Abstract
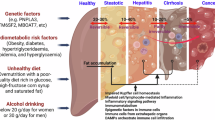
Metabolic dysfunction-associated steatotic liver disease (MASLD) and its severe subgroup metabolic dysfunction-associated steatohepatitis (MASH) have become a global epidemic and are driven by chronic overnutrition and multiple genetic susceptibility factors. The physiological outcomes include hepatocyte death, liver inflammation and cirrhosis. The first therapeutic for MASLD and MASH, resmetirom, has recently been approved for clinical use and has energized this therapeutic space. However, there is still much to learn in clinical studies of MASH, such as the scale of placebo responses, optimal trial end points, the time required for fibrosis reversal and side effect profiles. This Review introduces aspects of disease pathogenesis related to drug development and discusses two main therapeutic approaches. Thyroid hormone receptor-β agonists, such as resmetirom, as well as fatty acid synthase inhibitors, target the liver and enable it to function within a toxic metabolic environment. In parallel, incretin analogues such as semaglutide improve metabolism, allowing the liver to self-regulate and reversing many aspects of MASH. We also discuss how combinations of therapeutics could potentially be used to treat patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77, 1335–1347 (2023). This is a meta-analysis of studies conducted from 1990 to 2019 revealing the highest nonalcoholic fatty liver disease prevalence was in Latin America, 44.37%; then Middle East and North Africa, South Asia, 33.83%; South-East Asia, 33.07%; North America, 31.20%; East Asia, 29.71%; Asia Pacific, 28.02%; and Western Europe, 25.10%.
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Allen, A. M. et al. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology 67, 1726–1736 (2018).
Younossi, Z. M. et al. Patients with nonalcoholic steatohepatitis experience severe impairment of health-related quality of life. Am. J. Gastroenterol. 114, 1636–1641 (2019).
Wong, R. J. et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148, 547–555 (2015).
Younossi, Z. M. et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62, 1723–1730 (2015).
Hales, C. M., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018. NCHS data brief; no. 360 (Centers for Disease Control and Prevention, 2020).
Parcha, V. et al. Insulin resistance and cardiometabolic risk profile among nondiabetic American young adults: insights from NHANES. J. Clin. Endocrinol. Metab. 107, e25–e37 (2022).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 78, 1966–1986 (2023). This summarizes the process and the final consensus leading to the new nomenclature for steatotic liver disease.
Harrison, S. A. NASH, from diagnosis to treatment: where do we stand? Hepatology 62, 1652–1655 (2015).
Rinella, M. E., Tacke, F., Sanyal, A. J., Anstee, Q. M. & participants of the AASLD/EASL Workshop, Report on the AASLD/EASL joint workshop on clinical trial endpoints in NAFLD. J. Hepatol. 71, 823–833 (2019). This reviews the rationale and summarizes the main trial end points in clinical trials of MASH.
Anstee, Q. M., Reeves, H. L., Kotsiliti, E., Govaere, O. & Heikenwalder, M. From NASH to HCC: current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 16, 411–428 (2019).
Allen, A. M. et al. Clinical course of non-alcoholic fatty liver disease and the implications for clinical trial design. J. Hepatol. 77, 1237–1245 (2022).
Brennan, P. N. et al. Antifibrotic therapy in nonalcoholic steatohepatitis: time for a human-centric approach. Nat. Rev. Gastroenterol. Hepatol. 20, 679–688 (2023). The authors discuss why antifibrotic effects observed in nonalcoholic steatohepatitis pharmacotherapy trials have been underwhelming and outline potential approaches to improve the likelihood of future clinical success, with a focus on combination therapies.
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124 (2021). This phase II trial shows that semaglutide results in greater nonalcoholic steatohepatitis resolution than placebo, but it does not show a significant improvement in fibrosis stage.
Newsome, P. N. & Ambery, P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J. Hepatol. 79, 1557–1565 (2023). This review explores in detail the direct and indirect effect of a range of incretin molecules on the liver within the context of MASH biology.
Li, L., Song, Y., Shi, Y. & Sun, L. Thyroid hormone receptor-β agonists in NAFLD therapy: possibilities and challenges. J. Clin. Endocrinol. Metab. 108, 1602–1613 (2023).
