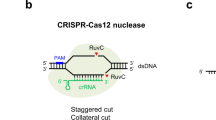
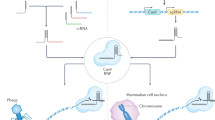
Abstract
Clustered regularly interspaced short palindromic repeats (CRISPR) technology has transformed molecular biology and the future of gene-targeted therapeutics. CRISPR systems comprise a CRISPR-associated (Cas) endonuclease and a guide RNA (gRNA) that can be programmed to guide sequence-specific binding, cleavage, or modification of complementary DNA or RNA. However, the application of CRISPR-based therapeutics is challenged by factors such as molecular size, prokaryotic or phage origins, and an essential gRNA cofactor requirement, which impact efficacy, delivery and safety. This Review focuses on chemical modification and engineering approaches for gRNAs to enhance or enable CRISPR-based therapeutics, emphasizing Cas9 and Cas12a as therapeutic paradigms. Issues that chemically modified gRNAs seek to address, including drug delivery, physiological stability, editing efficiency and off-target effects, as well as challenges that remain, are discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Porteus, M. H. A new class of medicines through DNA editing. N. Engl. J. Med. 380, 947–959 (2019).
Taylor, D. W. The final cut: Cas9 editing. Nat. Struct. Mol. Biol. 26, 669–670 (2019).
Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Fernández, A., Josa, S. & Montoliu, L. A history of genome editing in mammals. Mamm. Genome 28, 237–246 (2017).
Wiedenheft, B., Sternberg, S. H. & Doudna, J. A. RNA-guided genetic silencing systems in bacteria and archaea. Nature 482, 331–338 (2012).
Jinek, M. et al. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). A groundbreaking study demonstrating the effectiveness of CRISPR–Cas9 as a programmable genome-editing tool, leading to its recognition in the 2020 Nobel Prize in Chemistry.
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Brouns, S. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008).
Garneau, J. E. et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010).
Bolotin, A., Quinquis, B., Sorokin, A. & Ehrlich, S. D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151, 2551–2561 (2005).
Hsu, P. D. et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 (2013).
Frangoul, H. et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 384, 252–260 (2020). The first example of CRISPR technology to enter clinical trials for ex vivo gene editing, leading to the clinical approval of the first CRISPR-based therapeutic, exa-cel.
Jiang, F. & Doudna, J. A. CRISPR–Cas9 structures and mechanisms. Annu. Rev. Biophys. 46, 505–529 (2017).
Rouet, P., Smih, F. & Jasin, M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol. Cell. Biol. 14, 8096–8106 (1994).
Rouet, P., Smih, F. & Jasin, M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc. Natl Acad. Sci. USA 91, 6064–6068 (1994).
Platt, F. M., d’Azzo, A., Davidson, B. L., Neufeld, E. F. & Tifft, C. J. Lysosomal storage diseases. Nat. Rev. Dis. Primers 4, 27 (2018).
Jensen, N. M. et al. An update on targeted gene repair in mammalian cells: methods and mechanisms. J. Biomed. Sci. 18, 10 (2011).
Shim, G. et al. Therapeutic gene editing: delivery and regulatory perspectives. Acta Pharmacol. Sin. 38, 738–753 (2017).
Nidhi, S. et al. Novel CRISPR–Cas systems: an updated review of the current achievements, applications, and future research perspectives. Int. J. Mol. Sci. 22, 3327 (2021).
Li, Y., Glass, Z., Huang, M., Chen, Z.-Y. & Xu, Q. Ex vivo cell-based CRISPR/Cas9 genome editing for therapeutic applications. Biomaterials 234, 119711 (2020).
Carroll, D. Progress and prospects: zinc-finger nucleases as gene therapy agents. Gene Ther. 15, 1463–1468 (2008).
Allen, D., Rosenberg, M. & Hendel, A. Using synthetically engineered guide RNAs to enhance CRISPR genome editing systems in mammalian cells. Front. Genome Ed. 2, 617910 (2021).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771 (2015). The first study identifying Cas12a as an alternative effector enzyme to Cas9, expanding the range of available CRISPR–Cas systems capable of gene editing activity in mammalian cells.
Makarova, K. S. et al. An updated evolutionary classification of CRISPR–Cas systems. Nat. Rev. Microbiol. 13, 722–736 (2015).
Koonin, E. V., Gootenberg, J. S. & Abudayyeh, O. O. Discovery of diverse CRISPR-Cas systems and expansion of the genome engineering toolbox. Biochemistry 62, 3465–3487 (2023).
Egli, M. & Manoharan, M. Chemistry, structure and function of approved oligonucleotide therapeutics. Nucleic Acids Res. 51, 2529–2573 (2023).
Al Shaer, D., Al Musaimi, O., Albericio, F. & de la Torre, B. G. 2023 FDA TIDES (peptides and oligonucleotides) harvest. Pharmaceuticals 17, 243 (2024).
Vertex and CRISPR Therapeutics announce authorization of the first CRISPR/Cas9 gene-edited therapy, CASGEVY™ (exagamglogene autotemcel), by the United Kingdom MHRA for the treatment of sickle cell disease and transfusion-dependent beta thalassemia. Vertex Pharmaceuticals https://investors.vrtx.com/news-releases/news-release-details/vertex-and-crispr-therapeutics-announce-authorization-first (2023).
McKenzie, L. K., El-Khoury, R., Thorpe, J. D., Damha, M. J. & Hollenstein, M. Recent progress in non-native nucleic acid modifications. Chem. Soc. Rev. 50, 5126–5164 (2021).
Wan, W. B. & Seth, P. P. The medicinal chemistry of therapeutic oligonucleotides. J. Med. Chem. 59, 9645–9667 (2016).
Khvorova, A. & Watts, J. K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 35, 238–248 (2017).
Deleavey, G. F. & Damha, M. J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 19, 937–954 (2012).
Yamano, T. et al. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell 165, 949–962 (2016). This study presents the crystal structure of the Cas12a ribonucleoprotein complex, emphasizing key interactions and providing guidance for the development of modified CRISPR–Cas12a systems in subsequent investigations.
Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156, 935–949 (2014). Like Yamano et al. (2016), this research unveils the crystal structure of the Cas9 ribonucleoprotein complex, providing crucial insights for future studies.
