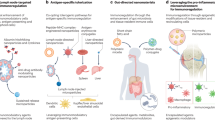
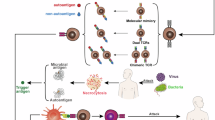
Abstract
Remarkable progress has been made in recent decades in engineering antibodies and other protein therapeutics, including enhancements to existing functions as well as the advent of novel molecules that confer biological activities previously unknown in nature. These protein therapeutics have brought major benefits to patients across multiple areas of medicine. One major ongoing challenge is that protein therapeutics can elicit unwanted immune responses (immunogenicity) in treated patients, including the generation of anti-drug antibodies. In rare and unpredictable cases, anti-drug antibodies can seriously compromise therapeutic safety and/or efficacy. Systematic deconvolution of this immunogenicity problem is confounded by the complexity of its many contributing factors and the inherent limitations of available experimental and computational methods. Nevertheless, continued progress with the assessment and mitigation of immunogenicity risk at the preclinical stage has the potential to reduce the incidence and severity of clinical immunogenicity events. This Review focuses on identifying key unsolved anti-drug antibody-related challenges and offers some pragmatic approaches towards addressing them. Examples are drawn mainly from antibodies, given that the majority of available clinical data are from this class of protein therapeutics. Plausible and seemingly tractable solutions are in sight for some immunogenicity problems, whereas other challenges will likely require completely new approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Walsh, G. & Walsh, E. Biopharmaceutical benchmarks 2022. Nat. Biotechnol. 40, 1722–1760 (2022).
Fülöp, T., Mészáros, T., Kozma, G. T., Szebeni, J. & Józsi, M. Infusion reactions associated with the medical application of monoclonal antibodies: the role of complement activation and possibility of inhibition by factor H. Antibodies 7, 14 (2018).
Rombouts, M. D., Swart, E. L., van den Eertwegh, A. J. M. & Crul, M. Systematic review on infusion reactions to and infusion rate of monoclonal antibodies used in cancer treatment. Anticancer Res. 40, 1201–1218 (2020).
Sala-Cunill, A., Luengo, O. & Cardona, V. Biologics and anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 19, 439–446 (2019).
Shimabukuro-Vornhagen, A. et al. Cytokine release syndrome. J. Immunother. Cancer 6, 56 (2018).
Krishna, M. & Nadler, S. G. Immunogenicity to biotherapeutics – the role of anti-drug immune complexes. Front. Immunol. 7, 21 (2016).
Gunn, G. R. et al. From the bench to clinical practice: understanding the challenges and uncertainties in immunogenicity testing for biopharmaceuticals. Clin. Exp. Immunol. 184, 137–146 (2016).
Cassotta, A. et al. A single T cell epitope drives the neutralizing anti-drug antibody response to natalizumab in multiple sclerosis patients. Nat. Med. 25, 1402–1407 (2019). This ABIRISK consortium study creates and characterizes panels of monoclonal antibodies from two anti-natalizumab antibody-positive patients.
Mehta, P. & Manson, J. J. What is the clinical relevance of TNF inhibitor immunogenicity in the management of patients with rheumatoid arthritis? Front. Immunol. 11, 589 (2020).
Ridker, P. M. et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N. Engl. J. Med. 376, 1517–1526 (2017). Multiple phase III clinical studies of a humanized antibody (bococizumab) in thousands of patients reveals high rates of ADAs that correlate with adverse effects on pharmacokinetics and efficacy.
Yu, R. J., Krantz, M. S., Phillips, E. J. & Stone, C. A. Emerging causes of drug-induced anaphylaxis: a review of anaphylaxis-associated reports in the FDA adverse event reporting system (FAERS). J. Allergy Clin. Immunol. Pract. 9, 819–829.e2 (2021).
Chung, C. H. et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N. Engl. J. Med. 358, 1109–1117 (2008).
Casadevall, N. et al. Pure red-cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N. Engl. J. Med. 346, 469–475 (2002). Ground-breaking report that demonstrates that ADAs to protein therapeutics can on rare occasions have a severe clinical impact.
Schellekens, H. & Jiskoot, W. Eprex-associated pure red cell aplasia and leachates. Nat. Biotechnol. 24, 613–614 (2006).
McKoy, J. M. et al. Epoetin‐associated pure red cell aplasia: past, present, and future considerations. Transfusion 48, 1754–1762 (2008).
Rubic-Schneider, T. et al. T-cell assays confirm immunogenicity of tungsten-induced erythropoietin aggregates associated with pure red cell aplasia. Blood Adv. 1, 367–379 (2017).
Gibbons, J. B., Laber, M. & Bennett, C. L. Humira: the first $20 billion drug. Am. J. Manag. Care 29, 78–80 (2023).
