. S. P. et al. Lipid kinases VPS34 and PIKfyve coordinate a phosphoinositide cascade to regulate retriever-mediated recycling on endosomes. eLife 11, e69709 (2022).
Rutherford, A. C. et al. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J. Cell Sci. 119, 3944–3957 (2006).
Cabezas, A., Pattni, K. & Stenmark, H. Cloning and subcellular localization of a human phosphatidylinositol 3-phosphate 5-kinase, PIKfyve/Fab1. Gene 371, 34–41 (2006).
Dong, X. et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat. Commun. 1, 38 (2010).
de Lartigue, J. et al. PIKfyve regulation of endosome-linked pathways. Traffic 10, 883–893 (2009).
Oppelt, A. et al. Production of phosphatidylinositol 5-phosphate via PIKfyve and MTMR3 regulates cell migration. EMBO Rep. 14, 57–64 (2013).
Bissig, C., Hurbain, I., Raposo, G. & van Niel, G. PIKfyve activity regulates reformation of terminal storage lysosomes from endolysosomes. Traffic 18, 747–757 (2017).
Choy, C. H. et al. Lysosome enlargement during inhibition of the lipid kinase PIKfyve proceeds through lysosome coalescence. J. Cell Sci. 131, jcs213587 (2018).
Sbrissa, D., Ikonomov, O. C. & Shisheva, A. Phosphatidylinositol 3-phosphate-interacting domains in PIKfyve. Binding specificity and role in PIKfyve. Endomenbrane localization. J. Biol. Chem. 277, 6073–6079 (2002).
Shisheva, A., Sbrissa, D. & Ikonomov, O. Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinositide kinase in insulin-sensitive cells. Mol. Cell Biol. 19, 623–634 (1999).
Sbrissa, D., Ikonomov, O. C. & Shisheva, A. PIKfyve lipid kinase is a protein kinase: downregulation of 5′-phosphoinositide product formation by autophosphorylation. Biochemistry 39, 15980–15989 (2000).
Ikonomov, O. C. et al. The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J. Biol. Chem. 286, 13404–13413 (2011).
Takasuga, S. et al. Critical roles of type III phosphatidylinositol phosphate kinase in murine embryonic visceral endoderm and adult intestine. Proc. Natl Acad. Sci. USA 110, 1726–1731 (2013).
Jin, N. et al. VAC14 nucleates a protein complex essential for the acute interconversion of PI3P and PI(3,5)P(2) in yeast and mouse. EMBO J. 27, 3221–3234 (2008).
Sbrissa, D. et al. A mammalian ortholog of Saccharomyces cerevisiae Vac14 that associates with and up-regulates PIKfyve phosphoinositide 5-kinase activity. Mol. Cell Biol. 24, 10437–10447 (2004).
Sbrissa, D. et al. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J. Biol. Chem. 282, 23878–23891 (2007).
Duex, J. E., Tang, F. & Weisman, L. S. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J. Cell Biol. 172, 693–704 (2006).
Rudge, S. A., Anderson, D. M. & Emr, S. D. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol. Biol. Cell 15, 24–36 (2004).
Zhang, Y. et al. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc. Natl Acad. Sci. USA 104, 17518–17523 (2007).
Chow, C. Y. et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 448, 68–72 (2007).
Lenk, G. M. et al. Pathogenic mechanism of the FIG4 mutation responsible for Charcot-Marie-Tooth disease CMT4J. PLoS Genet. 7, e1002104 (2011).
Chow, C. Y. et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am. J. Hum. Genet. 84, 85–88 (2009).
Zhang, X. et al. Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration. Brain 131, 1990–2001 (2008).
Lenk, G. M. et al. Biallelic mutations of VAC14 in pediatric-onset neurological disease. Am. J. Hum. Genet. 99, 188–194 (2016).
Lees, J. A., Li, P., Kumar, N., Weisman, L. S. & Reinisch, K. M. Insights into lysosomal PI(3,5)P2 homeostasis from a structural-biochemical analysis of the PIKfyve lipid kinase complex. Mol. Cell 80, 736–743.e4 (2020). This article provides the first molecular and biochemical insight into the assembly and regulation of the PIKfyve–Sac1–Vac14 signalling complex.
Hayakawa, M. et al. Synthesis and biological evaluation of 4-morpholino-2-phenylquinazolines and related derivatives as novel PI3 kinase p110alpha inhibitors. Bioorg. Med. Chem. 14, 6847–6858 (2006).
Jefferies, H. B. J. et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P2 production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 9, 164–170 (2008).
Wada, Y. et al. Selective abrogation of Th1 response by STA-5326, a potent IL-12/IL-23 inhibitor. Blood 109, 1156–1164 (2007).
Cai, X. et al. PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem. Biol. 20, 912–921 (2013).
Hayakawa, N. et al. Structure-activity relationship study, target identification, and pharmacological characterization of a small molecular IL-12/23 inhibitor, APY0201. Bioorg. Med. Chem. 22, 3021–3029 (2014).
Sharma, G. et al. A family of PIKFYVE inhibitors with therapeutic potential against autophagy-dependent cancer cells disrupt multiple events in lysosome homeostasis. Autophagy 15, 1694–1718 (2019).
Trabbic, C. J. et al. Synthesis and biological evaluation of indolyl-pyridinyl-propenones having either methuosis or microtubule disruption activity. J. Med. Chem. 58, 2489–2512 (2015).
Cho, H. et al. Indolyl-pyridinyl-propenone-induced methuosis through the inhibition of PIKFYVE. ACS Omega 3, 6097–6103 (2018).
Hudkins, R. L. et al. Synthesis and biological profile of the pan-vascular endothelial growth factor receptor/tyrosine kinase with immunoglobulin and epidermal growth factor-like homology domains 2 (VEGF-R/TIE-2) inhibitor 11-(2-methylpropyl)-12,13-dihydro-2-methyl-8-(pyrimidin-2-ylamino)-4H-indazolo[5,4-a]pyrrolo[3,4-c]carbazol-4-one (CEP-11981): a novel oncology therapeutic agent. J. Med. Chem. 55, 903–913 (2012).
Qiao, Y. et al. Autophagy inhibition by targeting PIKfyve potentiates response to immune checkpoint blockade in prostate cancer. Nat. Cancer 2, 978–993 (2021). This article identifies PIKfyve as the direct target of the anti-cancer candidate ESK981, with it inhibiting autophagy and resulting in an enhanced therapeutic response to immune checkpoint blockade.
Guerrero-Valero, M. et al. Dysregulation of myelin synthesis and actomyosin function underlies aberrant myelin in CMT4B1 neuropathy. Proc. Natl Acad. Sci. USA 118, e2009469118 (2021).
Sawade, L. et al. Rab35-regulated lipid turnover by myotubularins represses mTORC1 activity and controls myelin growth. Nat. Commun. 11, 2835 (2020).
Soto, C. & Pritzkow, S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340 (2018).
Soares, A. C. et al. PIKfyve activity is required for lysosomal trafficking of tau aggregates and tau seeding. J. Biol. Chem. 296, 100636 (2021).
