Abstract
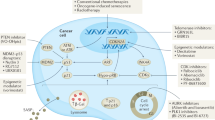
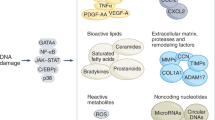
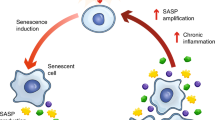
Cellular senescence is a stress response that restrains the growth of aged, damaged or abnormal cells. Thus, senescence has a crucial role in development, tissue maintenance and cancer prevention. However, lingering senescent cells fuel chronic inflammation through the acquisition of a senescence-associated secretory phenotype (SASP), which contributes to cancer and age-related tissue dysfunction. Recent progress in understanding senescence has spurred interest in the development of approaches to target senescent cells, known as senotherapies. In this Review, we evaluate the status of various types of senotherapies, including senolytics that eliminate senescent cells, senomorphics that suppress the SASP, interventions that mitigate senescence and strategies that harness the immune system to clear senescent cells. We also summarize how these approaches can be combined with cancer therapies, and we discuss the challenges and opportunities in moving senotherapies into clinical practice. Such therapies have the potential to address root causes of age-related diseases and thus open new avenues for preventive therapies and treating multimorbidities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961). This article is a pioneering study that defines the concept of cellular senescence.
d’Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Chang, B. D. et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 18, 4808–4818 (1999).
Schmitt, C. A. et al. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109, 335–346 (2002). Together with Chang et al. (1999), this work describes the concept of TIS in human cells and mouse models for the first time.
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Lee, S. et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature 599, 283–289 (2021).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Munoz-Espin, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013). Together with Storer et al. (2013), this work is the first study to describe developmental senescence.
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134, 657–667 (2008).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of PTEN-deficient tumorigenesis. Nature 436, 725–730 (2005).
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016). This article identifies the beneficial effects of senolysis in naturally aged mouse models.
Serrano, M. et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85, 27–37 (1996).
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 (2004).
Sharpless, N. E. & Sherr, C. J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 15, 397–408 (2015).
Kaplon, J. et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 498, 109–112 (2013).
Kondoh, H. et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 65, 177–185 (2005).
Young, A. R. et al. Autophagy mediates the mitotic senescence transition. Genes. Dev. 23, 798–803 (2009).
Chandra, T. & Narita, M. High-order chromatin structure and the epigenome in SAHFs. Nucleus 4, 23–28 (2013).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479, 547–551 (2011).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007). Together with Kang et al. (2011), this work is the first study to describe immune surveillance of SnCs.
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Munoz-Espin, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Jun, J. I. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12, 676–685 (2010).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Ritschka, B. et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes. Dev. 31, 172–183 (2017).
Mosteiro, L. et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354, eaaf4445 (2016).
Yu, Q. et al. Cellular senescence promotes progenitor cell expansion during axolotl limb regeneration. Dev. Cell 58, 2416–2427.e7 (2023).
Reyes, N. S. et al. Sentinel p16INK4a+ cells in the basement membrane form a reparative niche in the lung. Science 378, 192–201 (2022).
Grosse, L. et al. Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab. 378, 87–99.e6 (2020). Together with Reyes et al. (2022), this work is the first study to describe a detrimental effect associated with eliminating specific populations of SnCs.
Helman, A. et al. p16Ink4a-induced senescence of pancreatic β cells enhances insulin secretion. Nat. Med. 22, 412–420 (2016).
Wang, L., Lankhorst, L. & Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 22, 340–355 (2022). This important Review summarizes the use of senotherapies for cancer.
Di Micco, R. et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444, 638–642 (2006).
Bartkova, J. et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 (2006).
Faget, D. V., Ren, Q. & Stewart, S. A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 19, 439–453 (2019).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Gonzalez-Meljem, J. M. et al. Stem cell senescence drives age-attenuated induction of pituitary tumours in mouse models of paediatric craniopharyngioma. Nat. Commun. 8, 1819 (2017).
Laberge, R. M., Awad, P., Campisi, J. & Desprez, P. Y. Epithelial–mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. 5, 39–44 (2011).
Eggert, T. et al. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 30, 533–547 (2016).
Di Mitri, D. et al. Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515, 134–137 (2014). Together with Eggert et al. (2016), this work is the first study to describe the SASP-driven recruitment of immunosuppressive MDSCs.