Barrangou, R. & Doudna, J. A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 34, 933–941 (2016).
Fantoni, N. Z., El-Sagheer, A. H. & Brown, T. A hitchhiker’s guide to click-chemistry with nucleic acids. Chem. Rev. 121, 7122–7154 (2021).
McMahon, M. A., Prakash, T. P., Cleveland, D. W., Bennett, C. F. & Rahdar, M. Chemically modified Cpf1-CRISPR RNAs mediate efficient genome editing in mammalian cells. Mol. Ther. 26, 1228–1240 (2018).
O’Reilly, D. et al. Extensive CRISPR RNA modification reveals chemical compatibility and structure-activity relationships for Cas9 biochemical activity. Nucleic Acids Res. 47, 546–558 (2018). This study utilizes various chemical modifications to investigate structure–activity relationships within the CRISPR–Cas9 system and highlights the disparity between in vitro and cell-based assays in the examination of gene editing.
Doudna, J. A. & Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014).
Swarts, D. C. & Jinek, M. Cas9 versus Cas12a/Cpf1: structure–function comparisons and implications for genome editing. Wiley Interdiscip. Rev. RNA 9, e1481 (2018).
Cui, Y., Xu, J., Cheng, M., Liao, X. & Peng, S. Review of CRISPR/Cas9 sgRNA design tools. Interdiscip. Sci. 10, 455–465 (2018).
Zetsche, B., Abudayyeh, O. O., Gootenberg, J. S., Scott, D. A. & Zhang, F. A survey of genome editing activity for 16 Cas12a orthologs. Keio J. Med. 69, 59–65 (2020).
Swarts, D. C., van der Oost, J. & Jinek, M. Structural basis for guide RNA processing and seed-dependent DNA targeting by CRISPR-Cas12a. Mol. Cell 66, 221–233 (2017).
Dong, D. et al. The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 532, 522–526 (2016).
Hirano, H. et al. Structure and engineering of Francisella novicida Cas9. Cell 164, 950–961 (2016).
Fonfara, I., Richter, H., Bratovič, M., Le Rhun, A. & Charpentier, E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532, 517–521 (2016).
Park, H. M. et al. Extension of the crRNA enhances Cpf1 gene editing in vitro and in vivo. Nat. Commun. 9, 3313 (2018).
Ageely, E. A. et al. Gene editing with CRISPR-Cas12a guides possessing ribose-modified pseudoknot handles. Nat. Commun. 12, 6591 (2021). This research implements a structure-guided strategy to make extensive modifications to the Cas12a gRNA, with the primary goal of overcoming the 2′-OH barrier.
Sudhakar, S. et al. Binding to the conserved and stably folded guide RNA pseudoknot induces Cas12a conformational changes during ribonucleoprotein assembly. J. Biol. Chem. 299, 104700 (2023).
Sternberg, S. H., LaFrance, B., Kaplan, M. & Doudna, J. A. Conformational control of DNA target cleavage by CRISPR–Cas9. Nature 527, 110–113 (2015).
Pacesa, M. et al. Structural basis for Cas9 off-target activity. Cell 185, 4067–4081 (2022).
Szczelkun, M. D. et al. Direct observation of R-loop formation by single RNA-guided Cas9 and Cascade effector complexes. Proc. Natl Acad. Sci. USA 111, 9798–9803 (2014).
Dagdas, Y. S., Chen, J. S., Sternberg, S. H., Doudna, J. A. & Yildiz, A. A conformational checkpoint between DNA binding and cleavage by CRISPR-Cas9. Sci. Adv. 3, eaao0027 (2017).
Singh, A., Chakraborty, D. & Maiti, S. CRISPR/Cas9: a historical and chemical biology perspective of targeted genome engineering. Chem. Soc. Rev. 45, 6666–6684 (2016).
Wu, X., Kriz, A. J. & Sharp, P. A. Target specificity of the CRISPR-Cas9 system. Quant. Biol. 2, 59–70 (2014).
Strohkendl, I., Saifuddin, F. A., Rybarski, J. R., Finkelstein, I. J. & Russell, R. Kinetic basis for DNA target specificity of CRISPR-Cas12a. Mol. Cell 71, 816–824 (2018).
Finn, J. D. et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235 (2018).
Agard, N. J. & Bertozzi, C. R. Chemical approaches to perturb, profile, and perceive glycans. Acc. Chem. Res. 42, 788–797 (2009).
Aston, N. S., Watt, N., Morton, I. E., Tanner, M. S. & Evans, G. S. Copper toxicity affects proliferation and viability of human hepatoma cells (HepG2 line). Hum. Exp. Toxicol. 19, 367–376 (2000).
Taemaitree, L., Shivalingam, A., El-Sagheer, A. H. & Brown, T. An artificial triazole backbone linkage provides a split-and-click strategy to bioactive chemically modified CRISPR sgRNA. Nat. Commun. 10, 1610 (2019).
He, K., Chou, E. T., Begay, S., Anderson, E. M. & van Brabant Smith, A. Conjugation and evaluation of triazole-linked single guide RNA for CRISPR-Cas9 gene editing. ChemBioChem 17, 1809–1812 (2016).
Chen, Z. et al. Tetrazine-ligated CRISPR sgRNAs for efficient genome editing. ACS Chem. Biol. 17, 1045–1050 (2022).
Hoy, A., Zheng, Y. Y., Sheng, J. & Royzen, M. Bio-orthogonal chemistry conjugation strategy facilitates investigation of N-methyladenosine and thiouridine guide RNA modifications on CRISPR activity. CRISPR J. 5, 787–798 (2022).
Hendel, A. et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat. Biotechnol. 33, 985–989 (2015). The first study to investigate the impact of chemical modifications on the gene-editing activity of Cas9 gRNA for its eventual use in therapeutic applications.
Li, B. et al. Engineering CRISPR–Cpf1 crRNAs and mRNAs to maximize genome editing efficiency. Nat. Biomed. Eng. 1, 0066 (2017). One of the first studies to incorporate chemical modifications into the Cas12a gRNA.
Basila, M., Kelley, M. L. & van Brabant Smith, A. Minimal 2′-O-methyl phosphorothioate linkage modification pattern of synthetic guide RNAs for increased stability and efficient CRISPR-Cas9 gene editing avoiding cellular toxicity. PLoS ONE 12, e0188593 (2017).