Highlights of prescribing information, HUMIRA® (adalimumab) injection, for subcutaneous use. US Food and Drug Administration www.accessdata.fda.gov/drugsatfda_docs/label/2018/125057s410lbl.pdf (2018).
Bartelds, G. M. et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305, 1460–1468 (2011).
Murdaca, G. et al. Immunogenicity of infliximab and adalimumab: what is its role in hypersensitivity and modulation of therapeutic efficacy and safety? Expert Opin. Drug Saf. 15, 43–52 (2016).
Koren, E. et al. Recommendations on risk-based strategies for detection and characterization of antibodies against biotechnology products. J. Immunol. Methods 333, 1–9 (2008).
Mire-Sluis, A. R. et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J. Immunol. Methods 289, 1–16 (2004).
Shankar, G. et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. AAPS J. 16, 658–673 (2014).
Immunogenicity assessment for therapeutic protein products. Docket number: FDA-2013-D-0092. US Food and Drug Administration www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-assessment-therapeutic-protein-products (2014).
Immunogenicity testing of therapeutic protein products — developing and validating assays for anti-drug antibody detection. Docket number FDA-2009-D-0539. US Food and Drug Administration www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-testing-therapeutic-protein-products-developing-and-validating-assays-anti-drug (2019).
Immunogenicity assessment of biotechnology-derived therapeutic proteins — scientific guideline EMEA/CHMP/BMWP/14327/2006 Rev 1. European Medicines Agency www.ema.europa.eu/en/immunogenicity-assessment-biotechnology-derived-therapeutic-proteins-scientific-guideline (2017).
Carter, P. J. & Rajpal, A. Designing antibodies as therapeutics. Cell 185, 2789–2805 (2022).
Ebrahimi, S. B. & Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat. Commun. 14, 2411 (2023).
Abbas, A. K., Lichtman, A. H. & Pillai, S. in Cellular and Molecular Immunology 10th edn, Ch. 6 (Elsevier, 2021).
Cornaby, C. et al. B cell epitope spreading: mechanisms and contribution to autoimmune diseases. Immunol. Lett. 163, 56–68 (2015).
Fahlquist-Hagert, C. et al. Antigen presentation by B cells enables epitope spreading across an MHC barrier. Nat. Commun. 14, 6941 (2023).
Murphy, K. M., Weaver, C. & Berg, L. J. Janeway’s Immunobiology (eds Twitchell, B. & Barrett-Bressack, C.) 10th edn (W. W. Norton and Company, 2022).
Moore, W. V. & Leppert, P. Role of aggregated human growth hormone (hGH) in development of antibodies to hGH. J. Clin. Endocrinol. Metab. 51, 691–697 (1980). Early study demonstrating that the incidence of ADAs in patients treated with human growth hormone correlates with the level of aggregates.
Joubert, M. K. et al. Highly aggregated antibody therapeutics can enhance the in vitro innate and late-stage T-cell immune responses. J. Biol. Chem. 287, 25266–25279 (2012).
Bessa, J. et al. The immunogenicity of antibody aggregates in a novel transgenic mouse model. Pharm. Res. 32, 2344–2359 (2015).
Kretsinger, J. et al. Expectations for phase-appropriate drug substance and drug product specifications for early-stage protein therapeutics. J. Pharm. Sci. 108, 1442–1452 (2019).
Casasola-LaMacchia, A. et al. HLAII peptide presentation of infliximab increases when complexed with TNF. Front. Immunol. 13, 932252 (2022).
Kroenke, M. A. et al. Immune complex formation is associated with loss of tolerance and an antibody response to both drug and target. Front. Immunol. 12, 782788 (2021).
Wilkinson, I. et al. Fc-engineered antibodies with immune effector functions completely abolished. PLoS ONE 16, e0260954 (2021).
Siegel, M. et al. Validation of a dendritic cell and CD4+ T cell restimulation assay contributing to the immunogenicity risk evaluation of biotherapeutics. Pharmaceutics 14, 2672 (2022).
Tatarewicz, S. M. et al. Strategic characterization of anti-drug antibody responses for the assessment of clinical relevance and impact. Bioanalysis 6, 1509–1523 (2014).
Wadhwa, M. & Thorpe, R. Harmonization and standardization of immunogenicity assessment of biotherapeutic products. Bioanalysis 11, 1593–1604 (2019).
Gorovits, B. Antidrug antibody assay validation: industry survey results. AAPS J. 11, 133–138 (2009).
Starcevic Manning, M. et al. Comparison of titer and signal to noise (S/N) for determination of anti-drug antibody magnitude using clinical data from an industry consortium. AAPS J. 24, 81 (2022).