Harrison, S. et al. Primary results from MAESTRO-NASH a pivotal phase 3 52-week serial liver biopsy study in 966 patients with NASH and fibrosis. J. Hepatol. 78, s1 (2023).
Puengel, T. & Tacke, F. Efruxifermin, an investigational treatment for fibrotic or cirrhotic nonalcoholic steatohepatitis (NASH). Expert Opin. Investig. Drugs 32, 451–461 (2023). This review examines the biology of efruxifermin and other FGF-21 agonists as therapeutic agents in MASH.
Staels, B., Butruille, L. & Francque, S. Treating NASH by targeting peroxisome proliferator-activated receptors. J. Hepatol. 79, 1302–1316 (2023).
Linden, D. & Romeo, S. Therapeutic opportunities for the treatment of NASH with genetically validated targets. J. Hepatol. 79, 1056–1064 (2023).
Ratziu, V. & Charlton, M. Rational combination therapy for NASH: insights from clinical trials and error. J. Hepatol. 78, 1073–1079 (2023).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378.e5 (2015).
Musso, G., Gambino, R. & Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid Res. 52, 175–191 (2013).
Yamaguchi, K. et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 45, 1366–1374 (2007).
Angulo, P. et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397.e10 (2015). A retrospective study of data from the USA, Europe and Thailand, which demonstrates that liver fibrosis but no other feature is associated with overall mortality, liver transplantation and liver-related events.
Puri, P. et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46, 1081–1090 (2007).
Martinez-Arranz, I. et al. Metabolic subtypes of patients with NAFLD exhibit distinctive cardiovascular risk profiles. Hepatology 76, 1121–1134 (2022).
Smith, G. I. et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 130, 1453–1460 (2020). A detailed isotope-based examination of DNL in participants with MASH that demonstrates that hepatic DNL is an important regulator of liver triglyceride content and that increases in circulating glucose and insulin stimulate hepatic DNL.
Lomonaco, R. et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 55, 1389–1397 (2012).
Chitturi, S. et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 35, 373–379 (2002).
Magkos, F. et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 23, 591–601 (2016). A randomized controlled trial that demonstrates that moderate (5%) weight loss improves metabolic function in multiple organs simultaneously, and progressive weight loss causes positive dose-dependent alterations in key metabolic pathways.
Semple, R. K. et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J. Clin. Investig. 119, 315–322 (2009).
Pagano, G. et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology 35, 367–372 (2002).
Lambert, J. E., Ramos–Roman, M. A., Browning, J. D. & Parks, E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 146, 726–735 (2014).
Donnelly, K. L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 115, 1343–1351 (2005).
Softic, S., Cohen, D. E. & Kahn, C. R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 61, 1282–1293 (2016).
Mager, D. R., Iñiguez, I. R., Gilmour, S. & Yap, J. The effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD). J. Parenter. Enter. Nutr. 39, 73–84 (2015).
Lustig, R. H. et al. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity 24, 453–460 (2016).
Diehl, A. M. & Day, C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N. Engl. J. Med. 377, 2063–2072 (2017).
Abdelmalek, M. F. et al. Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 56, 952–960 (2012).
Abdelmalek, M. F. et al. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51, 1961–1971 (2010).
Younossi, Z. M. et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology 53, 1874–1882 (2011).
McPherson, S. et al. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J. Hepatol. 62, 1148–1155 (2015).
Wong, V. W.-S. et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 59, 969–974 (2010).
Zhang, X. J., Cai, J. & Li, H. Targeting ACC for NASH resolution. Trends Mol. Med. 28, 5–7 (2022).
Batchuluun, B., Pinkosky, S. L. & Steinberg, G. R. Lipogenesis inhibitors: therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 21, 283–305 (2022).
Brunt, E. M. et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 53, 810–820 (2011).
Hardy, T., Oakley, F., Anstee, Q. M. & Day, C. P. Nonalcoholic fatty liver disease: pathogenesis and disease spectrum. Annu. Rev. Pathol. 11, 451–496 (2016).
Dulai, P. S. et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta‐analysis. Hepatology 65, 1557–1565 (2017).
Schuppan, D., Surabattula, R. & Wang, X. Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 68, 238–250 (2018).