D’Ambrosio, M. & Gil, J. Reshaping of the tumor microenvironment by cellular senescence: an opportunity for senotherapies. Dev. Cell 58, 1007–1021 (2023).
Yasuda, T. et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep. 34, 108779 (2021).
Prieto, L. I. et al. Senescent alveolar macrophages promote early-stage lung tumorigenesis. Cancer Cell 41, 1261–1275.e6 (2023).
Haston, S. et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell 41, 1–19 (2023).
Maggiorani, D. et al. Senescence drives immunotherapy resistance by inducing an immunosuppressive tumor microenvironment. Nat. Commun. 15, 2435 (2024).
Wang, T. W. et al. Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes. Nature 611, 358–364 (2022).
Chaib, S. et al. The efficacy of chemotherapy is limited by intratumoral senescent cells expressing PD-L2. Nat. Cancer 5, 448–462 (2024). Together with Wang et al. (2022), this work is the first study to describe the effect of immune checkpoint inhibitors on SnCs.
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Kurz, D. J., Decary, S., Hong, Y. & Erusalimsky, J. D. Senescence-associated β-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J. Cell Sci. 113, 3613–3622 (2000).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Burd, C. E. et al. Monitoring tumorigenesis and senescence in vivo with a p16INK4a-luciferase model. Cell 152, 340–351 (2013).
Jeyapalan, J. C., Ferreira, M., Sedivy, J. M. & Herbig, U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 128, 36–44 (2007).
Tanaka, T. et al. Plasma proteomic signature of age in healthy humans. Aging Cell 17, e12799 (2018).
Omori, S. et al. Generation of a p16 reporter mouse and its use to characterize and target p16high cells in vivo. Cell Metab. 32, 814–828.e6 (2020).
Karin, O., Agrawal, A., Porat, Z., Krizhanovsky, V. & Alon, U. Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat. Commun. 10, 5495 (2019).
Schafer, M. J. et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 8, 14532 (2017).
Narasimhan, A., Flores, R. R., Robbins, P. D. & Niedernhofer, L. J. Role of cellular senescence in type II diabetes. Endocrinology 162, bqab136 (2021).
Gorenne, I., Kavurma, M., Scott, S. & Bennett, M. Vascular smooth muscle cell senescence in atherosclerosis. Cardiovasc. Res. 72, 9–17 (2006).
Minamino, T. et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 (2002).
Papatheodoridi, A. M., Chrysavgis, L., Koutsilieris, M. & Chatzigeorgiou, A. The role of senescence in the development of nonalcoholic fatty liver disease and progression to nonalcoholic steatohepatitis. Hepatology 71, 363–374 (2020).
Crespo-Garcia, S. et al. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 33, 818–832.e7 (2021).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
Herdy, J. R. et al. Increased post-mitotic senescence in aged human neurons is a pathological feature of Alzheimer’s disease. Cell Stem Cell 29, 1637–1652.e6 (2022).
Zhang, L. et al. Cellular senescence: a key therapeutic target in aging and diseases. J. Clin. Invest. 132, e158450 (2022).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Moiseeva, V. et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 613, 169–178 (2023).
Dungan, C. M. et al. Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell 21, e13528 (2022).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Wang, B. et al. An inducible p21-Cre mouse model to monitor and manipulate p21-highly-expressing senescent cells in vivo. Nat. Aging 1, 962–973 (2021).
Lee, S. et al. A guide to senolytic intervention in neurodegenerative disease. Mech. Ageing Dev. 200, 111585 (2021).
Zhang, P. et al. Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat. Neurosci. 22, 719–728 (2019).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Bigenwald, C. et al. BRAFV600E-induced senescence drives Langerhans cell histiocytosis pathophysiology. Nat. Med. 27, 851–861 (2021).
Wang, C. et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 574, 268–272 (2019).
Herranz, N. et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 17, 1205–1217 (2015).
Laberge, R. M. et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 17, 1049–1061 (2015).
Pospelova, T. V. et al. Suppression of replicative senescence by rapamycin in rodent embryonic cells. Cell Cycle 11, 2402–2407 (2012).
Xu, Q. et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 3, 1706–1726 (2021).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Khosla, S. Senescent cells, senolytics and tissue repair: the devil may be in the dosing. Nat. Aging 3, 139–141 (2023).
Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Res. 55, 2284–2292 (1995).