Rozners, E. Chemical modifications of CRISPR RNAs to improve gene-editing activity and specificity. J. Am. Chem. Soc. 144, 12584–12594 (2022).
Kotikam, V., Gajula, P. K., Coyle, L. & Rozners, E. Amide internucleoside linkages are well tolerated in protospacer adjacent motif-distal region of CRISPR RNAs. ACS Chem. Biol. 17, 509–512 (2022).
Gu, C. et al. Chemical synthesis of stimuli-responsive guide RNA for conditional control of CRISPR-Cas9 gene editing. Chem. Sci. 12, 9934–9945 (2021).
Wang, Y., Liu, Y., Xie, F., Lin, J. & Xu, L. Photocontrol of CRISPR/Cas9 function by site-specific chemical modification of guide RNA. Chem. Sci. 11, 11478–11484 (2020).
Arzumanian, V. A., Dolgalev, G. V., Kurbatov, I. Y., Kiseleva, O. I. & Poverennaya, E. V. Epitranscriptome: review of top 25 most-studied RNA modifications. Int. J. Mol. Sci. 23, 13851 (2022).
Moosavi, A. & Motevalizadeh Ardekani, A. Role of epigenetics in biology and human diseases. Iran. Biomed. J. 20, 246–258 (2016).
Lorenz, C., Lünse, C. E. & Mörl, M. tRNA modifications: impact on structure and thermal adaptation. Biomolecules 7, 35 (2017).
Shen, X. & Corey, D. R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 46, 1584–1600 (2018).
Zhang, G., Tang, T., Chen, Y., Huang, X. & Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 8, 365 (2023).
Nance, K. D. & Meier, J. L. Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent. Sci. 7, 748–756 (2021).
Prokhorova, D. V. et al. Natural nucleoside modifications in guide RNAs can modulate the activity of the CRISPR-Cas9 system in vitro. CRISPR J. 5, 799–812 (2022).
Prokhorova, D., Matveeva, A., Zakabunin, A., Ryabchenko, A. & Stepanov, G. Influence of N1-methylpseudouridine in guide RNAs on CRISPR/Cas9 activity. Int. J. Mol. Sci. 24, 17116 (2023).
Vaidyanathan, S. et al. Uridine depletion and chemical modification increase Cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol. Ther. Nucleic Acids 12, 530–542 (2018).
Hu, Z. et al. Regulation of the CRISPR-Cas12a system by methylation and demethylation of guide RNA. Chem. Sci. 14, 5945–5955 (2023).
Kim, K. Q. et al. N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products. Cell Rep. 40, 111300 (2022).
Roehr, B. Fomivirsen approved for CMV retinitis. J. Int. Assoc. Physicians AIDS Care 4, 14–16 (1998).
Grillone, L. R. & Lanz, R. Fomivirsen. Drugs Today 37, 245–255 (2001).
Scott, L. J. Givosiran: first approval. Drugs 80, 335–339 (2020).
Liebow, A. et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J. Am. Soc. Nephrol. 28, 494–503 (2017).
Gales, L. Tegsedi (inotersen): an antisense oligonucleotide approved for the treatment of adult patients with hereditary transthyretin amyloidosis. Pharmaceuticals 12, 78 (2019).
Merki, E. et al. Antisense oligonucleotide directed to human apolipoprotein B-100 reduces lipoprotein(a) levels and oxidized phospholipids on human apolipoprotein B-100 particles in lipoprotein(a) transgenic mice. Circulation 118, 743–753 (2008).
Paik, J. & Duggan, S. Volanesorsen: first global approval. Drugs 79, 1349–1354 (2019).
Lim, K. R., Maruyama, R. & Yokota, T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des. Dev. Ther. 11, 533–545 (2017).
Summerton, J. E. in Morpholino Oligomers: Methods and Protocols, Vol. 1565 (eds Moulton, H. M. & Moulton, J. D.) 1–15 (Humana, 2017).
Heo, Y. A. Golodirsen: first approval. Drugs 80, 329–333 (2020).
Kartje, Z. J., Barkau, C. L., Rohilla, K. J., Ageely, E. A. & Gagnon, K. T. Chimeric guides probe and enhance Cas9 biochemical activity. Biochemistry 57, 3027–3031 (2018).
Yin, H. et al. Partial DNA-guided Cas9 enables genome editing with reduced off-target activity. Nat. Chem. Biol. 14, 311–316 (2018).
Kim, H. et al. Enhancement of target specificity of CRISPR–Cas12a by using a chimeric DNA–RNA guide. Nucleic Acids Res. 48, 8601–8616 (2020).
Rueda, F. O. et al. Mapping the sugar dependency for rational generation of a DNA-RNA hybrid-guided Cas9 endonuclease. Nat. Commun. 8, 1610 (2017).
Bishani, A. & Chernolovskaya, E. L. Activation of innate immunity by therapeutic nucleic acids. Int. J. Mol. Sci. 22, 13360 (2021).
Sakovina, L., Vokhtantsev, I., Vorobyeva, M., Vorobyev, P. & Novopashina, D. Improving stability and specificity of CRISPR/Cas9 system by selective modification of guide RNAs with 2′-fluoro and locked nucleic acid nucleotides. Int. J. Mol. Sci. 23, 13460 (2022).
Mir, A. et al. Heavily and fully modified RNAs guide efficient SpyCas9-mediated genome editing. Nat. Commun. 9, 2641 (2018). This study suggests the potential to develop a completely chemically modified Cas9 gRNA, similar to the approach used for other oligonucleotide therapeutics.
Yin, H. et al. Structure-guided chemical modification of guide RNA enables potent non-viral in vivo genome editing. Nat. Biotechnol. 35, 1179–1187 (2017). One of the first studies to incorporate chemical modifications throughout the Cas9 gRNA and combine them with LNP formulations for in vivo gene-editing applications.
Rahdar, M. et al. Synthetic CRISPR RNA-Cas9–guided genome editing in human cells. Proc. Natl Acad. Sci. USA 112, E7110–E7117 (2015). Like Yin et al. (2017), this research is one of the first to explore the use of chemical modifications throughout the Cas9 gRNA with the aim of creating therapeutically viable CRISPR-based systems.
Cromwell, C. R. et al. Incorporation of bridged nucleic acids into CRISPR RNAs improves Cas9 endonuclease specificity. Nat. Commun. 9, 1448 (2018).