Bowles, K. R. et al. ELAVL4, splicing, and glutamatergic dysfunction precede neuron loss in MAPT mutation cerebral organoids. Cell 184, 4547–4563.e17 (2021). This article shows that treatment of organoids expressing mutant microtubule-associated protein tau with the PIKfyve inhibitor apilimod prevented glutamatergic toxicity, suggesting a potential therapeutic approach for FTD.
See, S. K. et al. PIKfyve inhibition blocks endolysosomal escape of α-synuclein fibrils and spread of α-synuclein aggregation. Preprint available at bioRxiv https://doi.org/10.1101/2021.01.21.427704 (2021).
Shi, Y. et al. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat. Med. 24, 313–325 (2018). This article shows using patient-derived human induced motor neurons expressing C9ORF72, which is a model of ALS, that treatment with a PIKfyve inhibitor apilimod or YM201636 improves neuron survival, and ameliorates neurodegeneration in mouse models.
Lenk, G. M. et al. Cerebral hypomyelination associated with biallelic variants of FIG4. Hum. Mutat. 40, 619–630 (2019).
Lakkaraju, A. K. K. et al. Loss of PIKfyve drives the spongiform degeneration in prion diseases. EMBO Mol. Med. 13, e14714 (2021).
Carette, J. E. et al. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1. Nature 477, 340–343 (2011).
Nelson, E. A. et al. The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection. PLoS Negl. Trop. Dis. 11, e0005540 (2017).
Qiu, S. et al. Ebola virus requires phosphatidylinositol (3,5) bisphosphate production for efficient viral entry. Virology 513, 17–28 (2018).
Kang, Y.-L. et al. Inhibition of PIKfyve kinase prevents infection by Zaire Ebolavirus and SARS-CoV-2. Proc. Natl Acad. Sci. USA 117, 20803–20813 (2020).
Ou, X. et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 11, 1620 (2020).
Riva, L. et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature 586, 113–119 (2020).
Bouhaddou, M. et al. The global phosphorylation landscape of SARS-CoV-2 Infection. Cell 182, 685–712.e19 (2020). Together, articles 61–64 provide the first insight into the role of PIKfyve as a crucial host factor in cell entry for SARS coronaviruses, and identified inhibitors of PIKfyve as potential antivirals.
Dayam, R. M. et al. The lipid kinase PIKfyve coordinates the neutrophil immune response through the activation of the Rac GTPase. J. Immunol. 199, 2096–2105 (2017).
Gayle, S. et al. Identification of apilimod as a first-in-class PIKfyve kinase inhibitor for treatment of B-cell non-Hodgkin lymphoma. Blood 129, 1768–1778 (2017). This article described the application of the PIKfyve specific inhibitor apilimod as a potential therapeutic in B-NHL through disruption of lysosome homeostasis.
de Campos, C. B. et al. Identification of PIKfyve kinase as a target in multiple myeloma. Haematologica 105, 1641–1649 (2020).
Milan, E., Fabbri, M. & Cenci, S. Autophagy in plasma cell ontogeny and malignancy. J. Clin. Immunol. 36 (Suppl. 1), 18–24 (2016).
Hou, J.-Z. et al. Inhibition of PIKfyve using YM201636 suppresses the growth of liver cancer via the induction of autophagy. Oncol. Rep. 41, 1971–1979 (2019).
Loijens, J. C., Boronenkov, I. V., Parker, G. J. & Anderson, R. A. The phosphatidylinositol 4-phosphate 5-kinase family. Adv. Enzym. Regul. 36, 115–140 (1996).
Rameh, L. E., Tolias, K. F., Duckworth, B. C. & Cantley, L. C. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 390, 192–196 (1997).
Bazenet, C. E., Ruano, A. R., Brockman, J. L. & Anderson, R. A. The human erythrocyte contains two forms of phosphatidylinositol-4-phosphate 5-kinase which are differentially active toward membranes. J. Biol. Chem. 265, 18012–18022 (1990).
Ling, L. E., Schulz, J. T. & Cantley, L. C. Characterization and purification of membrane-associated phosphatidylinositol-4-phosphate kinase from human red blood cells. J. Biol. Chem. 264, 5080–5088 (1989).
Lundquist, M. R. et al. Phosphatidylinositol-5-phosphate 4-kinases regulate cellular lipid metabolism by facilitating autophagy. Mol. Cell 70, 531–544.e9 (2018). This article shows defects in fasting autophagy in mice lacking both PI5P4Kα and PI5P4Kβ and in Caenorhabditis elegans lacking PI5P4K owing to a defect in clearing of autophagosomes, and highlights an important evolutionarily conserved role of the PI5P4Ks.
Hu, A. et al. PIP4K2A regulates intracellular cholesterol transport through modulating PI(4,5)P2 homeostasis. J. Lipid Res. 59, 507–514 (2018).
Ishihara, H. et al. Cloning of cDNAs encoding two isoforms of 68-kDa type I phosphatidylinositol-4-phosphate 5-kinase. J. Biol. Chem. 271, 23611–23614 (1996).
Loijens, J. C. & Anderson, R. A. Type I phosphatidylinositol-4-phosphate 5-kinases are distinct members of this novel lipid kinase family. J. Biol. Chem. 271, 32937–32943 (1996).
Oude Weernink, P. A., Schmidt, M. & Jakobs, K. H. Regulation and cellular roles of phosphoinositide 5-kinases. Eur. J. Pharmacol. 500, 87–99 (2004).
van den Bout, I. & Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J. Cell. Sci. 122, 3837–3850 (2009).
Rao, V. D., Misra, S., Boronenkov, I. V., Anderson, R. A. & Hurley, J. H. Structure of type II beta phosphatidylinositol phosphate kinase: a protein kinase fold flattened for interfacial phosphorylation. Cell 94, 829–839 (1998).
Clarke, J. H. & Irvine, R. F. The activity, evolution and association of phosphatidylinositol 5-phosphate 4-kinases. Adv. Biol. Regul. 52, 40–45 (2012).
Emerling, B. M. et al. Depletion of a putatively druggable class of phosphatidylinositol kinases inhibits growth of p53-null tumors. Cell 155, 844–857 (2013). This article shows that PI5P4Kα and PI5P4Kβ are essential for tumour survival in the absence of the tumour suppressor p53.
Shim, H. et al. Deletion of the gene Pip4k2c, a novel phosphatidylinositol kinase, results in hyperactivation of the immune system. Proc. Natl Acad. Sci. USA 113, 7596–7601 (2016).
Lamia, K. A. et al. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase beta-/- mice. Mol. Cell Biol. 24, 5080–5087 (2004).
Clarke, J. H. & Irvine, R. F. Evolutionarily conserved structural changes in phosphatidylinositol 5-phosphate 4-kinase (PI5P4K) isoforms are responsible for differences in enzyme activity and localization. Biochem. J. 454, 49–57 (2013).
Bulley, S. J. et al. In B cells, phosphatidylinositol 5-phosphate 4-kinase-α synthesizes PI(4,5)P2 to impact mTORC2 and Akt signaling. Proc. Natl Acad. Sci. USA 113, 10571–10576 (2016).