Yosef, R. et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 7, 11190 (2016).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016). Together with Chang et al. (2016) and Yosef et al. (2016), this study is the first description of the use of BCL-2 inhibitors as senolytics.
Gandhi, L. et al. Phase I study of navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J. Clin. Oncol. 29, 909–916 (2011).
Munoz-Espin, D. et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 10, e9355 (2018).
Gonzalez-Gualda, E. et al. Galacto-conjugation of navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 19, e13142 (2020).
Jia, Y. et al. Co-targeting BCL-XL and BCL-2 by PROTAC 753B eliminates leukemia cells and enhances efficacy of chemotherapy by targeting senescent cells. Haematologica 108, 2626–2638 (2023).
He, Y. et al. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat. Commun. 11, 1996 (2020).
Zhu, Y. et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging 9, 955–963 (2017).
Thompson, P. J. et al. Targeted elimination of senescent β cells prevents type 1 diabetes. Cell Metab. 29, 1045–1060.e10 (2019).
Troiani, M. et al. Single-cell transcriptomics identifies Mcl-1 as a target for senolytic therapy in cancer. Nat. Commun. 13, 2177 (2022).
Wang, L. et al. cFLIP suppression and DR5 activation sensitize senescent cancer cells to senolysis. Nat. Cancer 3, 1284–1299 (2022).
Agostini, A. et al. Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angew. Chem. Int. Ed. Engl. 51, 10556–10560 (2012).
Guerrero, A. et al. Galactose-modified duocarmycin prodrugs as senolytics. Aging Cell 19, e13133 (2020).
Cai, Y. et al. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 30, 574–589 (2020).
Efeyan, A. et al. Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin. Cancer Res. 67, 7350–7357 (2007).
Dolgin, E. Send in the senolytics. Nat. Biotechnol. 38, 1371–1377 (2020).
Li, M., Brooks, C. L., Kon, N. & Gu, W. A dynamic role of HAUSP in the p53–Mdm2 pathway. Mol. Cell 13, 879–886 (2004).
He, Y. et al. Inhibition of USP7 activity selectively eliminates senescent cells in part via restoration of p53 activity. Aging Cell 19, e13117 (2020).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147.e16 (2017).
Le, H. H. et al. Molecular modelling of the FOXO4–TP53 interaction to design senolytic peptides for the elimination of senescent cancer cells. EBioMedicine 73, 103646 (2021).
Wakita, M. et al. A BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nat. Commun. 11, 1935 (2020).
Dorr, J. R. et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 501, 421–425 (2013). This article provides the first description of how targeting specific vulnerabilities can selectively eliminate SnCs.
Correia-Melo, C. et al. Mitochondria are required for pro-ageing features of the senescent phenotype. EMBO J. 35, 724–742 (2016).
Pluquet, O., Pourtier, A. & Abbadie, C. The unfolded protein response and cellular senescence. A review in the theme: cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am. J. Physiol. Cell Physiol. 308, C415–C425 (2015).
Fuhrmann-Stroissnigg, H. et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 8, 422 (2017).
McHugh, D. et al. COPI vesicle formation and N-myristoylation are targetable vulnerabilities of senescent cells. Nat. Cell Biol. 25, 1804–1820 (2023).
Jackson, L. P. Structure and mechanism of COPI vesicle biogenesis. Curr. Opin. Cell Biol. 29, 67–73 (2014).
Narita, M. et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 332, 966–970 (2011).
Anerillas, C. et al. The YAP–TEAD complex promotes senescent cell survival by lowering endoplasmic reticulum stress. Nat. Aging 3, 1237–1250 (2023).
Johmura, Y. et al. Senolysis by glutaminolysis inhibition ameliorates various age-associated disorders. Science 371, 265–270 (2021).
Guerrero, A. et al. Cardiac glycosides are broad-spectrum senolytics. Nat. Metab. 1, 1074–1088 (2019).
Triana-Martinez, F. et al. Identification and characterization of cardiac glycosides as senolytic compounds. Nat. Commun. 10, 4731 (2019).
Smer-Barreto, V. et al. Discovery of senolytics using machine learning. Nat. Commun. 14, 3445 (2023).
Therien, A. G. & Blostein, R. Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol. 279, C541–C566 (2000).
Russo, G. L. et al. Mechanisms of aging and potential role of selected polyphenols in extending healthspan. Biochem. Pharmacol. 173, 113719 (2020).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015). This article provides the first description of the combination of D + Q as senolytics.
Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016).