Pratt, K. P. Anti-drug antibodies: emerging approaches to predict, reduce or reverse biotherapeutic immunogenicity. Antibodies 7, 19 (2018).
Hofbauer, C. J. et al. Affinity of FVIII-specific antibodies reveals major differences between neutralizing and nonneutralizing antibodies in humans. Blood 125, 1180–1188 (2015).
Wu, B. et al. Strategies to determine assay format for the assessment of neutralizing antibody responses to biotherapeutics. AAPS J. 18, 1335–1350 (2016).
Büttel, I. C. et al. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals 39, 100–109 (2011).
Deal, C. L. et al. Efficacy and safety of weekly somatrogon vs daily somatropin in children with growth hormone deficiency: a phase 3 study. J. Clin. Endocrinol. Metab. 107, e2717–e2728 (2022).
Fineberg, S. E. et al. Immunological responses to exogenous insulin. Endocr. Rev. 28, 625–652 (2007).
Gorovits, B. Current considerations for immunoglobulin isotype characterization of antibody response against biotherapeutics. AAPS J. 22, 144 (2020).
Carrasco-Triguero, M. et al. Immunogenicity assays for antibody-drug conjugates: case study with ado-trastuzumab emtansine. Bioanalysis 5, 1007–1023 (2013).
Myler, H. et al. Anti-drug antibody validation testing and reporting harmonization. AAPS J. 24, 4 (2021).
Lundahl, M. L. E., Fogli, S., Colavita, P. E. & Scanlan, E. M. Aggregation of protein therapeutics enhances their immunogenicity: causes and mitigation strategies. RSC Chem. Biol. 2, 1004–1020 (2021).
Jawa, V., Maamary, J., Swanson, M., Zhang, S. & Montgomery, D. Implementing a clinical immunogenicity strategy using preclinical risk assessment outputs. J. Pharm. Sci. 111, 960–969 (2022).
Ducret, A. et al. Assay format diversity in pre-clinical immunogenicity risk assessment: toward a possible harmonization of antigenicity assays. mAbs 14, 1993522 (2022).
Ulitzka, M. et al. Engineering therapeutic antibodies for patient safety: tackling the immunogenicity problem. Protein Eng. Des. Sel. 33, gzaa025 (2020).
Nilsson, J. B. et al. Accurate prediction of HLA class II antigen presentation across all loci using tailored data acquisition and refined machine learning. Sci. Adv. 9, eadj6367 (2023). In silico tool (NetMHCIIpan-4.3) refined by machine learning for high-accuracy prediction of antigen presentation across all human leukocyte antigen class II allotypes.
Chen, B. et al. Predicting HLA class II antigen presentation through integrated deep learning. Nat. Biotechnol. 37, 1332–1343 (2019).
Thrift, W. J. et al. Graph-pMHC: graph neural network approach to MHC class II peptide presentation and antibody immunogenicity. Brief. Bioinform. 25, bbae123 (2024).
Prihoda, D. et al. BioPhi: a platform for antibody design, humanization, and humanness evaluation based on natural antibody repertoires and deep learning. mAbs 14, 2020203 (2022).
Attermann, A. S. et al. Improved prediction of HLA antigen presentation hotspots: applications for immunogenicity risk assessment of therapeutic proteins. Immunology 162, 208–219 (2021).
Ramon, A. et al. Assessing antibody and nanobody nativeness for hit selection and humanization with AbNatiV. Nat. Mach. Intell. 6, 74–91 (2024).
Racle, J. et al. Machine learning predictions of MHC-II specificities reveal alternative binding mode of class II epitopes. Immunity 56, 1359–1375.e13 (2023).
Melendez, R. et al. Introducing dendritic cell antibody internalization as an immunogenicity risk assessment tool. Bioanalysis 14, 703–713 (2022).
Wen, Y. et al. Development of a FRET-based assay for analysis of mAbs internalization and processing by dendritic cells in preclinical immunogenicity risk assessment. AAPS J. 22, 68 (2020).
Röhn, T. A. et al. A novel strategy for the discovery of MHC class II–restricted tumor antigens: identification of a melanotransferrin helper T-cell epitope. Cancer Res. 65, 10068–10078 (2005).
Steiner, G. et al. Enabling routine MHC-II-associated peptide proteomics for risk assessment of drug-induced immunogenicity. J. Proteome Res. 19, 3792–3806 (2020).
Lee, M. V. et al. Development of a semi-automated MHC-associated peptide proteomics (MAPPs) method using streptavidin bead-based immunoaffinity capture and nano LC-MS/MS to support immunogenicity risk assessment in drug development. Front. Immunol. 14, 1295285 (2023).