Sumita, K. et al. The lipid kinase PI5P4Kβ is an intracellular GTP sensor for metabolism and tumorigenesis. Mol. Cell 61, 187–198 (2016).
Bultsma, Y., Keune, W.-J. & Divecha, N. PIP4Kbeta interacts with and modulates nuclear localization of the high-activity PtdIns5P-4-kinase isoform PIP4Kalpha. Biochem. J. 430, 223–235 (2010).
Jones, D. R. et al. Nuclear PtdIns5P as a transducer of stress signaling: an in vivo role for PIP4Kbeta. Mol. Cell 23, 685–695 (2006).
Keune, W.-J. et al. Regulation of phosphatidylinositol-5-phosphate signaling by Pin1 determines sensitivity to oxidative stress. Sci. Signal. 5, ra86 (2012).
Mackey, A. M., Sarkes, D. A., Bettencourt, I., Asara, J. M. & Rameh, L. E. PIP4kγ is a substrate for mTORC1 that maintains basal mTORC1 signaling during starvation. Sci. Signal. 7, ra104–ra104 (2014).
Semenas, J. et al. The role of PI3K/AKT-related PIP5K1α and the discovery of its selective inhibitor for treatment of advanced prostate cancer. Proc. Natl Acad. Sci. USA 111, E3689–E3698 (2014).
Wright, B. D. et al. The lipid kinase PIP5K1C regulates pain signaling and sensitization. Neuron 82, 836–847 (2014).
Andrews, D. M. et al. Identification and optimization of a novel series of selective PIP5K inhibitors. Bioorg. Med. Chem. 54, 116557 (2022).
Sarwar, M. et al. The role of PIP5K1α/pAKT and targeted inhibition of growth of subtypes of breast cancer using PIP5K1α inhibitor. Oncogene 38, 375–389 (2019).
Adhikari, H. & Counter, C. M. Interrogating the protein interactomes of RAS isoforms identifies PIP5K1A as a KRAS-specific vulnerability. Nat. Commun. 9, 3646 (2018).
Choi, S., Chen, M., Cryns, V. L. & Anderson, R. A. A nuclear phosphoinositide kinase complex regulates p53. Nat. Cell Biol. 21, 462–475 (2019).
Muscolini, M. et al. Phosphatidylinositol 4-phosphate 5-kinase α activation critically contributes to CD28-dependent signaling responses. J. Immunol. 190, 5279–5286 (2013).
Muscolini, M. et al. Phosphatidylinositol 4-phosphate 5-kinase α and Vav1 mutual cooperation in CD28-mediated actin remodeling and signaling functions. J. Immunol. 194, 1323–1333 (2015).
Camperio, C. et al. CD28 ligation in the absence of TCR stimulation up-regulates IL-17A and pro-inflammatory cytokines in relapsing-remitting multiple sclerosis T lymphocytes. Immunol. Lett. 158, 134–142 (2014).
Chen, S. et al. Pharmacological inhibition of PI5P4Kα/β disrupts cell energy metabolism and selectively kills p53-null tumor cells. Proc. Natl Acad. Sci. USA 118, e2002486118 (2021).
Ravi, A. et al. PI5P4Ks drive metabolic homeostasis through peroxisome-mitochondria interplay. Dev. Cell 56, 1661–1676.e10 (2021).
Kitagawa, M. et al. Dual blockade of the lipid kinase PIP4Ks and mitotic pathways leads to cancer-selective lethality. Nat. Commun. 8, 2200 (2017).
Sivakumaren, S. C. et al. Targeting the PI5P4K lipid kinase family in cancer using covalent inhibitors. Cell Chem. Biol. 27, 525–537.e6 (2020).
Manz, T. D. et al. Discovery and structure-activity relationship study of (Z)-5-methylenethiazolidin-4-one derivatives as potent and selective Pan-phosphatidylinositol 5-phosphate 4-kinase inhibitors. J. Med. Chem. 63, 4880–4895 (2020).
Davis, M. I. et al. A homogeneous, high-throughput assay for phosphatidylinositol 5-phosphate 4-kinase with a novel, rapid substrate preparation. PLoS ONE 8, e54127 (2013).
Wortmann, L. et al. Discovery and characterization of the potent and highly selective 1,7-naphthyridine-based inhibitors BAY-091 and BAY-297 of the kinase PIP4K2A. J. Med. Chem. 64, 15883–15911 (2021).
Voss, M. D. et al. Discovery and pharmacological characterization of a novel small molecule inhibitor of phosphatidylinositol-5-phosphate 4-kinase, type II, beta. Biochem. Biophys. Res. Commun. 449, 327–331 (2014).
Clarke, J. H. et al. The function of phosphatidylinositol 5-phosphate 4-kinase γ (PI5P4Kγ) explored using a specific inhibitor that targets the PI5P-binding site. Biochem. J. 466, 359–367 (2015).
Al-Ramahi, I. et al. Inhibition of PIP4Kγ ameliorates the pathological effects of mutant huntingtin protein. eLife 6, e29123 (2017).
Manz, T. D. et al. Structure-activity relationship study of covalent Pan-phosphatidylinositol 5-phosphate 4-kinase inhibitors. ACS Med. Chem. Lett. 11, 346–352 (2020).
See, C. S. et al. Discovery of the cancer cell selective dual acting anti-cancer agent (Z)-2-(1H-indol-3-yl)-3-(isoquinolin-5-yl)acrylonitrile (A131). Eur. J. Med. Chem. 156, 344–367 (2018).
Boffey, H. K. et al. Development of selective phosphatidylinositol 5-phosphate 4-kinase γ inhibitors with a non-ATP-competitive, allosteric binding mode. J. Med. Chem. 65, 3359–3370 (2022).
Arora, G. K., Palamiuc, L. & Emerling, B. M. Expanding role of PI5P4Ks in cancer: a promising druggable target. FEBS Lett. 596, 3–16 (2022).
Luoh, S.-W., Venkatesan, N. & Tripathi, R. Overexpression of the amplified Pip4k2beta gene from 17q11-12 in breast cancer cells confers proliferation advantage. Oncogene 23, 1354–1363 (2004).
Keune, W.-J. et al. Low PIP4K2B expression in human breast tumors correlates with reduced patient survival: a role for PIP4K2B in the regulation of E-cadherin expression. Cancer Res. 73, 6913–6925 (2013).
Lima, K. et al. PIP4K2A and PIP4K2C transcript levels are associated with cytogenetic risk and survival outcomes in acute myeloid leukemia. Cancer Genet. 233–234, 56–66 (2019).
Jude, J. G. et al. A targeted knockdown screen of genes coding for phosphoinositide modulators identifies PIP4K2A as required for acute myeloid leukemia cell proliferation and survival. Oncogene 34, 1253–1262 (2015).
Shin, Y. J. et al. PIP4K2A as a negative regulator of PI3K in PTEN-deficient glioblastoma. J. Exp. Med. 216, 1120–1134 (2019).