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019). First in human study using D + Q as a senolytic therapy.
Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 (2018).
Wang, Y. et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging 8, 2915–2926 (2016).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018).
Prieto, L. I., Sturmlechner, I., Goronzy, J. J. & Baker, D. J. Senescent cells as thermostats of antitumor immunity. Sci. Transl. Med. 15, eadg7291 (2023).
Hasegawa, T. et al. Cytotoxic CD4+ T cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell 186, 1417–1431.e20 (2023).
Chen, H. A. et al. Senescence rewires microenvironment sensing to facilitate antitumor immunity. Cancer Discov. 13, 432–453 (2023).
Shahbandi, A. et al. Breast cancer cells survive chemotherapy by activating targetable immune-modulatory programs characterized by PD-L1 or CD80. Nat. Cancer 3, 1513–1533 (2022).
Pereira, B. I. et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 10, 2387 (2019).
Munoz, D. P. et al. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight 5, e124716 (2019).
Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 11, 3801 (2020).
Arora, S. et al. Invariant natural killer T cells coordinate removal of senescent cells. Med 2, 938–950 (2021).
Kim, K. M. et al. Identification of senescent cell surface targetable protein DPP4. Genes. Dev. 31, 1529–1534 (2017).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature https://doi.org/10.1038/s41586-020-2403-9 (2020). This study is the first to report the use of senolytic CAR-T cells.
Poblocka, M. et al. Targeted clearance of senescent cells using an antibody–drug conjugate against a specific membrane marker. Sci. Rep. 11, 20358 (2021).
Suda, M. et al. Glycoprotein nonmetastatic melanoma protein B regulates lysosomal integrity and lifespan of senescent cells. Sci. Rep. 12, 6522 (2022).
Sagiv, A. et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 8, 328–344 (2016).
Yang, D. et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Sci. Transl. Med. 15, eadd1951 (2023).
Yoshida, S. et al. The CD153 vaccine is a senotherapeutic option for preventing the accumulation of senescent T cells in mice. Nat. Commun. 11, 2482 (2020).
Marin, I. et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 13, 410–431 (2023).
Birch, J. & Gil, J. Senescence and the SASP: many therapeutic avenues. Genes. Dev. 34, 1565–1576 (2020).
Gluck, S. et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 19, 1061–1070 (2017).
Dou, Z. et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 550, 402–406 (2017). Together with Gluck et al. (2017), this work highlights the role of cGAS–STING on SASP and senescence regulation.
Vizioli, M. G. et al. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes. Dev. 34, 428–445 (2020).
Rodier, F. et al. DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J. Cell Sci. 124, 68–81 (2011).
Freund, A., Patil, C. K. & Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 30, 1536–1548 (2011).
Tasdemir, N. et al. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discov. 6, 612–629 (2016).
Kuilman, T. et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133, 1019–1031 (2008).
Acosta, J. C. et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018 (2008).
Gulen, M. F. et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature 620, 374–380 (2023).
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA 112, E6301–E6310 (2015).
Georgilis, A. et al. PTBP1-mediated alternative splicing regulates the inflammatory secretome and the pro-tumorigenic effects of senescent cells. Cancer Cell 34, 85–102.e9 (2018).
Bird, T. G. et al. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci. Transl. Med. 10, eaan1230 (2018).
Widjaja, A. A. et al. Inhibition of IL-11 signalling extends mammalian healthspan and lifespan. Nature 632, 157–165 (2024).
Ogata, A., Kato, Y., Higa, S. & Yoshizaki, K. IL-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review. Mod. Rheumatol. 29, 258–267 (2019).
Mariette, X. et al. Effectiveness of tocilizumab in patients hospitalized with COVID-19: a follow-up of the CORIMUNO-TOCI-1 randomized clinical trial. JAMA Intern. Med. 181, 1241–1243 (2021).
Schmitt, C. A. et al. COVID-19 and cellular senescence. Nat. Rev. Immunol. 23, 251–263 (2023).
Del Rey, M. J. et al. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immun. Ageing 16, 29 (2019).
Basisty, N. et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18, e3000599 (2020).
Hoare, M. et al. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat. Cell Biol. 18, 979–992 (2016).
Toso, A. et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 9, 75–89 (2014).
Franceschi, C. et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 128, 92–105 (2007).
Ruscetti, M. et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 362, 1416–1422 (2018).