Hartman, K. et al. Expanding the MAPPs assay to accommodate MHC-II pan receptors for improved predictability of potential T cell epitopes. Biology 12, 1265 (2023).
Jiang, W. & Boder, E. T. High-throughput engineering and analysis of peptide binding to class II MHC. Proc. Natl Acad. Sci. USA 107, 13258–13263 (2010).
Arata, Y. et al. Rapid in vitro assessment of the immunogenicity potential of engineered antibody therapeutics through detection of CD4+ T cell interleukin-2 secretion. mAbs 15, 2253570 (2023).
Cohen, S. et al. Immunogenicity risk assessment for biotherapeutics through in vitro detection of CD134 and CD137 on T helper cells. mAbs 13, 1898831 (2021).
Walsh, R. E. et al. Post hoc assessment of the immunogenicity of three antibodies reveals distinct immune stimulatory mechanisms. mAbs 12, 1764829 (2020).
Ito, S. et al. In vitro human helper T-cell assay to screen antibody drug candidates for immunogenicity. J. Immunotoxicol. 16, 125–132 (2019).
Ponce, R. et al. Immunogenicity of biologically-derived therapeutics: assessment and interpretation of nonclinical safety studies. Regul. Toxicol. Pharmacol. 54, 164–182 (2009).
Swanson, S. J. & Bussiere, J. Immunogenicity assessment in non-clinical studies. Curr. Opin. Microbiol. 15, 337–347 (2012).
van Meer, P. J. et al. Immunogenicity of mAbs in non-human primates during nonclinical safety assessment. mAbs 5, 810–816 (2013).
Egli, J. et al. Enhanced immunogenic potential of cancer immunotherapy antibodies in human IgG1 transgenic mice. mAbs 14, 2143009 (2022).
Brennan, F. R. et al. Safety testing of monoclonal antibodies in non-human primates: case studies highlighting their impact on human risk assessment. mAbs 10, 1–17 (2017).
Gokemeijer, J. et al. Survey outcome on immunogenicity risk assessment tools for biotherapeutics: an insight into consensus on methods, application, and utility in drug development. AAPS J. 25, 55 (2023).
Quarmby, V., Phung, Q. T. & Lill, J. R. MAPPs for the identification of immunogenic hotspots of biotherapeutics; an overview of the technology and its application to the biopharmaceutical arena. Expert Rev. Proteomics 15, 733–748 (2018).
Sekiguchi, N. et al. MHC-associated peptide proteomics enabling highly sensitive detection of immunogenic sequences for the development of therapeutic antibodies with low immunogenicity. mAbs 10, 1168–1181 (2018).
Egholm Bruun Jensen, E., Reynisson, B., Barra, C. & Nielsen, M. New light on the HLA-DR immunopeptidomic landscape. J. Leukoc. Biol. 115, 913–925 (2024).
Tsai, W. K. et al. Nonclinical immunogenicity risk assessment for knobs-into-holes bispecific IgG1 antibodies. mAbs 16, 2362789 (2024). First direct evidence to support the validity of the widespread practice of using research-grade antibody preparations to investigate the immunogenicity risk of their pharmaceutical counterparts.
Xue, L., Hickling, T., Song, R., Nowak, J. & Rup, B. Contribution of enhanced engagement of antigen presentation machinery to the clinical immunogenicity of a human interleukin (IL)-21 receptor-blocking therapeutic antibody. Clin. Exp. Immunol. 183, 102–113 (2016).
Zinsli, L. V., Stierlin, N., Loessner, M. J. & Schmelcher, M. Deimmunization of protein therapeutics — recent advances in experimental and computational epitope prediction and deletion. Comput. Struct. Biotechnol. J. 19, 315–329 (2021).
Kearns, J. D. et al. A root cause analysis to identify the mechanistic drivers of immunogenicity against the anti-VEGF biotherapeutic brolucizumab. Sci. Transl. Med. 15, eabq5068 (2023).
Kuroda, D. & Tsumoto, K. Engineering stability, viscosity, and immunogenicity of antibodies by computational design. J. Pharm. Sci. 109, 1631–1651 (2020).
Sampei, Z. et al. Identification and multidimensional optimization of an asymmetric bispecific IgG antibody mimicking the function of factor VIII cofactor activity. PLoS ONE 8, e57479 (2013).
Schmitt, C. et al. Low immunogenicity of emicizumab in persons with haemophilia A. Haemophilia 27, 984–992 (2021). Together with Sampei et al. (2013), demonstrates that it is possible to extensively engineer a protein therapeutic (bispecific antibody) with low incidence of ADAs.