Wang, D. G. et al. PIP4Ks suppress insulin signaling through a catalytic-independent mechanism. Cell Rep. 27, 1991–2001.e5 (2019). This article shows the crucial catalytic-independent roles of the PI5P4Ks in decreasing insulin signalling through suppressing PIP2 generation by the PI4P5Ks.
Fagerberg, L. et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell Proteom. 13, 397–406 (2014).
Poli, A. et al. PIP4Ks impact on PI3K, FOXP3, and UHRF1 signaling and modulate human regulatory T cell proliferation and immunosuppressive activity. Proc. Natl Acad. Sci. USA 118, e2010053118 (2021).
He, Z. et al. The PIP5K2A gene and schizophrenia in the Chinese population-a case-control study. Schizophr. Res. 94, 359–365 (2007).
Thiselton, D. L. et al. Association analysis of the PIP4K2A gene on chromosome 10p12 and schizophrenia in the Irish study of high density schizophrenia families (ISHDSF) and the Irish case-control study of schizophrenia (ICCSS). Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 323–331 (2010).
Schwab, S. G. et al. Evidence for association of DNA sequence variants in the phosphatidylinositol-4-phosphate 5-kinase IIalpha gene (PIP5K2A) with schizophrenia. Mol. Psychiatry 11, 837–846 (2006).
Noch, E. K., Yim, I., Milner, T. A. & Cantley, L. C. Distribution and localization of phosphatidylinositol 5-phosphate, 4-kinase alpha and beta in the brain. J. Comp. Neurol. 529, 434–449 (2021).
Balla, T. et al. Isolation and molecular cloning of wortmannin-sensitive bovine type III phosphatidylinositol 4-kinases. J. Biol. Chem. 272, 18358–18366 (1997).
Boura, E. & Nencka, R. Phosphatidylinositol 4-kinases: function, structure, and inhibition. Exp. Cell Res. 337, 136–145 (2015).
Dornan, G. L., McPhail, J. A. & Burke, J. E. Type III phosphatidylinositol 4 kinases: structure, function, regulation, signalling and involvement in disease. Biochemical Soc. Trans. 44, 260–266 (2016).
Tan, J. & Brill, J. A. Cinderella story: PI4P goes from precursor to key signaling molecule. Crit. Rev. Biochem. Mol. Biol. 49, 33–58 (2014).
Burke, J. E. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol. Cell 71, 653–673 (2018).
Nakatsu, F. et al. PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J. Cell Biol. 199, 1003–1016 (2012).
Bojjireddy, N. et al. Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J. Biol. Chem. 289, 6120–6132 (2014). This article shows that knock-down of PI4KA activity at the plasma membrane either pharmacologically or genetically is lethal owing to severe intestinal necrosis, revealing the essential role of PI4KA in maintaining PI4P and PIP2 levels at the plasma membrane.
Nakagawa, T., Goto, K. & Kondo, H. Cloning, expression, and localization of 230-kDa phosphatidylinositol 4-kinase. J. Biol. Chem. 271, 12088–12094 (1996).
Lees, J. A. et al. Architecture of the human PI4KIIIα lipid kinase complex. Proc. Natl Acad. Sci. USA 114, 13720–13725 (2017). This study reveals molecular insight into the architechture and assembly of the PI4KA–TTC7–FAM126 complex that generates PI4P at the plasma membrane.
Wu, X. et al. Structural insights into assembly and regulation of the plasma membrane phosphatidylinositol 4-kinase complex. Dev. Cell 28, 19–29 (2014).
Baskin, J. M. et al. The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nat. Cell Biol. 18, 132–138 (2016).
Raghu, P., Joseph, A., Krishnan, H., Singh, P. & Saha, S. Phosphoinositides: regulators of nervous system function in health and disease. Front. Mol. Neurosci. 12, 208 (2019).
Pagnamenta, A. T. et al. Germline recessive mutations in PI4KA are associated with perisylvian polymicrogyria, cerebellar hypoplasia and arthrogryposis. Hum. Mol. Genet. 24, 3732–3741 (2015).
Salter, C. G. et al. Biallelic PI4KA variants cause neurological, intestinal and immunological disease. Brain 144, 3597–3610 (2021).
Bigorgne, A. E. et al. TTC7A mutations disrupt intestinal epithelial apicobasal polarity. J. Clin. Invest. 124, 328–337 (2014).
Avitzur, Y. et al. Mutations in tetratricopeptide repeat domain 7A result in a severe form of very early onset inflammatory bowel disease. Gastroenterology 146, 1028–1039 (2014).
Chen, R. et al. Whole-exome sequencing identifies tetratricopeptide repeat domain 7A (TTC7A) mutations for combined immunodeficiency with intestinal atresias. J. Allergy Clin. Immunol. 132, 656–664.e17 (2013).
Berger, K. L. et al. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc. Natl Acad. Sci. USA 106, 7577–7582 (2009). This is one of the first studies to identify the role of PI4KA and its product PI4P as essential in the viral replication of HCV.
Dorobantu, C. M. et al. Modulation of the host lipid landscape to promote RNA virus replication: the picornavirus encephalomyocarditis virus converges on the pathway used by hepatitis C virus. PLoS Pathog. 11, e1005185 (2015).
Burke, J. E. et al. Structures of PI4KIIIβ complexes show simultaneous recruitment of Rab11 and its effectors. Science 344, 1035–1038 (2014). This study reveals molecular insight into the structure and inhibition of PI4KB, and how it can be regulated by protein-binding partners.
McPhail, J. A., Ottosen, E. H., Jenkins, M. L. & Burke, J. E. The molecular basis of Aichi virus 3A protein activation of phosphatidylinositol 4 kinase IIIβ, PI4KB, through ACBD3. Structure 25, 121–131 (2017).
Klima, M. et al. Structural insights and in vitro reconstitution of membrane targeting and activation of human PI4KB by the ACBD3 protein. Sci. Rep. 6, 23641 (2016).
Hausser, A. et al. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat. Cell Biol. 7, 880–886 (2005).
Chalupská, D. et al. Phosphatidylinositol 4-kinase IIIβ (PI4KB) forms highly flexible heterocomplexes that include ACBD3, 14-3-3, and Rab11 proteins. Sci. Rep. 9, 567 (2019).
McPhail, J. A. et al. Characterization of the c10orf76-PI4KB complex and its necessity for Golgi PI4P levels and enterovirus replication. EMBO Rep. 21, e48441 (2020).
Mesmin, B. et al. Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP. EMBO J. 36, 3156–3174 (2017).
Brill, J. A., Hime, G. R., Scharer-Schuksz, M. & Fuller, M. T. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development 127, 3855–3864 (2000).
Baba, T. et al. Myelination of peripheral nerves is controlled by PI4KB through regulation of Schwann cell Golgi function. Proc. Natl Acad. Sci. USA 117, 28102–28113 (2020).
van der Schaar, H. M., Dorobantu, C. M., Albulescu, L., Strating, J. R. P. M. & van Kuppeveld, F. J. M. Fat(al) attraction: picornaviruses usurp lipid transfer at membrane contact sites to create replication organelles. Trends Microbiol. 24, 535–546 (2016).