Noncirrhotic nonalcoholic steatohepatitis with liver fibrosis: developing drugs for treatment guidance for industry. US Food and Drug Administration (FDA) fda.gov/media/119044/download (2018).
Nonalcoholic steatohepatitis with compensated cirrhosis: developing drugs for treatment guidance for industry. US Food and Drug Administration (FDA) fda.gov/media/127738/download (2019).
Farrell, G. C., van Rooyen, D., Gan, L. & Chitturi, S. NASH is an inflammatory disorder: pathogenic, prognostic and therapeutic implications. Gut Liver 6, 149–171 (2012).
Sutti, S. et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 59, 886–897 (2014).
Tomita, K. et al. Tumour necrosis factor α signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut 55, 415–424 (2006).
Arrese, M., Cabrera, D., Kalergis, A. M. & Feldstein, A. E. Innate immunity and inflammation in NAFLD/NASH. Dig. Dis. Sci. 61, 1294–1303 (2016).
Galic, S., Oakhill, J. S. & Steinberg, G. R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 316, 129–139 (2010).
Fontana, L., Eagon, J. C., Trujillo, M. E., Scherer, P. E. & Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56, 1010–1013 (2007).
Catalan, V. et al. Increased interleukin-32 levels in obesity promote adipose tissue inflammation and extracellular matrix remodeling: effect of weight loss. Diabetes 65, 3636–3648 (2016).
Fadaei, R. et al. Serum levels of IL-32 in patients with type 2 diabetes mellitus and its relationship with TNF-α and IL-6. Cytokine 125, 154832 (2020).
Moschen, A. R. et al. Interleukin-32: a new proinflammatory cytokine involved in hepatitis C virus-related liver inflammation and fibrosis. Hepatology 53, 1819–1829 (2011).
Kim, D. H. et al. Intracellular interleukin-32γ mediates antiviral activity of cytokines against hepatitis B virus. Nat. Commun. 9, 3284 (2018).
Baselli, G. A. et al. Liver transcriptomics highlights interleukin-32 as novel NAFLD-related cytokine and candidate biomarker. Gut 69, 1855–1866 (2020).
Shoda, H. et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res. Ther. 8, R166 (2006).
Garcia-Martinez, I. et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J. Clin. Investig. 126, 859–864 (2016).
Miura, K. et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology 139, 323–334.e7 (2010).
Sanyal, A. J. et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 385, 1559–1569 (2021). A prospective study of 1773 patients with MASLD over a median of 4 years demonstrating that having fibrosis stages F3 and F4 is associated with increased risks of liver-related complications and death.
Anania, F. A., Dimick-Santos, L., Mehta, R., Toerner, J. & Beitz, J. Nonalcoholic steatohepatitis: current thinking from the division of hepatology and nutrition at the Food and Drug Administration. Hepatology 73, 2023–2027 (2020).
Singh, S. et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 13, 643–654.e9 (2015).
Kisseleva, T. & Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18, 151–166 (2021).
Iwakiri, Y. & Trebicka, J. Portal hypertension in cirrhosis: pathophysiological mechanisms and therapy. JHEP Rep. 3, 100316 (2021).
Fickert, P. Is this the last requiem for simtuzumab? Hepatology 69, 476–479 (2019).
Schumacher, J. D. & Guo, G. L. Regulation of hepatic stellate cells and fibrogenesis by fibroblast growth factors. BioMed. Res. Int. 2016, 8323747 (2016).
Jung, M. Y. et al. Fatty acid synthase is required for profibrotic TGF-β signaling. FASEB J. 32, 3803–3815 (2018).
Alonso-Merino, E. et al. Thyroid hormones inhibit TGF-β signaling and attenuate fibrotic responses. Proc. Natl Acad. Sci. USA 113, E3451–E3460 (2016).
Harrison, S. A. et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J. Hepatol. 73, 26–39 (2020).
Chen, W. et al. Lysyl oxidase (LOX) family members: rationale and their potential as therapeutic targets for liver fibrosis. Hepatology 72, 729–741 (2020).
Sanyal, A. J. et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 362, 1675–1685 (2010).
Cheung, A. et al. Defining improvement in nonalcoholic steatohepatitis for treatment trial endpoints: recommendations from the liver forum. Hepatology 70, 1841–1855 (2019).