Mazor, R. et al. Elimination of murine and human T-cell epitopes in recombinant immunotoxin eliminates neutralizing and anti-drug antibodies in vivo. Cell. Mol. Immunol. 14, 432–442 (2017). Demonstration of immunogenicity risk mitigation by engineering out T cell epitopes in a recombinant immunotoxin to prevent the formation of ADAs in immune-competent mice.
Mazor, R. & Pastan, I. Immunogenicity of immunotoxins containing Pseudomonas exotoxin A: causes, consequences, and mitigation. Front. Immunol. 11, 1261 (2020).
Therapeutic monoclonal antibodies approved or in regulatory review. The Antibody Society www.antibodysociety.org/antibody-therapeutics-product-data (accessed 3 May 2024).
Boulianne, G. L., Hozumi, N. & Shulman, M. J. Production of functional chimaeric mouse/human antibody. Nature 312, 643–646 (1984).
Morrison, S. L., Johnson, M. J., Herzenberg, L. A. & Oi, V. T. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc. Natl Acad. Sci. USA 81, 6851–6855 (1984).
Jones, P. T., Dear, P. H., Foote, J., Neuberger, M. S. & Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986).
Riechmann, L., Clark, M., Waldmann, H. & Winter, G. Reshaping human antibodies for therapy. Nature 332, 323–327 (1988).
Verhoeyen, M., Milstein, C. & Winter, G. Reshaping human antibodies: grafting an antilysozyme activity. Science 239, 1534–1536 (1988).
Foote, J. & Winter, G. Antibody framework residues affecting the conformation of the hypervariable loops. J. Mol. Biol. 224, 487–499 (1992).
Vaughan, T. J. et al. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat. Biotechnol. 14, 309–314 (1996).
Fishwild, D. M. et al. High-avidity human IgGκ monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat. Biotechnol. 14, 845–851 (1996).
Mendez, M. J. et al. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat. Genet. 15, 146–156 (1997).
Lee, E. C. et al. Complete humanization of the mouse immunoglobulin loci enables efficient therapeutic antibody discovery. Nat. Biotechnol. 32, 356–363 (2014).
Murphy, A. J. et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc. Natl Acad. Sci. USA 111, 5153–5158 (2014).
Baek, D. S. & Kim, Y. S. Construction of a large synthetic human Fab antibody library on yeast cell surface by optimized yeast mating. J. Microbiol. Biotechnol. 24, 408–420 (2014).
Kelly, R. L., Le, D., Zhao, J. & Wittrup, K. D. Reduction of nonspecificity motifs in synthetic antibody libraries. J. Mol. Biol. 430, 119–130 (2018).
Gieselmann, L. et al. Effective high-throughput isolation of fully human antibodies targeting infectious pathogens. Nat. Protoc. 16, 3639–3671 (2021).
Marks, C., Hummer, A. M., Chin, M. & Deane, C. M. Humanization of antibodies using a machine learning approach on large-scale repertoire data. Bioinformatics 37, 4041–4047 (2021). Demonstration of a negative correlation between humanness score and clinical ADA incidence for antibodies.
Jain, T., Boland, T. & Vasquez, M. Identifying developability risks for clinical progression of antibodies using high-throughput in vitro and in silico approaches. mAbs 15, 2200540 (2023).
Jain, T. et al. Biophysical properties of the clinical-stage antibody landscape. Proc. Natl Acad. Sci. USA 114, 944–949 (2017). Pioneering developability assessment of a large panel (n = 137) of clinical-stage antibodies to empirically define the boundaries of drug-like behaviour.
Hua, F. et al. Anti-IL21 receptor monoclonal antibody (ATR-107): safety, pharmacokinetics, and pharmacodynamic evaluation in healthy volunteers: a phase I, first-in-human study. J. Clin. Pharmacol. 54, 14–22 (2014).
Lowe, S. L. et al. Donanemab (LY3002813) dose-escalation study in Alzheimer’s disease. Alzheimers Dement. 7, e12112 (2021).
Baker, M., Reynolds, H. M., Lumicisi, B. & Bryson, C. J. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself 1, 314–322 (2010).
Harris, C. T. & Cohen, S. Reducing immunogenicity by design: approaches to minimize immunogenicity of monoclonal antibodies. BioDrugs 38, 205–226 (2024).
Myler, H. et al. Report on the AAPS immunogenicity guidance forum. AAPS J. 21, 55 (2019).
Wyant, T., Yang, L. & Rosario, M. Comparison of the ELISA and ECL assay for vedolizumab anti-drug antibodies: assessing the impact on pharmacokinetics and safety outcomes of the phase 3 GEMINI trials. AAPS J. 23, 3 (2020).
Davda, J. et al. Immunogenicity of immunomodulatory, antibody-based, oncology therapeutics. J. Immunother. Cancer 7, 105 (2019).