Altan-Bonnet, N. & Balla, T. Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms. Trends Biochem. Sci. 37, 293–302 (2012).
Hsu, N.-Y. et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 141, 799–811 (2010). This is one of the first studies to identify the role of PI4KB and its product PI4P as essential in the viral replication of enteroviruses.
Nakanishi, S., Catt, K. J. & Balla, T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl Acad. Sci. USA 92, 5317–5321 (1995).
Nakanishi, S., Catt, K. J. & Balla, T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl Acad. Sci. USA 92, 5317–5321 (1995).
Meyers, R. & Cantley, L. C. Cloning and characterization of a wortmannin-sensitive human phosphatidylinositol 4-kinase. J. Biol. Chem. 272, 4384–4390 (1997).
Bianco, A. et al. Metabolism of phosphatidylinositol 4-kinase IIIα-dependent PI4P Is subverted by HCV and is targeted by a 4-anilino quinazoline with antiviral activity. PLoS Pathog. 8, e1002576 (2012).
Vaillancourt, F. H. et al. Evaluation of phosphatidylinositol-4-kinase IIIα as a hepatitis C virus drug target. J. Virol. 86, 11595–11607 (2012).
Leivers, A. L. et al. Discovery of selective small molecule type III phosphatidylinositol 4-kinase alpha (PI4KIIIα) inhibitors as anti hepatitis C (HCV) agents. J. Med. Chem. 57, 2091–2106 (2014).
Raubo, P. et al. Discovery of potent, selective small molecule inhibitors of α-subtype of type III phosphatidylinositol-4-kinase (PI4KIIIα). Bioorg. Med. Chem. Lett. 25, 3189–3193 (2015).
Waring, M. J. et al. Potent, selective small molecule inhibitors of type III phosphatidylinositol-4-kinase α- but not β-inhibit the phosphatidylinositol signaling cascade and cancer cell proliferation. Chem. Commun. 50, 5388–5390 (2014).
Knight, Z. et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125, 733–747 (2006).
Rutaganira, F. U. et al. Design and structural characterization of potent and selective inhibitors of phosphatidylinositol 4 kinase IIIβ. J. Med. Chem. 59, 1830–1839 (2016).
Phillpotts, R. J., Wallace, J., Tyrrell, D. A. & Tagart, V. B. Therapeutic activity of enviroxime against rhinovirus infection in volunteers. Antimicrob. Agents Chemother. 23, 671–675 (1983).
Arita, M. et al. Phosphatidylinositol 4-kinase III beta is a target of enviroxime-like compounds for antipoliovirus activity. J. Virol. 85, 2364–2372 (2011).
van der Schaar, H. M. et al. A novel, broad-spectrum inhibitor of enterovirus replication that targets host cell factor phosphatidylinositol 4-kinase IIIβ. Antimicrob. Agents Chemother. 57, 4971–4981 (2013).
Mejdrová, I. et al. Highly selective phosphatidylinositol 4-Kinase IIIβ inhibitors and structural insight into their mode of action. J. Med. Chem. 58, 3767–3793 (2015).
Mejdrová, I. et al. Rational design of novel highly potent and selective phosphatidylinositol 4-Kinase IIIβ (PI4KB) inhibitors as broad-spectrum antiviral agents and tools for chemical biology. J. Med. Chem. 60, 100–118 (2017).
McPhail, J. A. & Burke, J. E. Molecular mechanisms of PI4K regulation and their involvement in viral replication. Traffic https://doi.org/10.1111/tra.12841 (2022).
Lamarche, M. J. et al. Anti-hepatitis C virus activity and toxicity of type III phosphatidylinositol-4-kinase beta inhibitors. Antimicrob. Agents Chemother. 56, 5149–5156 (2012).
Reuberson, J. et al. Discovery of a potent, orally bioavailable PI4KIIIβ inhibitor (UCB9608) able to significantly prolong allogeneic organ engraftment in vivo. J. Med. Chem. 61, 6705–6723 (2018).
Spickler, C. et al. Phosphatidylinositol 4-kinase III beta is essential for replication of human rhinovirus and its inhibition causes a lethal phenotype in vivo. Antimicrob. Agents Chemother. 57, 3358–3368 (2013).
McNamara, C. W. et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504, 248–253 (2013). This is the first study to report that inhibition of the Plasmodium variant of PI4KB was a potential therapeutic approach for malaria across all Plasmodium lifecycle stages.
Manjunatha, U. H. et al. A Cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature 546, 376–380 (2017). This article shows that small-molecule inhibition of the Cryptosporidium variant of PI4K shows therapeutic promise in the treatment of cryptosporidiosis.
Ghidelli-Disse, S. et al. Identification of Plasmodium PI4 kinase as target of MMV390048 by chemoproteomics. Malar. J. 13, P38 (2014).
Kato, N. et al. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 538, 344–349 (2016).
Paquet, T. et al. Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase. Sci. Transl. Med. 9, eaad9735 (2017). This article describes the pre-clinical efficacy of the first plasmodium PI4K inhibitor to enter clinical trials as a potential therapeutic towards malaria.
Fienberg, S. et al. Structural basis for inhibitor potency and selectivity of Plasmodium falciparum phosphatidylinositol 4-kinase inhibitors. ACS Infect. Dis. 6, 3048–3063 (2020).
Arendse, L. B., Wyllie, S., Chibale, K. & Gilbert, I. H. Plasmodium kinases as potential drug targets for malaria: challenges and opportunities. ACS Infect. Dis. 7, 518–534 (2021).
Brunschwig, C. et al. UCT943, a next-generation Plasmodium falciparum PI4K inhibitor preclinical candidate for the treatment of malaria. Antimicrob. Agents Chemother. 62, 62 (2018).
Adhikari, H. et al. Oncogenic KRAS is dependent upon an EFR3A-PI4KA signaling axis for potent tumorigenic activity. Nat. Commun. 12, 5248 (2021).
Kattan, W. E. et al. Components of the phosphatidylserine endoplasmic reticulum to plasma membrane transport mechanism as targets for KRAS inhibition in pancreatic cancer. Proc. Natl Acad. Sci. USA 118, e2114126118 (2021).
Shi, L. et al. Addiction to Golgi-resident PI4P synthesis in chromosome 1q21.3-amplified lung adenocarcinoma cells. Proc. Natl Acad. Sci. USA 118, e2023537118 (2021).
Tan, X. et al. PI4KIIIβ is a therapeutic target in chromosome 1q-amplified lung adenocarcinoma. Sci. Transl. Med. 12, eaax3772 (2020).
Gupta, S. et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell 129, 957–968 (2007).
Gulluni, F., De Santis, M. C., Margaria, J. P., Martini, M. & Hirsch, E. Class II PI3K functions in cell biology and disease. Trends Cell Biol. 29, 339–359 (2019).
Lo, W.-T. et al. Structural basis of phosphatidylinositol 3-kinase C2α function. Nat. Struct. Mol. Biol. 29, 218–228 (2022). This study describes the first molecular insight into how the class II PI3Ks are regulated by inhibitory interactions with their regulatory domains, and into their inhibition by small-molecule inhibitors.