Mangos, M. M. et al. Efficient RNase H-directed cleavage of RNA promoted by antisense DNA or 2′F-ANA constructs containing acyclic nucleotide inserts. J. Am. Chem. Soc. 125, 654–661 (2003).
Laursen, M. B. et al. Utilization of unlocked nucleic acid (UNA) to enhance siRNA performance in vitro and in vivo. Mol. Biosyst. 6, 862–870 (2010).
Noronha, A. M. et al. Synthesis and biophysical properties of arabinonucleic acids (ANA): circular dichroic spectra, melting temperatures, and ribonuclease H susceptibility of ANA•RNA hybrid duplexes. Biochemistry 39, 7050–7062 (2000).
El-Khoury, R. & Damha, M. J. 2′-Fluoro-arabinonucleic acid (FANA): a versatile tool for probing biomolecular interactions. Acc. Chem. Res. 54, 2287–2297 (2021).
Horlbeck, M. A. et al. Nucleosomes impede Cas9 access to DNA in vivo and in vitro. eLife 5, e12677 (2016).
Watts, J. K. et al. Differential stability of 2′F-ANA•RNA and ANA•RNA hybrid duplexes: roles of structure, pseudohydrogen bonding, hydration, ion uptake and flexibility. Nucleic Acids Res. 38, 2498–2511 (2010).
Egli, M. The steric hypothesis for DNA replication and fluorine hydrogen bonding revisited in light of structural data. Acc. Chem. Res. 45, 1237–1246 (2012).
Ryan, D. E. et al. Phosphonoacetate modifications enhance the stability and editing yields of guide RNAs for Cas9 editors. Biochemistry 62, 3512–3520 (2022).
Ryan, D. E. et al. Improving CRISPR–Cas specificity with chemical modifications in single-guide RNAs. Nucleic Acids Res. 46, 792–803 (2018).
Sheng, J. et al. Structural insights into the effects of 2′-5′ linkages on the RNA duplex. Proc. Natl Acad. Sci. USA 111, 3050–3055 (2014).
Kandimalla, E. R. et al. Mixed backbone antisense oligonucleotides: design, biochemical and biological properties of oligonucleotides containing 2′-5′-ribo- and 3′-5′-deoxyribonucleotide segments. Nucleic Acids Res. 25, 370–378 (1997).
Prokhorova, D. V. et al. Effect of the phosphoryl guanidine modification in chimeric DNA–RNA crRNAs on the activity of the CRISPR-Cas9 system in vitro. ACS Chem. Biol. 19, 1311–1319 (2024).
Saito-Tarashima, N., Ueno, M., Murai, A., Matsuo, A. & Minakawa, N. Cas9-mediated DNA cleavage guided by enzymatically prepared 4′-thio-modified RNA. Org. Biomol. Chem. 20, 5245–5248 (2022).
Zhang, H. et al. Self-delivering, chemically modified CRISPR RNAs for AAV co-delivery and genome editing in vivo. Nucleic Acids Res. 52, 977–997 (2024).
Chen, K. et al. Engineering self-deliverable ribonucleoproteins for genome editing in the brain. Nat. Commun. 15, 1727 (2024).
Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 24, 1020–1027 (2014).
Durrant, M. G. et al. Bridge RNAs direct programmable recombination of target and donor DNA. Nature 630, 984–993 (2024).
Wei, T. et al. Delivery of tissue-targeted scalpels: opportunities and challenges for in vivo CRISPR/Cas-based genome editing. ACS Nano 14, 9243–9262 (2020).
Madigan, V., Zhang, F. & Dahlman, J. E. Drug delivery systems for CRISPR-based genome editors. Nat. Rev. Drug Discov. 22, 875–894 (2023).
Nelson, C. E. et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat. Med. 25, 427–432 (2019).
Wang, H. et al. Engineering of a compact, high-fidelity EbCas12a variant that can be packaged with its crRNA into an all-in-one AAV vector delivery system. PLoS Biol. 22, e3002619 (2024).
Kabadi, A. M., Ousterout, D. G., Hilton, I. B. & Gersbach, C. A. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 42, e147 (2014).
Ortinski, P. I., O’Donovan, B., Dong, X. & Kantor, B. Integrase-deficient lentiviral vector as an all-in-one platform for highly efficient CRISPR/Cas9-mediated gene editing. Mol. Ther. Methods Clin. Dev. 5, 153–164 (2017).
Wang, D. et al. Adenovirus-mediated somatic genome editing of Pten by CRISPR/Cas9 in mouse liver in spite of Cas9-specific immune responses. Hum. Gene Ther. 26, 432–442 (2015).
Li, C. et al. Inhibition of HIV-1 infection of primary CD4+ T-cells by gene editing of CCR5 using adenovirus-delivered CRISPR/Cas9. J. Gen. Virol. 96, 2381–2393 (2015).
Jin, Y.-H. et al. Streamlined procedure for gene knockouts using all-in-one adenoviral CRISPR-Cas9. Sci. Rep. 9, 277 (2019).
Ghosh, S., Brown, A. M., Jenkins, C. & Campbell, K. Viral vector systems for gene therapy: a comprehensive literature review of progress and biosafety challenges. Appl. Biosaf. 25, 7–18 (2020).
Schmelas, C. & Grimm, D. Split Cas9, not hairs — advancing the therapeutic index of CRISPR technology. Biotechnol. J. 13, e1700432 (2018).
Villiger, L. et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 24, 1519–1525 (2018).
Nooraei, S. et al. Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 19, 59 (2021).
Liu, B.-Y. et al. Peptide and aptamer decorated delivery system for targeting delivery of Cas9/sgRNA plasmid to mediate antitumor genome editing. ACS Appl. Mater. Interfaces 11, 23870–23879 (2019).
Coelho, T. et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 369, 819–829 (2013).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).
Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2020).
Kenjo, E. et al. Low immunogenicity of LNP allows repeated administrations of CRISPR-Cas9 mRNA into skeletal muscle in mice. Nat. Commun. 12, 7101 (2021).
Intellia and Regeneron present updated interim data from phase 1 study of CRISPR-based NTLA-2001 for the treatment of transthyretin (ATTR) amyloidosis demonstrating that deep serum TTR reductions remained durable after a single dose. Intellia Therapeutics https://ir.intelliatx.com/news-releases/news-release-details/intellia-and-regeneron-present-updated-interim-data-phase-1 (2022).