Table of surrogate endpoints that were the basis of drug approval or licensure. US Food and Drug Administration (FDA) fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure (2019).
Anania, F. A., Dimick-Santos, L., Mehta, R., Toerner, J. & Beitz, J. Nonalcoholic steatohepatitis: current thinking from the division of hepatology and nutrition at the food and drug administration. Hepatology 73, 2023–2027 (2021).
Sanyal, A. J. et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis. Hepatology 75, 1235–1246 (2022).
Bedossa, P. & Poynard, T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24, 289–293 (1996).
Thanapirom, K. et al. Impact of compensated cirrhosis on survival in patients with acute-on-chronic liver failure. Hepatol. Int. 16, 171–182 (2022).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
Loomba, R., Ratziu, V., Harrison, S. A. & NASH Clinical Trial Design International Working Group, Expert panel review to compare FDA and EMA guidance on drug development and endpoints in nonalcoholic steatohepatitis. Gastroenterology 162, 680–688 (2022).
Davison, B. A. et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J. Hepatol. 73, 1322–1332 (2020).
Han, M. A. T. et al. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 17, 616–629.e26 (2019).
Polyzos, S. A., Kountouras, J. & Mantzoros, C. S. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metab. Clin. Exp. 92, 82–97 (2019).
Belew, G. D. & Jones, J. G. De novo lipogenesis in non-alcoholic fatty liver disease: quantification with stable isotope tracers. Eur. J. Clin. Invest. 52, e13733 (2022).
Hydes, T. J., Ravi, S., Loomba, R. & Gray, M. E. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin. Mol. Hepatol. 26, 383–400 (2020).
Holst, J. J. The incretin system in healthy humans: the role of GIP and GLP-1. Metab. Clin. Exp. 96, 46–55 (2019).
McLean, B. A. et al. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr. Rev. 42, 101–132 (2021).
McLean, B. A., Wong, C. K., Kaur, K. D., Seeley, R. J. & Drucker, D. J. Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic versus hepatic actions of semaglutide. JCI Insight 6, e153732 (2021).
Hsieh, J. et al. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 53, 552–561 (2010).
Yabut, J. M. & Drucker, D. J. Glucagon-like peptide-1 receptor-based therapeutics for metabolic liver disease. Endocr. Rev. 44, 14–32 (2023). A review of the biology of GLP-1R agonists with a focus on how this relates to therapy for MASH.
Baggio, L. L. et al. The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice. Mol. Metab. 6, 1339–1349 (2017).
Seghieri, M. et al. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia 56, 156–161 (2013).
Bernsmeier, C. et al. Glucose-induced glucagon-like peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS ONE 9, e87488 (2014).
Bozzetto, L. et al. Insulin resistance, postprandial GLP-1 and adaptive immunity are the main predictors of NAFLD in a homogeneous population at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 26, 623–629 (2016).
Nevola, R. et al. GLP-1 receptor agonists in non-alcoholic fatty liver disease: current evidence and future perspectives. Int. J. Mol. Sci. 24, 1703 (2023).
Panjwani, N. et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− mice. Endocrinology 154, 127–139 (2013).
Perakakis, N., Stefanakis, K., Feigh, M., Veidal, S. S. & Mantzoros, C. S. Elafibranor and liraglutide improve differentially liver health and metabolism in a mouse model of non-alcoholic steatohepatitis. Liver Int. 41, 1853–1866 (2021).
Trevaskis, J. L. et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G762–G772 (2012).
Armstrong, M. J. et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 64, 399–408 (2016).
Wang, Y. et al. Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br. J. Pharmacol. 171, 723–734 (2014).
Rodbard, H. W. et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care 42, 2272–2281 (2019).
Loomba, R. et al. Semaglutide 2.4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 8, 511–522 (2023).
Gasbjerg, L. S. et al. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides 125, 170183 (2020).
El, K. & Campbell, J. E. The role of GIP in α-cells and glucagon secretion. Peptides 125, 170213 (2020).
Sun, B. et al. Structural determinants of dual incretin receptor agonism by tirzepatide. Proc. Natl Acad. Sci. USA 119, e2116506119 (2022).