Gaidarov, I., Smith, M. E., Domin, J. & Keen, J. H. The class II phosphoinositide 3-kinase C2alpha is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol. Cell 7, 443–449 (2001).
Wang, H. et al. Autoregulation of class II Alpha PI3K activity by its lipid-binding PX-C2 domain module. Mol. Cell 71, 343–351.e4 (2018).
Chen, K.-E., Tillu, V. A., Chandra, M. & Collins, B. M. Molecular basis for membrane recruitment by the PX and C2 domains of class II phosphoinositide 3-kinase-C2α. Structure 26, 1612–1625.e4 (2018).
Posor, Y. et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3, 4-bisphosphate. Nature 499, 233–237 (2013).
Yoshioka, K. et al. Endothelial PI3K-C2α, a class II PI3K, has an essential role in angiogenesis and vascular barrier function. Nat. Med. 18, 1560–1569 (2012). This study reveals the crucial role of the PIK3C2A isoform of class II PI3K in angiogenesis, and as a potential therapeutic target in vascular disease.
Franco, I. et al. PI3K class II α controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev. Cell 28, 647–658 (2014).
Alliouachene, S. et al. Inactivation of class II PI3K-C2α induces leptin resistance, age-dependent insulin resistance and obesity in male mice. Diabetologia 59, 1503–1512 (2016).
Boukhalfa, A. et al. PI3KC2α-dependent and VPS34-independent generation of PI3P controls primary cilium-mediated autophagy in response to shear stress. Nat. Commun. 11, 294 (2020).
Tiosano, D. et al. Mutations in PIK3C2A cause syndromic short stature, skeletal abnormalities, and cataracts associated with ciliary dysfunction. PLoS Genet. 15, e1008088 (2019). This work identifies loss-of-function mutations in PIK3C2A in patients that lead to developmental disorders, highlighting a crucial role of class II PI3K in growth, vision and skeletal and/or neurological development.
Gulluni, F. et al. PI(3,4)P2-mediated cytokinetic abscission prevents early senescence and cataract formation. Science 374, eabk0410 (2021). This work identifies the crucial role of PIK3C2A in cytokinetic abscission in the lens of the eye, identifying the pathogenic mechanism of cataract development in patients with loss-of-function mutations in PIK3C2A.
Alliouachene, S. et al. Inactivation of the class II PI3K-C2β potentiates insulin signaling and sensitivity. Cell Rep. 13, 1881–1894 (2015).
Anquetil, T. et al. PI3KC2β inactivation stabilizes VE-cadherin junctions and preserves vascular integrity. EMBO Rep. 22, e51299 (2021).
Posor, Y. et al. Local synthesis of the phosphatidylinositol-3,4-bisphosphate lipid drives focal adhesion turnover. Dev. Cell 57, 1694–1711.e7 (2022).
Sabha, N. et al. PIK3C2B inhibition improves function and prolongs survival in myotubular myopathy animal models. J. Clin. Invest. 126, 3613–3625 (2016).
Marat, A. L. et al. mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science 356, 968–972 (2017).
Gozzelino, L. et al. Defective lipid signalling caused by mutations in PIK3C2B underlies focal epilepsy. Brain 145, 2313–2331 (2022).
Domin, J. et al. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J. 326, 139–147 (1997).
Selvadurai, M. V. et al. Disrupting the platelet internal membrane via PI3KC2α inhibition impairs thrombosis independently of canonical platelet activation. Sci. Transl. Med. 12, eaar8430 (2020). This study reveals the potential of class II PI3K inhibitors as potent anti-thrombotic agents, with limited effect on activation-dependent platelet function.
Li, H. et al. Phosphoinositide conversion inactivates R-RAS and drives metastases in breast cancer. Adv. Sci. 9, e2103249 (2022).
Lo, W.-T. et al. Development of selective inhibitors of phosphatidylinositol 3-kinase C2α. Nat. Chem. Biol. https://doi.org/10.1038/s41589-022-01118-z (2022). This study reports the identification and structural characterization of the first truly selective class II PI3K inhibitors, opening up the possibilities of class II PI3K inhibitors as therapeutics.
Mountford, J. K. et al. The class II PI 3-kinase, PI3KC2α, links platelet internal membrane structure to shear-dependent adhesive function. Nat. Commun. 6, 6535 (2015).
Valet, C. et al. Essential role of class II PI3K-C2α in platelet membrane morphology. Blood 126, 1128–1137 (2015).
Gulluni, F. et al. Mitotic spindle assembly and genomic stability in breast cancer require PI3K-C2α scaffolding function. Cancer Cell 32, 444–459.e7 (2017).
Ryan, E. L., Shelford, J., Massam-Wu, T., Bayliss, R. & Royle, S. J. Defining endogenous TACC3-chTOG-clathrin-GTSE1 interactions at the mitotic spindle using induced relocalization. J. Cell Sci. 134, jcs255794 (2021).
Ohashi, Y., Tremel, S. & Williams, R. L. VPS34 complexes from a structural perspective. J. Lipid Res. 60, 229–241 (2019).
Rostislavleva, K. et al. Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science 350, aac7365 (2015). This study reveals the first molecular insight into the assembly of complex II of the class III PI3K complex, providing unique insight into its architechture and regulation.
Stjepanovic, G., Baskaran, S., Lin, M. G. & Hurley, J. H. Vps34 kinase domain dynamics regulate the autophagic PI 3-kinase complex. Mol. Cell 67, 528–534.e3 (2017).
Young, L. N., Goerdeler, F. & Hurley, J. H. Structural pathway for allosteric activation of the autophagic PI 3-kinase complex I. Proc. Natl Acad. Sci. USA 116, 21508–21513 (2019).
Tremel, S. et al. Structural basis for VPS34 kinase activation by Rab1 and Rab5 on membranes. Nat. Commun. 12, 1564 (2021). This article reports on the molecular basis for how class III PI3Ks are activated on membranes by Rab GTPases, and reveals crucial differences between complex I and complex II that may be exploited for complex-specific targeting.
Petiot, A., Ogier-Denis, E., Blommaart, E. F., Meijer, A. J. & Codogno, P. Distinct classes of phosphatidylinositol 3’-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992–998 (2000).
Bago, R. et al. Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem. J. 463, 413–427 (2014).
Dowdle, W. E. et al. Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16, 1069–1079 (2014).
Ronan, B. et al. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat. Chem. Biol. 10, 1013–1019 (2014).
Dyczynski, M. et al. Targeting autophagy by small molecule inhibitors of vacuolar protein sorting 34 (Vps34) improves the sensitivity of breast cancer cells to Sunitinib. Cancer Lett. 435, 32–43 (2018).
Hu, D. X. et al. Structure-based design of potent, selective, and orally bioavailable VPS34 kinase inhibitors. J. Med. Chem. 65, 11500–11512 (2022).
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, e108863 (2021).