Kasiewicz, L. N. et al. GalNAc-lipid nanoparticles enable non-LDLR dependent hepatic delivery of a CRISPR base editing therapy. Nat. Commun. 14, 2776 (2023).
Fitzgerald, K. et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N. Engl. J. Med. 376, 41–51 (2016).
Viney, N. J. et al. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: preclinical and phase 1 data. ESC Heart Fail. 8, 652–661 (2021).
Brown, C. R. et al. Investigating the pharmacodynamic durability of GalNAc–siRNA conjugates. Nucleic Acids Res. 48, 11827–11844 (2020).
Chen, S., Lee, B., Lee, A. Y.-F., Modzelewski, A. J. & He, L. Highly efficient mouse genome editing by CRISPR ribonucleoprotein electroporation of zygotes. J. Biol. Chem. 291, 14457–14467 (2016).
Ferguson, C. M. et al. Comparative route of administration studies using therapeutic siRNAs show widespread gene modulation in Dorset sheep. JCI Insight 6, e152203 (2021).
Luo, N. et al. Hepatic stellate cell reprogramming via exosome-mediated CRISPR/dCas9-VP64 delivery. Drug Deliv. 28, 10–18 (2021).
Ye, Y. et al. An engineered exosome for delivering sgRNA:Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomater. Sci. 8, 2966–2976 (2020).
Koonin, E. V. Evolution of RNA- and DNA-guided antivirus defense systems in prokaryotes and eukaryotes: common ancestry vs convergence. Biol. Direct 12, 5 (2017).
Wu, X. et al. Genome-wide binding of the CRISPR endonuclease Cas9 in mammalian cells. Nat. Biotechnol. 32, 670–676 (2014).
Kosicki, M., Tomberg, K. & Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 36, 765–771 (2018).
Cullot, G. et al. CRISPR-Cas9 genome editing induces megabase-scale chromosomal truncations. Nat. Commun. 10, 1136 (2019).
Leibowitz, M. L. et al. Chromothripsis as an on-target consequence of CRISPR–Cas9 genome editing. Nat. Genet. 53, 895–905 (2021).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Heidersbach, A. J., Dorighi, K. M., Gomez, J. A., Jacobi, A. M. & Haley, B. A versatile, high-efficiency platform for CRISPR-based gene activation. Nat. Commun. 14, 902 (2023).
Casas-Mollano, J. A., Zinselmeier, M. H., Erickson, S. E. & Smanski, M. J. CRISPR-Cas activators for engineering gene expression in higher eukaryotes. CRISPR J. 3, 350–364 (2020).
Carroll, M. S. & Giacca, M. CRISPR activation and interference as investigative tools in the cardiovascular system. Int. J. Biochem. Cell Biol. 155, 106348 (2023).
Larson, M. H. et al. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat. Protoc. 8, 2180–2196 (2013).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
Villiger, L. et al. CRISPR technologies for genome, epigenome and transcriptome editing. Nat. Rev. Mol. Cell Biol. 25, 464–487 (2024).
Méndez-Mancilla, A. et al. Chemically modified guide RNAs enhance CRISPR-Cas13 knockdown in human cells. Cell Chem. Biol. 29, 321–327 (2022).
Palaz, F. et al. CRISPR-Cas13 system as a promising and versatile tool for cancer diagnosis, therapy, and research. ACS Synth. Biol. 10, 1245–1267 (2021).
Marina, R. J., Brannan, K. W., Dong, K. D., Yee, B. A. & Yeo, G. W. Evaluation of engineered CRISPR-Cas-mediated systems for site-specific RNA editing. Cell Rep. 33, 108350 (2020).
Fiflis, D. N. et al. Repurposing CRISPR-Cas13 systems for robust mRNA trans-splicing. Nat. Commun. 15, 2325 (2024).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Jiang, T. et al. Chemical modifications of adenine base editor mRNA and guide RNA expand its application scope. Nat. Commun. 11, 1979 (2020). This study aims to enhance the efficacy of Cas9 base editors through chemical modifications of both mRNA and gRNA for correcting single-nucleotide mutations.
Matsoukas, I. G. Prime editing: genome editing for rare genetic diseases without double-strand breaks or donor DNA. Front. Genet. 11, 528 (2020).
Nelson, J. W. et al. Engineered pegRNAs improve prime editing efficiency. Nat. Biotechnol. 40, 402–410 (2022). This research involves engineering of pegRNAs to protect them from degradation and improve prime editing efficiency.
Zhang, W. et al. Enhancing CRISPR prime editing by reducing misfolded pegRNA interactions. eLife 12, RP90948 (2024).
Li, X. et al. Enhancing prime editing efficiency by modified pegRNA with RNA G-quadruplexes. J. Mol. Cell Biol. 14, mjac002 (2022).
Zhang, G. et al. Enhancement of prime editing via xrRNA motif-joined pegRNA. Nat. Commun. 13, 1856 (2022).
Chen, Z. et al. In vivo prime editing by lipid nanoparticle co-delivery of chemically modified pegRNA and prime editor mRNA. GEN Biotechnol. 2, 490–502 (2023).
Liu, B. et al. Targeted genome editing with a DNA-dependent DNA polymerase and exogenous DNA-containing templates. Nat. Biotechnol. 42, 1039–1045 (2024).
Maeder, M. L. et al. CRISPR RNA–guided activation of endogenous human genes. Nat. Methods 10, 977–979 (2013).
Perez-Pinera, P. et al. RNA-guided gene activation by CRISPR-Cas9–based transcription factors. Nat. Methods 10, 973–976 (2013).
Chavez, A. et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 12, 326–328 (2015).
Tanenbaum, M. E., Gilbert, L. A., Qi, L. S., Weissman, J. S. & Vale, R. D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159, 635–646 (2014).
Chavez, A. et al. Comparison of Cas9 activators in multiple species. Nat. Methods 13, 563–567 (2016).
Groner, A. C. et al. KRAB–zinc finger proteins and KAP1 can mediate long-range transcriptional repression through heterochromatin spreading. PLoS Genet. 6, e1000869 (2010).
Yeo, N. C. et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods 15, 611–616 (2018).