Willard, F. S. et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 5, e140532 (2020).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022). A phase III double-blind, randomized trial of weekly tirzepatide in individuals with a BMI greater than 30 demonstrating substantial and sustained reductions in body weight.
Gastaldelli, A. et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 10, 393–406 (2022).
Loomba, R. et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N. Engl. J. Med. 391, 299–310 (2024).
Doggrell, S. A. Is retatrutide (LY3437943), a GLP-1, GIP, and glucagon receptor agonist a step forward in the treatment of diabetes and obesity? Expert Opin. Investig. Drugs 32, 355–359 (2023).
Rosenstock, J. et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 402, 529–544 (2023).
Jastreboff, A. M. et al. Triple–hormone-receptor agonist retatrutide for obesity — a phase 2 trial. N. Engl. J. Med. 389, 514–526 (2023). A phase II double-blind, randomized trial of weekly retatrutide in individuals with a BMI greater than 30 demonstrating substantial and sustained reductions in body weight.
Hædersdal, S., Andersen, A., Knop, F. K. & Vilsbøll, T. Revisiting the role of glucagon in health, diabetes mellitus and other metabolic diseases. Nat. Rev. Endocrinol. 19, 321–335 (2023).
Winther-Sørensen, M. et al. Glucagon acutely regulates hepatic amino acid catabolism and the effect may be disturbed by steatosis. Mol. Metab. 42, 101080 (2020).
Kleinert, M., Sachs, S., Habegger, K. M., Hofmann, S. M. & Muller, T. D. Glucagon regulation of energy expenditure. Int. J. Mol. Sci. 20, 5407 (2019).
Habegger, K. M. et al. The metabolic actions of glucagon revisited. Nat. Rev. Endocrinol. 6, 689–697 (2010).
Parker, V. E. R. et al. Cotadutide promotes glycogenolysis in people with overweight or obesity diagnosed with type 2 diabetes. Nat. Metab. 5, 2086–2093 (2023).
Sanyal, A. J. et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N. Engl. J. Med. 391, 311–319 (2024).
Jastreboff, A. M., Kaplan, L. M. & Hartman, M. L. Triple-hormone-receptor agonist retatrutide for obesity. Reply. N. Engl. J. Med. 389, 1629–1630 (2023).
Tillman, E. J. & Rolph, T. FGF21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front. Endocrinol. 11, 601290 (2020).
Geng, L., Lam, K. S. L. & Xu, A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat. Rev. Endocrinol. 16, 654–667 (2020).
Xu, P. et al. Fibroblast growth factor 21 attenuates hepatic fibrogenesis through TGF-β/smad2/3 and NF-κB signaling pathways. Toxicol. Appl. Pharmacol. 290, 43–53 (2016).
Adachi, M. & Brenner, D. A. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology 47, 677–685 (2008).
Harrison, S. A. et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 27, 1262–1271 (2021).
Wei, W. et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc. Natl Acad. Sci. USA 109, 3143–3148 (2012).
Talukdar, S. & Kharitonenkov, A. FGF19 and FGF21: in NASH we trust. Mol. Metab. 46, 101152 (2021). An analysis of the mechanism of action and efficacy data on several FGF-1 and FGF-21 assets in the development for MASH therapy.
Harrison, S. A. et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Gastroenterol. Hepatol. 7, 603–616 (2022).
Rinella, M. E. et al. A randomized, double-blind, placebo-controlled trial of aldafermin in patients with NASH and compensated cirrhosis. Hepatology 79, 674–689 (2024).
Iglesias, P. et al. Hyperthyroidism and cardiovascular disease: an association study using big data analytics. Endocrine 83, 405–413 (2024).
Yorke, E. Hyperthyroidism and liver dysfunction: a review of a common comorbidity. Clin. Med. Insights Endocrinol. Diabetes 15, 11795514221074672 (2022).
Bano, A. et al. Thyroid function and the risk of nonalcoholic fatty liver disease: the Rotterdam Study. J. Clin. Endocrinol. Metab. 101, 3204–3211 (2016).
Bohinc, B. N. et al. Repair-related activation of hedgehog signaling in stromal cells promotes intrahepatic hypothyroidism. Endocrinology 155, 4591–4601 (2014).
Sinha, R. A., Singh, B. K. & Yen, P. M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 14, 259–269 (2018).