Ruscetti, M. et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell 181, 424–441.e21 (2020).
Chibaya, L. et al. EZH2 inhibition remodels the inflammatory senescence-associated secretory phenotype to potentiate pancreatic cancer immune surveillance. Nat. Cancer 4, 872–892 (2023).
Blagosklonny, M. V. Rapamycin, proliferation and geroconversion to senescence. Cell Cycle 17, 2655–2665 (2018).
Fumagalli, M. et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 14, 355–365 (2012).
Aguado, J. et al. Inhibition of DNA damage response at telomeres improves the detrimental phenotypes of Hutchinson–Gilford progeria syndrome. Nat. Commun. 10, 4990 (2019).
Bousset, L. & Gil, J. Targeting senescence as an anticancer therapy. Mol. Oncol. 16, 3855–3880 (2022).
Ou, H. L. et al. Cellular senescence in cancer: from mechanisms to detection. Mol. Oncol. 15, 2634–2671 (2021).
Chambers, C. R., Ritchie, S., Pereira, B. A. & Timpson, P. Overcoming the senescence-associated secretory phenotype (SASP): a complex mechanism of resistance in the treatment of cancer. Mol. Oncol. 15, 3242–3255 (2021).
Wang, L. & Bernards, R. Taking advantage of drug resistance, a new approach in the war on cancer. Front. Med. 12, 490–495 (2018).
Fleury, H. et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat. Commun. 10, 2556 (2019).
Jochems, F. et al. The cancer SENESCopedia: a delineation of cancer cell senescence. Cell Rep. 36, 109441 (2021).
Kolodkin-Gal, D. et al. Senolytic elimination of Cox2-expressing senescent cells inhibits the growth of premalignant pancreatic lesions. Gut 71, 345–355 (2021).
Alvarez-Fernandez, M. & Malumbres, M. Mechanisms of sensitivity and resistance to CDK4/6 inhibition. Cancer Cell 37, 514–529 (2020).
Wang, L. et al. High-throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep. 21, 773–783 (2017).
Baell, J. B. et al. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature 560, 253–257 (2018).
Heckenbach, I. et al. Nuclear morphology is a deep learning biomarker of cellular senescence. Nat. Aging 2, 742–755 (2022).
Kusumoto, D. et al. Anti-senescent drug screening by deep learning-based morphology senescence scoring. Nat. Commun. 12, 257 (2021).
Duran, I. et al. Detection of senescence using machine learning algorithms based on nuclear features. Nat. Commun. 15, 1041 (2024). Together with Heckenbach et al. (2022) and Kusumoto et al. (2021), this work reports the use of a machine learning analysis of morphological parameters to detect SnCs.
Cui, W., Liu, L., Kodibagkar, V. D. & Mason, R. P. S-Gal, a novel 1H MRI reporter for β-galactosidase. Magn. Reson. Med. 64, 65–71 (2010).
Schafer, M. J. et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 5, e133668 (2020).
Wiley, C. D. et al. Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Cell Metab. 33, 1124–1136.e5 (2021). This article provides a description of a biomarker of senolysis that can be detected in the blood.
Farr, J. N. et al. Local senolysis in aged mice only partially replicates the benefits of systemic senolysis. J. Clin. Invest. 133, e162519 (2023).
Nambiar, A. et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine 90, 104481 (2023).
Hashimoto, M. et al. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight 1, e87732 (2016).
Chandra, A. et al. Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity. Aging Cell 21, e13602 (2022).
Ohtani, N., Yamakoshi, K., Takahashi, A. & Hara, E. Real-time in vivo imaging of p16 gene expression: a new approach to study senescence stress signaling in living animals. Cell Div. 5, 1 (2010).
Ohtani, N. et al. Visualizing the dynamics of p21Waf1/Cip1 cyclin-dependent kinase inhibitor expression in living animals. Proc. Natl Acad. Sci. USA 104, 15034–15039 (2007).
Liu, J. Y. et al. Cells exhibiting strong p16INK4a promoter activation in vivo display features of senescence. Proc. Natl Acad. Sci. USA 116, 2603–2611 (2019).
Hori, N. et al. Shaving black fur uncovers hidden issues in p16-3MR mice. Preprint at bioRxiv https://doi.org/10.1101/2024.06.24.600181 (2024).