Liu, X. et al. Simultaneous inhibition of Vps34 kinase would enhance PI3Kδ inhibitor cytotoxicity in the B-cell malignancies. Oncotarget 7, 53515–53525 (2016).
Meunier, G. et al. Antileukemic activity of the VPS34-IN1 inhibitor in acute myeloid leukemia. Oncogenesis 9, 94 (2020).
Noman, M. Z. et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti–PD-1/PD-L1 immunotherapy. Sci. Adv. 6, eaax7881 (2020). This article describes the potential of class III PI3K inhibitors as anti-cancer therapeutics based on mouse models of melanoma and colorectal cancer tumours, specifically in combination with immunotherapy approaches.
Schlütermann, D. et al. Targeting urothelial carcinoma cells by combining cisplatin with a specific inhibitor of the autophagy-inducing class III PtdIns3K complex. Urol. Oncol. 36, 160.e1–160.e13 (2018).
Henley, Z. A. et al. Optimization of orally bioavailable PI3Kδ inhibitors and identification of Vps34 as a key selectivity target. J. Med. Chem. 63, 638–655 (2020).
Bilanges, B. et al. Vps34 PI 3-kinase inactivation enhances insulin sensitivity through reprogramming of mitochondrial metabolism. Nat. Commun. 8, 1804 (2017).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Varadi, M. et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Ikonomov, O. C., Sbrissa, D., Fenner, H. & Shisheva, A. PIKfyve-ArPIKfyve-Sac3 core complex: contact sites and their consequence for Sac3 phosphatase activity and endocytic membrane homeostasis. J. Biol. Chem. 284, 35794–35806 (2009).
Botelho, R. J., Efe, J. A., Teis, D. & Emr, S. D. Assembly of a Fab1 phosphoinositide kinase signaling complex requires the Fig4 phosphoinositide phosphatase. Mol. Biol. Cell 19, 4273–4286 (2008).
Hansen, S. D., Lee, A. A., Duewell, B. R. & Groves, J. T. Membrane-mediated dimerization potentiates PIP5K lipid kinase activity. eLife 11, e73747 (2022).
Kunz, J. et al. The activation loop of phosphatidylinositol phosphate kinases determines signaling specificity. Mol. Cell 5, 1–11 (2000).
Kunz, J., Fuelling, A., Kolbe, L. & Anderson, R. A. Stereo-specific substrate recognition by phosphatidylinositol phosphate kinases is swapped by changing a single amino acid residue. J. Biol. Chem. 277, 5611–5619 (2002).
Hu, J. et al. Resolution of structure of PIP5K1A reveals molecular mechanism for its regulation by dimerization and dishevelled. Nat. Commun. 6, 8205 (2015).
Wills, R. C. et al. A novel homeostatic mechanism tunes PI(4,5)P 2-dependent signaling at the plasma membrane. Preprint available at bioRxiv https://doi.org/10.1101/2022.06.30.498262 (2022).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Lawrence, M. S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014).
Rathinaswamy, M. K. et al. Structure of the phosphoinositide 3-kinase (PI3K) p110γ-p101 complex reveals molecular mechanism of GPCR activation. Sci. Adv. 7, eabj4282 (2021).
Vadas, O., Burke, J. E., Zhang, X., Berndt, A. & Williams, R. L. Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci. Signal. 4, 1–13 (2011).
Burke, J. E. & Williams, R. L. Synergy in activating class I PI3Ks. Trends Biochem. Sci. 40, 88–100 (2015).
Dornan, G. L. & Burke, J. E. Molecular mechanisms of human disease mediated by oncogenic and primary immunodeficiency mutations in class IA phosphoinositide 3-kinases. Front. Immunol. 9, 575 (2018).
Dyment, D. A. et al. Mutations in PIK3R1 cause SHORT syndrome. Am. J. Hum. Genet. 93, 158–166 (2013).
Thauvin-Robinet, C. et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am. J. Hum. Genet. 93, 141–149 (2013).
Chudasama, K. K. et al. SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling. Am. J. Hum. Genet. 93, 150–157 (2013).
Thian, M. et al. Germline biallelic PIK3CG mutations in a multifaceted immunodeficiency with immune dysregulation. Haematologica 105, e488 (2020).
Takeda, A. J. et al. Human PI3Kγ deficiency and its microbiota-dependent mouse model reveal immunodeficiency and tissue immunopathology. Nat. Commun. 10, 4364 (2019).
Samuels, Y. et al. High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 (2004).
Samuels, Y. et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7, 561–573 (2005).
Urick, M. E. et al. PIK3R1 (p85α) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 71, 4061–4067 (2011).
Jaiswal, B. S. et al. Somatic mutations in p85α promote tumorigenesis through class IA PI3K activation. Cancer Cell 16, 463–474 (2009).
Madsen, R. R. et al. Oncogenic PIK3CA promotes cellular stemness in an allele dose-dependent manner. Proc. Natl Acad. Sci. USA 116, 8380–8389 (2019).
Vasan, N. et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science 366, 714–723 (2019).
Castillo, S. D. et al. Somatic activating mutations in Pik3ca cause sporadic venous malformations in mice and humans. Sci. Transl. Med. 8, 332ra43 (2016).
Lindhurst, M. J. et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat. Genet. 44, 928–933 (2012).
Jia, S. et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454, 776–779 (2008).
Wee, S. et al. PTEN-deficient cancers depend on PIK3CB. Proc. Natl Acad. Sci. USA 105, 13057–13062 (2008).
Angulo, I. et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science 342, 866–871 (2013).
Lucas, C. L. et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 15, 88–97 (2014).
Takeda, A. J. et al. Novel PIK3CD mutations affecting N-terminal residues of p110δ cause activated PI3Kδ syndrome (APDS) in humans. J. Allergy Clin. Immunol. 140, 1152–1156.e10 (2017).
Lucas, C. L. et al. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J. Exp. Med. 211, 2537–2547 (2014).
Deau, M.-C. et al. A human immunodeficiency caused by mutations in the PIK3R1 gene. J. Clin. Invest. 124, 3923–3928 (2014).
Dornan, G. L. et al. Conformational disruption of PI3Kδ regulation by immunodeficiency mutations in PIK3CD and PIK3R1. Proc. Natl Acad. Sci. Usa. 114, 1982–1987 (2017).
Kaneda, M. M. et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 539, 437–442 (2016).
De Henau, O. et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 539, 443–447 (2016).
Campa, C. C. et al. Inhalation of the prodrug PI3K inhibitor CL27c improves lung function in asthma and fibrosis. Nat. Commun. 9, 5232 (2018).
Liu, N. et al. BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol. Cancer Ther. 12, 2319–2330 (2013).
Venot, Q. et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 558, 540–546 (2018).
Gopal, A. K. et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N. Engl. J. Med. 370, 1008–1018 (2014).
Brown, J. R. et al. Idelalisib, an inhibitor of phosphatidylinositol 3 kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood 123, 3390–3397 (2014).
Flinn, I. W. et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood 123, 3406–3413 (2014).