Mills, C. et al. A novel CRISPR interference effector enabling functional gene characterization with synthetic guide RNAs. CRISPR J. 5, 769–786 (2022).
Chavez, M., Chen, X., Finn, P. B. & Qi, L. S. Advances in CRISPR therapeutics. Nat. Rev. Nephrol. 19, 9–22 (2023).
Ai, Y., Liang, D. & Wilusz, J. E. CRISPR/Cas13 effectors have differing extents of off-target effects that limit their utility in eukaryotic cells. Nucleic Acids Res. 50, e65 (2022).
Cheng, X. et al. Modeling CRISPR-Cas13d on-target and off-target effects using machine learning approaches. Nat. Commun. 14, 752 (2023).
Borrajo, J. et al. Programmable multi-kilobase RNA editing using CRISPR-mediated trans-splicing. Preprint at bioRxiv https://doi.org/10.1101/2023.08.18.553620 (2023).
Canver, M. C. et al. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 527, 192–197 (2015).
Nagar, R., Sinha, S. & Raman, R. Genotype–phenotype correlation and report of novel mutations in β-globin gene in thalassemia patients. Blood Cells Mol. Dis. 55, 10–14 (2015).
Makis, A., Voskaridou, E., Papassotiriou, I. & Hatzimichael, E. Novel therapeutic advances in β-thalassemia. Biology 10, 546 (2021).
Piel, F. B., Steinberg, M. H. & Rees, D. C. Sickle cell disease. N. Engl. J. Med. 376, 1561–1573 (2017).
Positive results from pivotal trials of exa-cel for transfusion-dependent beta thalassemia and severe sickle cell disease presented at the 2023 Annual European Hematology Association (EHA) Congress. Vertex Pharmaceuticals https://investors.vrtx.com/news-releases/news-release-details/positive-results-pivotal-trials-exa-cel-transfusion-dependent (2023).
Vertex and CRISPR Therapeutics present new data on more patients with longer follow-up treated with exagamglogene autotemcel (exa-cel) at the 2022 European Hematology Association (EHA) Congress. Vertex Pharmaceuticals https://news.vrtx.com/news-releases/news-release-details/vertex-and-crispr-therapeutics-present-new-data-more-patients (2022).
De Dreuzy, E. et al. EDIT-301: an experimental autologous cell therapy comprising Cas12a-RNP modified mPB-CD34+ cells for the potential treatment of SCD. Blood 134, 4636 (2019).
Liu, N. et al. Transcription factor competition at the γ-globin promoters controls hemoglobin switching. Nat. Genet. 53, 511–520 (2021).
Canver, M. C. & Orkin, S. H. Customizing the genome as therapy for the β-hemoglobinopathies. Blood 127, 2536–2545 (2016).
Zhang, L. et al. AsCas12a ultra nuclease facilitates the rapid generation of therapeutic cell medicines. Nat. Commun. 12, 3908 (2021).
De Dreuzy, E. et al. Robust pre-clinical results and large-scale manufacturing process for Edit-301: an autologous cell therapy for the potential treatment of SCD. Blood 136, 45–46 (2020).
Hanna, R. et al. S264: EDIT-301 shows promising preliminary safety and efficacy results in the phase I/II clinical trial (RUBY) of patients with severe sickle cell disease using highly specific and efficient AsCas12a enzyme. HemaSphere 7, e05170e0 (2023).
Gillmore, J. D. et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021). The first example of a systemically administered CRISPR–Cas9 system to enter clinical trials for in vivo gene editing, representing an important milestone in the advancement of gene therapy.
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).
Intellia Therapeutics announces second quarter 2024 financial results and highlights recent company progress. Intellia Therapeutics https://ir.intelliatx.com/news-releases/news-release-details/intellia-therapeutics-announces-second-quarter-2024-financial (2024).
Zuraw, B. L. Hereditary angiodema: a current state-of-the-art review, IV: short- and long-term treatment of hereditary angioedema: out with the old and in with the new? Ann. Allergy Asthma Immunol. 100, S13–S18 (2008).
Riano, I. & Prasongdee, K. A rare cause of isolated prolonged activated partial thromboplastin time: an overview of prekallikrein deficiency and the contact system. J Investig. Med. High Impact Case Rep. 9, 23247096211012187 (2021).
Longhurst, H. J. et al. CRISPR-Cas9 in vivo gene editing of KLKB1 for hereditary angioedema. N. Engl. J. Med. 390, 432–441 (2024).
Banerji, A. et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA 320, 2108–2121 (2018).
Fijen, L. M. et al. Inhibition of prekallikrein for hereditary angioedema. N. Engl. J. Med. 386, 1026–1033 (2022).
Nordestgaard, B. G. et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart J. 34, 3478–3490a (2013).
Abifadel, M. et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003).
Goldstein, J. L. & Brown, M. S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29, 431–438 (2009).
Tybjærg-Hansen, A., Steffensen, R., Meinertz, H., Schnohr, P. & Nordestgaard, B. G. Association of mutations in the apolipoprotein B gene with hypercholesterolemia and the risk of ischemic heart disease. N. Engl. J. Med. 338, 1577–1584 (1998).
Defesche, J. C. et al. Familial hypercholesterolaemia. Nat. Rev. Dis. Primers 3, 17093 (2017).
Berberich, A. J. & Hegele, R. A. The complex molecular genetics of familial hypercholesterolaemia. Nat. Rev. Cardiol. 16, 9–20 (2019).
Zhao, Z. et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 79, 514–523 (2006).
Lee, R. G. et al. Efficacy and safety of an investigational single-course CRISPR base-editing therapy targeting PCSK9 in nonhuman primate and mouse models. Circulation 147, 242–253 (2023).
Hooper, A. J., Tang, X. L. & Burnett, J. R. VERVE-101, a CRISPR base-editing therapy designed to permanently inactivate hepatic PCSK9 and reduce LDL-cholesterol. Expert Opin. Investig. Drugs 33, 753–756 (2024).
Horie, T. & Ono, K. VERVE-101: a promising CRISPR-based gene editing therapy that reduces LDL-C and PCSK9 levels in HeFH patients. Eur. Heart J. Cardiovasc. Pharmacother. 10, 89–90 (2023).
Manalac, T. Verve halts trial for lead gene editing program due to safety concerns. BioSpace https://www.biospace.com/verve-halts-trial-for-lead-gene-editing-program-due-to-safety-concerns (2024).