Harrison, S. A. et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 390, 497–509 (2024). A prospective, randomized, double-blind phase III study on the efficacy of the thyroid receptor b agonist resmetirom in patients with MASH and F2–F3 fibrosis.
Viking Therapeutics announces positive 52-week histologic data from phase 2b VOYAGE study of VK2809 in patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH). Viking Therapeutics https://ir.vikingtherapeutics.com/2024-06-04-Viking-Therapeutics-Announces-Positive-52-Week-Histologic-Data-from-Phase-2b-VOYAGE-Study-of-VK2809-in-Patients-with-Biopsy-Confirmed-Non-Alcoholic-Steatohepatitis-NASH (2024).
Sun, L., Cai, J. & Gonzalez, F. J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 18, 335–347 (2021).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Younossi, Z. M. et al. Obeticholic acid impact on quality of life in patients with nonalcoholic steatohepatitis: REGENERATE 18-month interim analysis. Clin. Gastroenterol. Hepatol. 20, 2050–2058.e12 (2022).
Tyagi, S., Gupta, P., Saini, A. S., Kaushal, C. & Sharma, S. The peroxisome proliferator-activated receptor: a family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2, 236–240 (2011).
Fougerat, A., Montagner, A., Loiseau, N., Guillou, H. & Wahli, W. Peroxisome proliferator-activated receptors and their novel ligands as candidates for the treatment of non-alcoholic fatty liver disease. Cells 9, 1638 (2020).
Berthier, A., Johanns, M., Zummo, F. P., Lefebvre, P. & Staels, B. PPARs in liver physiology. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166097 (2021).
Dufour, J. F., Caussy, C. & Loomba, R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut 69, 1877–1884 (2020).
Francque, S. M. et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N. Engl. J. Med. 385, 1547–1558 (2021).
Lawitz, E. J. et al. Acetyl-CoA carboxylase inhibitor GS-0976 for 12 weeks reduces hepatic de novo lipogenesis and steatosis in patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 16, 1983–1991.e3 (2018).
Higuchi, N. et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol. Res. 38, 1122–1129 (2008).
Alexander, M. C., Kowaloff, E. M., Witters, L. A., Dennihy, D. T. & Avruch, J. Purification of a hepatic 123,000-dalton hormone-stimulated 32P-peptide and its identification as ATP-citrate lyase. J. Biol. Chem. 254, 8052–8056 (1979).
Pierce, M. W., Palmer, J. L., Keutmann, H. T. & Avruch, J. ATP-citrate lyase. Structure of a tryptic peptide containing the phosphorylation site directed by glucagon and the cAMP-dependent protein kinase. J. Biol. Chem. 256, 8867–8870 (1981).
Govindaraju, A. & Sabarathinam, S. Bempedoic acid: a nonstatin drug for the management of hypercholesterolemia. Health Sci. Rep. 4, e431 (2021).
Morrow, M. R. et al. Inhibition of ATP-citrate lyase improves NASH, liver fibrosis, and dyslipidemia. Cell Metab. 34, 919–936.e8 (2022).
Dolle, R. E. et al. Synthesis of novel thiol-containing citric acid analogues. Kinetic evaluation of these and other potential active-site-directed and mechanism-based inhibitors of ATP citrate lyase. J. Med. Chem. 38, 537–543 (1995).
Bar-Tana, J., Rose-Kahn, G. & Srebnik, M. Inhibition of lipid synthesis by beta beta’-tetramethyl-substituted, C14-C22, alpha, omega-dicarboxylic acids in the rat in vivo. J. Biol. Chem. 260, 8404–8410 (1985).
Mayorek, N., Kalderon, B., Itach, E. & Bar-Tana, J. Sensitization to insulin induced by β,β′-methyl-substituted hexadecanedioic acid (MEDICA 16) in obese Zucker rats in vivo. Diabetes 46, 1958–1964 (1997).
Lally, J. S. V. et al. Inhibition of acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 29, 174–182.e5 (2019).
Kim, C. W. et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab. 26, 394–406.e6 (2017).
Calle, R. A. et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: two parallel, placebo-controlled, randomized phase 2a trials. Nat. Med. 27, 1836–1848 (2021).