Enrico, D., Paci, A., Chaput, N., Karamouza, E. & Besse, B. Antidrug antibodies against immune checkpoint blockers: impairment of drug efficacy or indication of immune activation? Clin. Cancer Res. 26, 787–792 (2020).
Clavero-Alvarez, A., Di Mambro, T., Perez-Gaviro, S., Magnani, M. & Bruscolini, P. Humanization of antibodies using a statistical inference approach. Sci. Rep. 8, 14820 (2018).
Tourdot, S. & Hickling, T. P. Nonclinical immunogenicity risk assessment of therapeutic proteins. Bioanalysis 11, 1631–1643 (2019).
Dyson, M. R. et al. Beyond affinity: selection of antibody variants with optimal biophysical properties and reduced immunogenicity from mammalian display libraries. mAbs 12, 1829335 (2020). Demonstration that improving the biophysical properties of an antibody (bococizumab) correlates with a reduced immunogenicity risk in nonclinical assays.
Wen, Y. & Jawa, V. The impact of product and process related critical quality attributes on immunogenicity and adverse immunological effects of biotherapeutics. J. Pharm. Sci. 110, 1025–1041 (2021).
Joubert, M. K., Luo, Q., Nashed-Samuel, Y., Wypych, J. & Narhi, L. O. Classification and characterization of therapeutic antibody aggregates. J. Biol. Chem. 286, 25118–25133 (2011).
Dang, X. et al. Epitope mapping of monoclonal antibodies: a comprehensive comparison of different technologies. mAbs 15, 2285285 (2023).
Geraldes, D. C. et al. Protein drug delivery: current dosage form profile and formulation strategies. J. Drug Target. 28, 339–355 (2019).
Ghosh, I., Gutka, H., Krause, M. E., Clemens, R. & Kashi, R. S. A systematic review of commercial high concentration antibody drug products approved in the US: formulation composition, dosage form design and primary packaging considerations. mAbs 15, 2205540 (2023).
Strickley, R. G. & Lambert, W. J. A review of formulations of commercially available antibodies. J. Pharm. Sci. 110, 2590–2608.e56 (2021).
Davis, J. D. et al. Subcutaneous administration of monoclonal antibodies: pharmacology, delivery, immunogenicity, and learnings from applications to clinical development. Clin. Pharmacol. Ther. 115, 422–439 (2024).
Felderman, J., Ramaiah, L., Vazquez-Abad, M. D., Messing, D. & Chen, Y. Anti-drug antibody incidence comparison of therapeutic proteins administered via subcutaneous vs. intravenous route. AAPS J. 26, 60 (2024).
Hamuro, L. et al. Perspectives on subcutaneous route of administration as an immunogenicity risk factor for therapeutic proteins. J. Pharm. Sci. 106, 2946–2954 (2017).
Jarvi, N. L. & Balu-Iyer, S. V. Immunogenicity challenges associated with subcutaneous delivery of therapeutic proteins. BioDrugs 35, 125–146 (2021).
Holland, M. C. et al. Autoantibodies to variable heavy (VH) chain Ig sequences in humans impact the safety and clinical pharmacology of a VH domain antibody antagonist of TNF-α receptor 1. J. Clin. Immunol. 33, 1192–1203 (2013).
Cordy, J. C. et al. Specificity of human anti-variable heavy (VH) chain autoantibodies and impact on the design and clinical testing of a VH domain antibody antagonist of tumour necrosis factor-α receptor 1. Clin. Exp. Immunol. 182, 139–148 (2015). B cell epitope mapping is used to redesign VH domain antibodies with minimal reactivity to pre-existing autoantibodies.
Brezski, R. J. et al. Human anti-IgG1 hinge autoantibodies reconstitute the effector functions of proteolytically inactivated IgGs. J. Immunol. 181, 3183–3192 (2008).
Mellbye, O. J. & Natvig, J. B. Evidence for immune complexes containing antibody to the pepsin site of IgG in rheumatoid synovial fluids. Clin. Exp. Immunol. 8, 889–899 (1971).
Rispens, T. et al. Antibodies to constant domains of therapeutic monoclonal antibodies: anti-hinge antibodies in immunogenicity testing. J. Immunol. Methods 375, 93–99 (2012).
Terness, P. et al. The natural human IgG anti-F(ab’)2 antibody recognizes a conformational IgG1 hinge epitope. J. Immunol. 154, 6446–6452 (1995).
Kim, H. S. et al. Evading pre-existing anti-hinge antibody binding by hinge engineering. mAbs 8, 1536–1547 (2016). B cell epitope mapping that facilitates the design of Fab and F(ab’)2 fragments with minimal reactivity to pre-existing anti-hinge autoantibodies.