Roberts, R. & Crothers, D. Stability and properties of double and triple helices: dramatic effects of RNA or DNA backbone composition. Science 258, 1463–1466 (1992).
Salazar, M., Fedoroff, O. Y., Miller, J. M., Ribeiro, N. S. & Reid, B. R. The DNA strand in DNA.RNA hybrid duplexes is neither B-form nor A-form in solution. Biochemistry 32, 4207–4215 (1993).
Yang, Z., Wang, J., Huang, L., Lilley, D. M. J. & Ye, K. Functional organization of box C/D RNA-guided RNA methyltransferase. Nucleic Acids Res. 48, 5094–5105 (2020).
Wood, H. FDA approves patisiran to treat hereditary transthyretin amyloidosis. Nat. Rev. Neurol. 14, 570 (2018).
Patra, A. et al. 2′-Fluoro RNA shows increased Watson–Crick H-bonding strength and stacking relative to RNA: evidence from NMR and thermodynamic data. Angew. Chem. Int. Ed. 51, 11863–11866 (2012).
Pallan, P. S. et al. Unexpected origins of the enhanced pairing affinity of 2′-fluoro-modified RNA. Nucleic Acids Res. 39, 3482–3495 (2011).
Gaus, H. J. et al. Characterization of the interactions of chemically-modified therapeutic nucleic acids with plasma proteins using a fluorescence polarization assay. Nucleic Acids Res. 47, 1110–1122 (2019).
Brown, D. A. et al. Effect of phosphorothioate modification of oligodeoxynucleotides on specific protein binding. J. Biol. Chem. 269, 26801–26805 (1994).
Eckstein, F. Phosphorothioate oligodeoxynucleotides: what is their origin and what is unique about them? Antisense Nucleic Acid. Drug. Dev. 10, 117–121 (2000).
Oka, N. & Wada, T. Stereocontrolled synthesis of oligonucleotide analogs containing chiral internucleotidic phosphorus atoms. Chem. Soc. Rev. 40, 5829–5843 (2011).
Oka, N., Kondo, T., Fujiwara, S., Maizuru, Y. & Wada, T. Stereocontrolled synthesis of oligoribonucleoside phosphorothioates by an oxazaphospholidine approach. Org. Lett. 11, 967–970 (2009).
Malek-Adamian, E. et al. Adjusting the structure of 2′-modified nucleosides and oligonucleotides via C4′-α-F or C4′-α-OMe substitution: synthesis and conformational analysis. J. Org. Chem. 83, 9839–9849 (2018).
Malek-Adamian, E. et al. 4′-C-Methoxy-2′-deoxy-2′-fluoro modified ribonucleotides improve metabolic stability and elicit efficient RNAi-mediated gene silencing. J. Am. Chem. Soc. 139, 14542–14555 (2017).
Campbell, M. A. & Wengel, J. Locked vs. unlocked nucleic acids (LNA vs. UNA): contrasting structures work towards common therapeutic goals. Chem. Soc. Rev. 40, 5680–5689 (2011).
Langkjær, N., Pasternak, A. & Wengel, J. UNA (unlocked nucleic acid): a flexible RNA mimic that allows engineering of nucleic acid duplex stability. Bioorg. Med. Chem. 17, 5420–5425 (2009).
Fluiter, K. et al. Filling the gap in LNA antisense oligo gapmers: the effects of unlocked nucleic acid (UNA) and 4′-C-hydroxymethyl-DNA modifications on RNase H recruitment and efficacy of an LNA gapmer. Mol. Biosyst. 5, 838–843 (2009).
Trempe, J.-F. et al. NMR solution structure of an oligonucleotide hairpin with a 2′F-ANA/RNA stem: implications for RNase H specificity toward DNA/RNA hybrid duplexes. J. Am. Chem. Soc. 123, 4896–4903 (2001).
Berger, I., Tereshko, V., Ikeda, H., Marquez, V. E. & Egli, M. Crystal structures of B-DNA with incorporated 2′-deoxy-2′-fluoro-arabino-furanosyl thymines: implications of conformational preorganization for duplex stability. Nucleic Acids Res. 26, 2473–2480 (1998).
Anzahaee, M. Y., Watts, J. K., Alla, N. R., Nicholson, A. W. & Damha, M. J. Energetically important C–H···F–C pseudohydrogen bonding in water: evidence and application to rational design of oligonucleotides with high binding affinity. J. Am. Chem. Soc. 133, 728–731 (2011).
O’Reilly, D. et al. Exploring atypical fluorine–hydrogen bonds and their effects on nucleoside conformations. Chem. Eur. J. 24, 16432–16439 (2018).
Lok C.N. et al. Potent gene-specific inhibitory properties of mixed-backbone antisense oligonucleotides comprised of 2′-deoxy-2′-fluoro-D-arabinose and 2′-deoxyribose nucleotides. Biochemistry 41, 3457–3467 (2002).
Kalota, A. et al. 2′-Deoxy-2′-fluoro-β-D-arabinonucleic acid (2′F-ANA) modified oligonucleotides (ON) effect highly efficient, and persistent, gene silencing. Nucleic Acids Res. 34, 451–461 (2006).
Dowler, T. et al. Improvements in siRNA properties mediated by 2′-deoxy-2′-fluoro-β-D-arabinonucleic acid (FANA). Nucleic Acids Res. 34, 1669–1675 (2006).
Alves Ferreira-Bravo, I., Cozens, C., Holliger, P. & DeStefano, J. J. Selection of 2′-deoxy-2′-fluoroarabinonucleotide (FANA) aptamers that bind HIV-1 reverse transcriptase with picomolar affinity. Nucleic Acids Res. 43, 9587–9599 (2015).
Lietard, J. et al. Mapping the affinity landscape of thrombin-binding aptamers on 2′F-ANA/DNA chimeric G-quadruplex microarrays. Nucleic Acids Res. 45, 1619–1632 (2017).
Aurup, H., Tuschl, T., Benseler, F., Ludwig, J. & Eckstein, F. Oligonucleotide duplexes containing 2′-amino-2′-deoxycytidines: thermal stability and chemical reactivity. Nucleic Acids Res. 22, 20–24 (1994).