Huard, K. et al. Optimizing the benefit/risk of acetyl-CoA carboxylase inhibitors through liver targeting. J. Med. Chem. 63, 10879–10896 (2020).
Loomba, R. et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology 155, 1463–1473.e6 (2018).
Loomba, R. et al. Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis attributable to NASH. Hepatology 73, 625–643 (2021).
Alkhouri, N. et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: a randomised, open-label phase II trial. J. Hepatol. 77, 607–618 (2022).
Mizojiri, R. et al. Design and synthesis of a monocyclic derivative as a selective ACC1 inhibitor by chemical modification of biphenyl ACC1/2 dual inhibitors. Bioorg. Med. Chem. 35, 116056 (2021).
Gu, Y. G. et al. Synthesis and structure−activity relationships of N-{3-[2-(4-alkoxyphenoxy)thiazol-5-yl]-1-methylprop-2-ynyl}carboxy derivatives as selective acetyl-CoA carboxylase 2 inhibitors. J. Med. Chem. 49, 3770–3773 (2006).
Smith, S. J. et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25, 87–90 (2000).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 (2009).
Amin, N. B., Saxena, A. R., Somayaji, V. & Dullea, R. Inhibition of diacylglycerol acyltransferase 2 versus diacylglycerol acyltransferase 1: potential therapeutic implications of pharmacology. Clin. Ther. 45, 55–70 (2023).
White, S. W., Zheng, J., Zhang, Y. M. & Rock, C. O. The structural biology of type II fatty acid biosynthesis. Annu. Rev. Biochem. 74, 791–831 (2005).
Syed-Abdul, M. M. et al. Fatty acid synthase inhibitor TVB-2640 reduces hepatic de novo lipogenesis in males with metabolic abnormalities. Hepatology 72, 103–118 (2020). A study of the efficacy of the fatty acid synthase inhibitor TVB-2640 in 12 participants with obesity demonstrating a 90% reduction in DNL.
Beysen, C. et al. Inhibition of fatty acid synthase with FT-4101 safely reduces hepatic de novo lipogenesis and steatosis in obese subjects with non-alcoholic fatty liver disease: results from two early-phase randomized trials. Diabetes Obes. Metab. 23, 700–710 (2021). A study demonstrating that single and repeat dosing of FT-4101 is safe and well-tolerated, and it significantly reduces DNL.
Kelly, K. L. et al. De novo lipogenesis is essential for platelet production in humans. Nat. Metab. 2, 1163–1178 (2020).
Das, S. et al. ATP citrate lyase improves mitochondrial function in skeletal muscle. Cell Metab. 21, 868–876 (2015).
Huang, Z. et al. ACSS2 promotes systemic fat storage and utilization through selective regulation of genes involved in lipid metabolism. Proc. Natl Acad. Sci. USA 115, E9499–E9506 (2018).
Liu, X. et al. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 175, 502–513.e13 (2018). This study demonstrates that, in mammals, pyruvate, the end-product of glycolysis, quantitatively generates acetate, which can then be used for DNL.
Anstee, Q. M. et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort☆. J. Hepatol. 73, 505–515 (2020).
Abul-Husn, N. S. et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N. Engl. J. Med. 378, 1096–1106 (2018).
Emdin, C. A. et al. A missense variant in Mitochondrial Amidoxime Reducing Component 1 gene and protection against liver disease. PLoS Genet. 16, e1008629 (2020).
Schneider, C. V. et al. A genome-first approach to mortality and metabolic phenotypes in MTARC1 p.Ala165Thr (rs2642438) heterozygotes and homozygotes. Med 2, 851–863.e3 (2021).
Pedrosa, M. et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: study design of the TANDEM trial. Contemp. Clin. Trials 88, 105889 (2020).
Cho, Y. et al. Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: an open-label randomized controlled trial (ESSENTIAL study). BMC Med. 20, 93 (2022).
Amin, N. B. et al. Efficacy and safety of an orally administered DGAT2 inhibitor alone or coadministered with a liver-targeted ACC inhibitor in adults with non-alcoholic steatohepatitis (NASH): rationale and design of the phase II, dose-ranging, dose-finding, randomised, placebo-controlled MIRNA (Metabolic Interventions to Resolve NASH with fibrosis) study. BMJ Open 12, e056159 (2022).