Barker, D. J. et al. The IPD-IMGT/HLA database. Nucleic Acids Res. 51, D1053–D1060 (2023).
McKinney, D. M. et al. A strategy to determine HLA class II restriction broadly covering the DR, DP, and DQ allelic variants most commonly expressed in the general population. Immunogenetics 65, 357–370 (2013).
McGill, J. R., Yogurtcu, O. N., Verthelyi, D., Yang, H. & Sauna, Z. E. SampPick: selection of a cohort of subjects matching a population HLA distribution. Front. Immunol. 10, 2894 (2019).
Yogurtcu, O. N., Sauna, Z. E., McGill, J. R., Tegenge, M. A. & Yang, H. TCPro: an in silico risk assessment tool for biotherapeutic protein immunogenicity. AAPS J. 21, 96 (2019).
Mahlangu, J. N. et al. Changes in the amino acid sequence of the recombinant human factor VIIa analog, vatreptacog alfa, are associated with clinical immunogenicity. J. Thromb. Haemost. 13, 1989–1998 (2015).
Lamberth, K. et al. Post hoc assessment of the immunogenicity of bioengineered factor VIIa demonstrates the use of preclinical tools. Sci. Transl. Med. 9, eaag1286 (2017). A post hoc assessment showing that a new T cell epitope is inadvertently created when three amino acid substitutions are made in factor VIIa to create vatreptacog alfa.
Jaber, A. & Baker, M. Assessment of the immunogenicity of different interferon beta-1a formulations using ex vivo T-cell assays. J. Pharm. Biomed. Anal. 43, 1256–1261 (2007).
Jaber, A. et al. The Rebif new formulation story: it’s not trials and error. Drugs R D 8, 335–348 (2007).
Giovannoni, G. et al. Safety and immunogenicity of a new formulation of interferon β-1a (Rebif® New Formulation) in a Phase IIIb study in patients with relapsing multiple sclerosis: 96-week results. Mult. Scler. J. 15, 219–228 (2009).
Alt, N. et al. Determination of critical quality attributes for monoclonal antibodies using quality by design principles. Biologicals 44, 291–305 (2016).
Jawa, V. et al. Evaluating immunogenicity risk due to host cell protein impurities in antibody-based biotherapeutics. AAPS J. 18, 1439–1452 (2016).
Barra, C. et al. Immunopeptidomic data integration to artificial neural networks enhances protein-drug immunogenicity prediction. Front. Immunol. 11, 1304 (2020).
Duhazé, J. et al. A machine learning approach for high-dimensional time-to-event prediction with application to immunogenicity of biotherapies in the ABIRISK cohort. Front. Immunol. 11, 608 (2020).
Sun, R., Qian, M. G. & Zhang, X. T and B cell epitope analysis for the immunogenicity evaluation and mitigation of antibody-based therapeutics. mAbs 16, 2324836 (2024).
Vanderlaan, M. et al. Changes in manufacturing processes of biologic therapies can alter the immunogenicity profile of the product. Clin. Pharmacol. Ther. 107, 988–993 (2020).
Vagenende, V., Yap, M. G. & Trout, B. L. Mechanisms of protein stabilization and prevention of protein aggregation by glycerol. Biochemistry 48, 11084–11096 (2009).
Fleischman, M. L., Chung, J., Paul, E. P. & Lewus, R. A. Shipping-induced aggregation in therapeutic antibodies: utilization of a scale-down model to assess degradation in monoclonal antibodies. J. Pharm. Sci. 106, 994–1000 (2017).
Fischer, S. K. et al. Specific immune response to phospholipase B-like 2 protein, a host cell impurity in lebrikizumab clinical material. AAPS J. 19, 254–263 (2017).
Molden, R. et al. Host cell protein profiling of commercial therapeutic protein drugs as a benchmark for monoclonal antibody-based therapeutic protein development. mAbs 13, 1955811 (2021).
Jones, M. et al. “High-risk” host cell proteins (HCPs): a multi-company collaborative view. Biotechnol. Bioeng. 118, 2870–2885 (2021).
Gupta, S., Jiskoot, W., Schöneich, C. & Rathore, A. S. Oxidation and deamidation of monoclonal antibody products: potential impact on stability, biological activity, and efficacy. J. Pharm. Sci. 111, 903–918 (2022).
Chen, B. M., Cheng, T. L. & Roffler, S. R. Polyethylene glycol immunogenicity: theoretical, clinical, and practical aspects of anti-polyethylene glycol antibodies. ACS Nano 15, 14022–14048 (2021).