Jana, S. K. et al. Nucleoside analogues with a seven-membered sugar ring: synthesis and structural compatibility in DNA–RNA hybrids. J. Org. Chem. 87, 2367–2379 (2022).
Sheehan, D. et al. Biochemical properties of phosphonoacetate and thiophosphonoacetate oligodeoxyribonucleotides. Nucleic Acids Res. 31, 4109–4118 (2003).
Acknowledgements
We acknowledge funding from the National Institutes of Health (1R01GM135646-01) to K.T.G. and the National Science and Engineering Council of Canada (NSERC) Discovery Grant #2022-03372 to M.J.D. Additionally, H.M.B. and M.J.D. are members of the Centre de recherche en biologie structurale, funded by Fonds de Recherche du Québec (Health Sector) Research Centres Grant #288558 and H.M.B. received graduate funding support from Fonds de Recherche du Québec - Nature et Technologies doctoral scholarship #332295 and the NSERC CREATE Programmed Molecules for Therapeutics, Sensing, and Diagnostics (PROMOTE) training program.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching the literature, discussing the article’s content, preparing figures, and writing and/or substantial editing.
Corresponding authors
Ethics declarations
Competing interests
There are no competing interests to declare.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Michael Rosenberg, Ayal Hendel, Laura Sepp-Lorenzino, Sabin Mulepati and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- 2′-OH contacts
-
Hydrogen bonding interactions between the 2′-hydroxyl (2′-OH) groups of the gRNA nucleotides and the Cas amino acids, as well as between nucleotides.
- Base editors
-
(BEs). Systems consisting of a dCas9 fused to either a cytidine deaminase, for cytosine base editors, or an adenosine deaminase, for adenine base editors, which allow for the precise conversion of specific DNA bases (such as, C to T or A to G) without double-strand breaks.
- Chemical modification
-
Either a naturally occurring or synthetic modification to the phosphate, sugar or nucleobase of nucleotides.
- Click chemistry
-
A biocompatible chemical reaction that allows for efficient and specific joining of two biomolecules.
- CRISPR RNA
-
(crRNA). An RNA molecule composed of a spacer-derived guide region and a repeat-derived trans-activating CRISPR RNA-pairing or 5′ handle region.
- dCas9
-
A Cas9 protein with mutations in both nuclease domains (RuvC and HNH), resulting in a catalytically inactive enzyme still capable of binding to DNA via guide RNA.
- Double-strand breaks
-
(DSBs). DNA damage types in which both strands of the DNA double helix are cleaved, forming either a blunt or staggered break.
- Ex vivo gene editing
-
A therapeutic approach in which patient cells are isolated, edited outside of the body and subsequently reintroduced back into the patient.
- Guide RNA
-
(gRNA). A generalized term for an RNA molecule that directs the CRISPR–Cas effector enzyme to a specific DNA sequence through complementary base pairing.
- Homology-directed repair
-
(HDR) A precise DNA repair pathway guided by a homologous DNA template to repair double-strand breaks.
- In vivo gene editing
-
A therapeutic approach in which the gene editing components are delivered directly into a patient via local or systemic delivery.
- Lipid nanoparticle
-
(LNP). A vesicle composed of lipid moieties used to encapsulate nucleic acids or proteins and deliver these therapeutic agents into cells.
- nCas9
-
A Cas9 protein with either the RuvC or HNH catalytic domain mutated, resulting in a Cas9 that can introduce a single-strand break (that is, a nick) into DNA by cleaving only one DNA strand.
- Non-homologous end joining
-
(NHEJ). A DNA repair pathway in which double-strand breaks are repaired by directly joining the ends, often resulting in insertions or deletions at the site.
- Non-target strand
-
The DNA strand opposite the target strand that contains the protospacer adjacent motif and remains unbound during CRISPR–Cas-mediated cleavage.
- Off-target effects
-
Unintended cleavages or modifications of DNA at sites other than the intended target sequence.
- Prime editing guide RNA
-
(pegRNA). An RNA molecule used in prime editing that combines the properties of a gRNA, a reverse transcriptase template and a template sequence encoding the desired edit to direct the fusion protein to the target DNA site.
- Prime editors
-
Systems consisting of an nCas9 fused to a reverse transcriptase enzyme and guided by a pegRNA to enable precise edits without double-strand breaks.
- Protospacer adjacent motif
-
(PAM). A short, specific DNA sequence adjacent to the target site that is essential for the recognition and binding of the Cas enzyme.
- Ribonucleoprotein (RNP) complex
-
A complex composed of both RNA and protein.
- R-loop
-
A nucleic acid structure that forms when an RNA strand binds to one strand of a DNA double helix, creating an RNA:DNA hybrid duplex and displacing the other DNA strand.
- Seed region
-
The first 5–10 nucleotides directly adjacent to the PAM that initiate base pairing between the gRNA and the target strand.
- Single-guide RNA
-
(sgRNA). An RNA molecule created by fusing the Cas9 crRNA and trans-activating CRISPR RNA into a single construct.
- Sugar pucker conformations
-
Conformations of the ribose ring in a nucleotide, with C2′-endo corresponding to DNA-like sugars with B-form helical structures and C3′-endo corresponding to RNA-like sugars with A-form helical structures.
- Target strand
-
The DNA strand that is complementary to the gRNA.
- Trans-activating CRISPR RNA
-
(tracrRNA). An RNA molecule that hybridizes to the crRNA and anchors the gRNA construct to Cas9 through its stem loop structures.
- Transcriptional activation
-
(CRISPRa). A technique using dCas9 fused to transcriptional activation domains and directed by a gRNA to specific genomic loci to upregulate gene expression.
- Transcriptional interference
-
(CRISPRi). A method using dCas9 fused to repressor domains to repress gene expression when guided to specific genomic loci by a gRNA.
- Viral vectors
-
Modified viruses used to deliver nucleic acids into cells for therapeutic purposes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barber, H.M., Pater, A.A., Gagnon, K.T. et al. Chemical engineering of CRISPR–Cas systems for therapeutic application. Nat Rev Drug Discov 24, 209–230 (2025). https://doi.org/10.1038/s41573-024-01086-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-024-01086-0
This article is cited by
-
Application and optimization of bioengineering strategies in facilitating tendon–bone healing
BioMedical Engineering OnLine (2025)
-
RNA chemistry and therapeutics
Nature Reviews Drug Discovery (2025)