Newsome, P. et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment. Pharmacol. Ther. 50, 193–203 (2019).
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 389, 2221–2232 (2023). A multicentre, double-blind, randomized, placebo-controlled, event-driven superiority trial of over 17,000 participants demonstrating that semaglutide reduces the incidence of death from cardiovascular causes.
Furlan, A. et al. Comparison of 2D shear wave elastography, transient elastography, and MR elastography for the diagnosis of fibrosis in patients with nonalcoholic fatty liver disease. Am. J. Roentgenol. 214, W20–W26 (2020).
Younes, R. & Bugianesi, E. NASH in lean individuals. Semin. Liver Dis. 39, 86–95 (2019).
Nabi, O. et al. Lean individuals with NAFLD have more severe liver disease and poorer clinical outcomes (NASH-CO Study). Hepatology 78, 272–283 (2023).
Zhu, X. et al. Presence of sarcopenia identifies a special group of lean NAFLD in middle-aged and older people. Hepatol. Int. 17, 313–325 (2023).
Lin, H. et al. Association of genetic variations with NAFLD in lean individuals. Liver Int. 42, 149–160 (2022).
Sargeant, J. A. et al. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol. Metab. 34, 247–262 (2019).
Tufvesson-Alm, M., Shevchouk, O. T. & Jerlhag, E. Insight into the role of the gut-brain axis in alcohol-related responses: emphasis on GLP-1, amylin, and ghrelin. Front. Psychiatry 13, 1092828 (2023).
Klausen, M. K., Thomsen, M., Wortwein, G. & Fink-Jensen, A. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br. J. Pharmacol. 179, 625–641 (2022).
Estes, C. et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 69, 896–904 (2018).
Ussher, J. R., Greenwell, A. A., Nguyen, M. A. & Mulvihill, E. E. Cardiovascular effects of incretin-based therapies: integrating mechanisms with cardiovascular outcome trials. Diabetes 71, 173–183 (2022).
Gutierrez-Cuevas, J., Santos, A. & Armendariz-Borunda, J. Pathophysiological molecular mechanisms of obesity: a link between MAFLD and NASH with cardiovascular diseases. Int. J. Mol. Sci. 22, 11629 (2021).
Chia, C. W. & Egan, J. M. Incretins in obesity and diabetes. Ann. NY Acad. Sci. 1461, 104–126 (2020).
Seetharaman, R. & Pandit, S. Can small molecule GLP-1 agonists be the next first-line drugs in type-2 diabetes mellitus? J. Basic. Clin. Physiol. Pharmacol. 35, 1–4 (2024).
Harrison, S. A. et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 394, 2012–2024 (2019).
Acknowledgements
We are grateful to B. Banini, V. Gupta and J. Dranoff for their helpful comments during manuscript preparation. W.Z.M. is supported by a VA Merit Award.
Author information
Authors and Affiliations
Contributions
All authors (A.D., F.Z. and W.Z.M.) contributed to research, writing and editing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Brian Finck, Michael Trauner and John Ussher for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Do, A., Zahrawi, F. & Mehal, W.Z. Therapeutic landscape of metabolic dysfunction-associated steatohepatitis (MASH). Nat Rev Drug Discov 24, 171–189 (2025). https://doi.org/10.1038/s41573-024-01084-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-024-01084-2
This article is cited by
-
Hepatic targeting in ASCVD: integrating lipid lowering and inflammation modulation from statins to gene editing
Journal of Translational Medicine (2026)
-
TSP50 attenuates metabolic dysfunction-associated steatotic liver disease via SCD1 degradation-mediated suppression of hepatocyte lipogenesis
Cellular & Molecular Biology Letters (2026)
-
The metabolic dysfunction-associated steatohepatitis (MASH) drug resmetirom exhibits broad nuclear receptor activity with minimal functional impact
Scientific Reports (2026)
-
Betulinic acid as a novel AT1R inhibitor: attenuation of liver fibrosis via modulation of endothelial–mesenchymal transition in chronic hepatic injury
Journal of Translational Medicine (2025)
-
Characteristics of serum bile acid profiles among individuals with metabolic dysfunction-associated steatotic liver disease
BMC Gastroenterology (2025)