Highlights of prescribing information, Rituxan (rituximab) injection for intravenous use. US Food and Drug Administration www.accessdata.fda.gov/drugsatfda_docs/label/2010/103705s5311lbl.pdf (2010).
Papadopoulos, K. P. et al. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic Nanobody® targeting the DR5 receptor. Cancer Chemother. Pharmacol. 75, 887–895 (2015).
Ducourau, E. et al. Methotrexate effect on immunogenicity and long-term maintenance of adalimumab in axial spondyloarthritis: a multicentric randomised trial. RMD Open 6, e001047 (2020).
Peters, S. et al. Obinutuzumab pretreatment as a novel approach to mitigate formation of anti-drug antibodies against cergutuzumab amunaleukin in patients with solid tumors. Clin. Cancer Res. 30, 1630–1641 (2024).
Reynisson, B., Alvarez, B., Paul, S., Peters, B. & Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 48, W449–W454 (2020).
Desai, A. K., Kazi, Z. B., Bali, D. S. & Kishnani, P. S. Characterization of immune response in cross-reactive immunological material (CRIM)-positive infantile Pompe disease patients treated with enzyme replacement therapy. Mol. Genet. Metab. Rep. 20, 100475 (2019).
Messinger, Y. H. et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet. Med. 14, 135–142 (2012).
Sands, E. et al. Tolerogenic nanoparticles mitigate the formation of anti-drug antibodies against pegylated uricase in patients with hyperuricemia. Nat. Commun. 13, 272 (2022).
Cia, G., Pucci, F. & Rooman, M. Critical review of conformational B-cell epitope prediction methods. Brief. Bioinform. 24, bbac567 (2023).
Lin, J. et al. A structure-based engineering approach to abrogate pre-existing antibody binding to biotherapeutics. PLoS ONE 16, e0254944 (2021).
Acknowledgements
We thank the following Genentech colleagues for their critical feedback of this article: M. Balazs, S. Cohen, J. Koerber, J. Lill, K. Peng, O. Saad, C. Spiess, S. Swanson, W.-T. Tsai and P. Wu.
Author information
Authors and Affiliations
Contributions
P.J.C. and V.Q. researched data for this article and wrote it.
Corresponding authors
Ethics declarations
Competing interests
The authors are current (P.J.C.) or former (V.Q.) employees of Genentech, Inc., which develops and commercializes therapeutics, including antibodies and other proteins.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks John Desjarlais, George Georgiou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
IPD-IMGT/HLA database: https://www.ebi.ac.uk/ipd/imgt/hla/about/statistics/
Glossary
- Antigen
-
Any molecule that can bind specifically to an antibody and/or generate peptide fragments that bind to a major histocompatibility complex molecule and are specifically recognized by a T cell receptor.
- B cell epitopes
-
Linear or conformational parts of an antigen that bind to an antibody.
- Critical quality attribute
-
(CQA). A physical, chemical, biological or microbiological property or characteristic that should be within an appropriate limit, range or distribution to ensure the desired product quality.
- Deimmunization
-
Modification of protein sequence to remove B cell and/or T cell epitopes.
- Developability
-
Broad set of desirable drug-like properties of protein therapeutics that includes feasibility of manufacture, stability during storage, ease of administration and favourable pharmacological behaviour in patients, and excludes target binding.
- Epitope spreading
-
Diversification of the B and T cell epitopes recognized by the immune system.
- Poly-reactivity
-
Ability of an antibody to bind multiple self and/or foreign antigens that are unrelated to the cognate antigen(s).
- Product-related variants
-
Truncated and other modified forms, aggregates, precursors, and degradation products arising during manufacturing and/or storage.
- Replacement protein therapeutic
-
Protein therapeutic that is intended to substitute or augment the deficiency of a specific endogenous protein.
- Target candidate profile
-
Set of desired biochemical, biological and biophysical attributes for therapeutic candidates.
- T cell epitopes
-
Peptides that are derived from an antigen that binds to a major histocompatibility complex molecule and are then recognized by cognate T cell receptors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carter, P.J., Quarmby, V. Immunogenicity risk assessment and mitigation for engineered antibody and protein therapeutics. Nat Rev Drug Discov 23, 898–913 (2024). https://doi.org/10.1038/s41573-024-01051-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-024-01051-x
This article is cited by
-
Redosing of anti-CD3 antibodies in NOD mice with new-onset diabetes does not alter the effect of a single treatment course
Diabetologia (2026)
-
Fifty years of monoclonals: the past, present and future of antibody therapeutics
Nature Reviews Immunology (2025)
-
Brain-Derived Exosomes in Neurodevelopmental and Neuropsychiatric Disorders: Molecular Insights, Therapeutic Potential, and Translational Challenges
Molecular Neurobiology (2025)