Acknowledgements
Figures have been partially generated with Biorender (Biorender.com) and Smart Servier Medical ART (https://smart.servier.com). For open access, the author has applied a Creative Commons Attribution (CC BY) licence. Core support from the Medical Research Council (MRC) (MC_U120085810) and a grant from Cancer Research UK (CRUK) (C15075/A28647) funded research in the Gil laboratory.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed to the discussion of the content and organization of the review and wrote the article.
Corresponding author
Ethics declarations
Competing interests
J.G. has acted as a consultant for Unity Biotechnology, Geras Bio, Myricx Pharma Ltd. and Merck KGaA; owns equity in Geras Bio and share options in Myricx Pharma Ltd; is a named inventor in Medical Research Council (MRC) patents related to senolytic therapies; and has in the past received funding from Pfizer and Unity Biotechnology to work on senolytics. D.M. and J.G. are named inventors in an Imperial College patent related to senolytic therapies. I.D. declares no competing interest.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Christopher Wiley and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Apical ectodermal ridge
-
A structure formed by a thickening of the ectoderm around the distal part of limb buds during development.
- Cardiac glycosides
-
(CGs). A family of natural compounds found in plants and amphibians that inhibit Na+/K+-ATPases.
- Coat protein complex I
-
(COPI). A protein complex for which one of its main functions is the formation of vesicles that transport proteins from the Golgi to the endoplasmic reticulum.
- DNA segments with chromatin alterations reinforcing senescence
-
(DNA-SCARS). Nuclear structures present in senescent cells (SnCs) with persistent DNA damage.
- INK-ATTAC mice
-
A mouse model in which a chimeric form of caspase 8 is expressed under the INK4a promoter, making possible the selective elimination of senescent cells (SnCs) expressing high levels of p16INK4a upon addition of a drug (AP20187) that activates the chimeric caspase 8 construct. INK refers to INK4a (encoding for p16INK4a), ATTAC is short for apoptosis through targeted activation of caspase.
- Invariant natural killer T cells
-
A class of lymphocytes expressing T cell receptors that are activated by lipid antigens.
- Langerhans cell histiocytosis
-
A rare neoplasia caused by the abnormal accumulation of a type of immune cells called Langerhans cells.
- Mesoporous silica nanoparticles
-
A type of silica-based nanostructures with well-defined pore diameters (2–50 nm, in the ‘mesoscale’) that can be loaded with drugs or cargo which can be released in a controlled fashion if functionalized.
- Multimorbidities
-
Co-occurrence of two or more chronic illnesses, more common in aged individuals.
- Myeloid-derived suppressor cells
-
(MDSCs). Myeloid cells that inhibit the activity of natural killer cells (NK cells) and CD8+ T cells.
- Non-homologous end joining
-
A mechanism to repair double-strand breaks in the DNA through direct ligation of the break ends and without the need for a homologous DNA template.
- Preneoplastic cells
-
Cells harbouring oncogenic lesions which have a higher risk of becoming transformed.
- Progeroid mice
-
A mouse model (such as BubR1H/H mice) that displays characteristics associated with ageing at an early age.
- Proteolysis-targeting chimaera
-
(PROTAC). A class of drugs that causes the selective degradation of their targets.
- Senescence-associated β-galactosidase
-
(SA-β-gal). Lysosomal β-galactosidase activity that is assayed at an acid pH and that is used as a senescence marker.
- Surfaceome
-
A collective name which is used to refer to the surface proteins expressed by a cell.
- Telomeric attrition
-
Progressive shortening of the chromosome ends (telomeres) that happens after every cell division as a consequence of the ‘end replication problem’.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McHugh, D., Durán, I. & Gil, J. Senescence as a therapeutic target in cancer and age-related diseases. Nat Rev Drug Discov 24, 57–71 (2025). https://doi.org/10.1038/s41573-024-01074-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-024-01074-4
This article is cited by
-
Senescent fibroblasts drive CD8+ T cell dysfunction in colorectal cancer via CD36-mediated lipid transfer and peroxidation
Journal of Translational Medicine (2026)
-
A synthetic system for RNA-responsive pyroptosis based on type III-E CRISPR nuclease-protease
Nature Communications (2026)
-
Rejuvenating the aging gut by targeting senescence
Nature Aging (2026)
-
Pore-forming venoms as a senolytic strategy
Nature Aging (2026)
-
Cytokine-induced senescence in tumors is based on sustained activation of STAT1- and NFκB-dependent gene regulatory signatures
GeroScience (2026)