Kahl, B. S. et al. Results of a phase I study of idelalisib, a PI3Kδ inhibitor, in patients with relapsed or refractory mantle cell lymphoma (MCL). Blood 123, 3398–3405 (2014).
Fowler, N. H. et al. Umbralisib, a dual PI3Kδ/CK1ε inhibitor in patients with relapsed or refractory indolent lymphoma. J. Clin. Oncol. 39, 1609–1618 (2021).
Flinn, I. W. et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ,γ, is clinically active in advanced hematologic malignancies. Blood 131, 877–887 (2018).
Chandarlapaty, S. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 (2011).
Chakrabarty, A., Sánchez, V., Kuba, M. G., Rinehart, C. & Arteaga, C. L. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc. Natl Acad. Sci. USA 109, 2718–2723 (2012).
Bosch, A. et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor–positive breast cancer. Sci. Transl. Med. 7, 283ra51 (2015).
Toska, E. et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science 355, 1324–1330 (2017).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).
Eschweiler, S. et al. Intermittent PI3Kδ inhibition sustains anti-tumour immunity and curbs irAEs. Nature 605, 741–746 (2022).
Song, K. W. et al. RTK-dependent inducible degradation of mutant PI3Ka drives GDC-0077 (Inavolisib) efficacy. Cancer Discov. 12, 204–219 (2022).
Brown, J. R. & Auger, K. R. Phylogenomics of phosphoinositide lipid kinases: perspectives on the evolution of second messenger signaling and drug discovery. BMC Evolut. Biol. 11, 4 (2011).
Burke, J. E. et al. Dynamics of the phosphoinositide 3-kinase p110δ interaction with p85α and membranes reveals aspects of regulation distinct from p110α. Structure 19, 1127–1137 (2011).
Burke, J. E., Perisic, O., Masson, G. R., Vadas, O. & Williams, R. L. Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110α (PIK3CA). Proc. Natl Acad. Sci. USA 109, 15259–15264 (2012).
Dbouk, H. A. et al. G protein-coupled receptor-mediated activation of p110β by Gβγ is required for cellular transformation and invasiveness. Sci. Signal. 5, ra89 (2012).
Vadas, O. et al. Molecular determinants of PI3Kγ-mediated activation downstream of G-protein-coupled receptors (GPCRs). Proc. Natl Acad. Sci. USA 110, 18862–18867 (2013).
Acknowledgements
J.E.B. is supported by the Natural Science and Engineering Research Council (Discovery grant NSERC-2020-04241), the Michael Smith Foundation for Health Research (Scholar Award 17686), the Canadian Institute of Health Research (CIHR Project grant 168998) and the Cancer Research Society (operating grant CRS-843232). J.T. is supported by the Swiss National Science Foundation (#310030_207635). B.M.E. receives research funding from NCI (R01 CA237536, CBC under contract no. 75N91019D00024, task order no. 75N91020F00003) and ACS (RSG-20-064-01-TBE, TLC-21-156-01). G.R.V.H. receives funding from NIGMS (R35 GM119412) and NCATS (R03TR003624). The authors are grateful to R. Wills, A. Shaw and C. Weckerly for valuable discussions and suggestions for the manuscript. They apologize to all authors whose work could not be mentioned because of the limitations on the number of references that could be cited.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
J.E.B. reports personal fees from Scorpion Therapeutics and Olema Oncology; and research grants from Novartis. J.T., B.M.E. and G.R.V.H. declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Acute myeloid leukaemia
-
(AML). A fast-growing cancer of the blood and bone marrow.
- Alzheimer disease
-
A progressive brain disorder that affects memory and other cognitive abilities.
- Amyotrophic lateral sclerosis
-
(ALS). A rare neurological disease that affects neurons responsible for controlling voluntary muscle movement.
- B cell non-Hodgkin lymphoma
-
(B-NHL). Cancer that arises when B cells, a type of lymphocyte that normally fights infections by producing antibodies to neutralize foreign invaders, start to grow uncontrollably, eventually overwhelming healthy cells.
- Charcot–Marie–Tooth disease
-
A group of inherited disorders that cause nerve damage. Also referred to as hereditary motor and sensory neuropathy.
- Creutzfeldt–Jacob disease
-
(CJD). A rapidly progressive degenerative brain disorder that leads to dementia and death.
- Cryptosporidiosis
-
A diarrhoeal disease caused by a microscopic parasite called Cryptosporidium, with particular prevalence in children and immunocompromised individuals.
- Endocytosis
-
A cellular process by which cells internalize substances from their external environment.
- Frontotemporal dementia
-
(FTD). An uncommon type of dementia that is a result of damage to neurons in the frontal and temporal lobes of the brain, which causes problems in behaviour and language.
- Glioblastoma
-
(GBM). A fast-growing type of central nervous system tumour that forms from the glial tissue of the brain and spinal cord.
- Macroautophagy
-
A fundamental degradative pathway for cytosolic components, such as proteins, RNA, DNA, lipids and organelles. It involves the sequestering of such cytosolic material in double-membraned structures called autophagosomes, followed by membrane trafficking to the lysosome for degradation and recycling.
- Malaria
-
A disease transmitted by the bite of infected female mosquitoes caused by Plasmodium parasites (five species infect humans, with two species, Plasmodium vivax and Plasmodium falciparum, posing the largest threat).
- Parkinson disease
-
A central nervous system disorder which is associated with a deficiency of the neurotransmitter dopamine and affects movement often resulting in tremors.
- Primary lateral sclerosis
-
(PLS). A rare neuromuscular disease that affects the central motor neurons and is characterized by the gradual weakness and stiffness of muscles.
- p53 null tumours
-
Tumours that are deficient in either the tumour suppressor TP53 gene or the functional p53 protein product, which induces apoptosis, cell cycle arrest or senescence in response to distinct stimuli.
- TTC7A deficiency
-
A rare genetic disorder caused by dysfunction of a gene (TTC7A), which causes diarrhoea, inflammation of the intestines, bowel obstructions, immune dysfunction and an inability to absorb nutrients.
- Viral pathogens
-
Viruses that can infect and replicate within human cells and cause disease.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burke, J.E., Triscott, J., Emerling, B.M. et al. Beyond PI3Ks: targeting phosphoinositide kinases in disease. Nat Rev Drug Discov 22, 357–386 (2023). https://doi.org/10.1038/s41573-022-00582-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-022-00582-5
This article is cited by
-
In silico discovery and experimental validation of natural derivatives as inhibitor of phosphoinositide Kinase, PIKfyve
Journal of Computer-Aided Molecular Design (2026)
-
Induction of the p21/CDK6 pathway and alteration of the immune microenvironment by the stem cell marker CBX3 in melanoma
Stem Cell Research & Therapy (2025)
-
The role of FAM111B in the malignant progression and molecular regulation of human glioma through the PI3K/Akt pathway
Chinese Neurosurgical Journal (2025)
-
P4-ATPases control phosphoinositide membrane asymmetry and neomycin resistance
Nature Cell Biology (2025)
-
Phosphoinositide kinases in cancer: from molecular mechanisms to therapeutic opportunities
Nature Reviews Cancer (2025)