Abstract
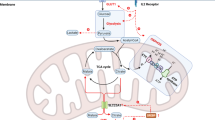
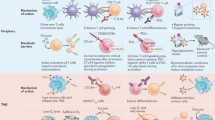
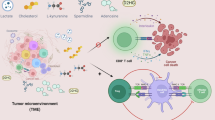
The importance of metabolic pathways in regulating immune responses is now well established, and a mapping of the bioenergetic metabolism of different immune cell types is under way. CD8 T cells and natural killer (NK) cells contribute to cancer immunosurveillance through their cytotoxic functions and secretion of cytokines and chemokines, complementing each other in target recognition mechanisms. Several immunotherapies leverage these cell types by either stimulating their activity or redirecting their specificity against tumour cells. However, the anticancer activity of CD8 T cells and NK cells is rapidly diminished in the tumour microenvironment, closely linked to a decline in their metabolic capacities. Various strategies have been developed to restore cancer immunosurveillance, including targeting bioenergetic metabolism or genetic engineering. This Review provides an overview of metabolic dysfunction in CD8 T cells and NK cells within the tumour microenvironment, highlighting current therapies aiming to overcome these issues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bruni, D., Angell, H. K. & Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 20, 662–680 (2020).
Kishton, R. J., Sukumar, M. & Restifo, N. P. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 26, 94–109 (2017).
Chiossone, L., Dumas, P.-Y., Vienne, M. & Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 18, 671 (2018).
Huntington, N. D., Cursons, J. & Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 20, 437–454 (2020).
Wolf, N. K., Kissiov, D. U. & Raulet, D. H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 23, 90–105 (2023).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Vivier, E. et al. Natural killer cell therapies. Nature 626, 727–736 (2024).
O’Brien, K. L. & Finlay, D. K. Immunometabolism and natural killer cell responses. Nat. Rev. Immunol. 19, 282–290 (2019).
Pearce, E. L. & Pearce, E. J. Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643 (2013).
van de Windt, G. J. W. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Makowski, L., Chaib, M. & Rathmell, J. C. Immunometabolism: from basic mechanisms to translation. Immunol. Rev. 295, 5–14 (2020).
Mattiola, I. & Diefenbach, A. Regulation of innate immune system function by the microbiome: consequences for tumor immunity and cancer immunotherapy. Semin. Immunol. 66, 101724 (2023).
Matson, V., Chervin, C. S. & Gajewski, T. F. Cancer and the microbiome-influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology 160, 600–613 (2021).
Bell, H. N. & Zou, W. Beyond the barrier: unraveling the mechanisms of immunotherapy resistance. Annu. Rev. Immunol. 42, 521–550 (2024).
Drijvers, J. M., Sharpe, A. H. & Haigis, M. C. The effects of age and systemic metabolism on anti-tumor T cell responses. eLife 9, e62420 (2020).
Møller, S. H., Hsueh, P.-C., Yu, Y.-R., Zhang, L. & Ho, P.-C. Metabolic programs tailor T cell immunity in viral infection, cancer, and aging. Cell Metab. 34, 378–395 (2022).
Warburg, O., Gawehn, K. & Geissler, A. W. Metabolism of leukocytes. Z. Naturforsch. B 13B, 515–516 (1958).
Hedeskov, C. J. Early effects of phytohaemagglutinin on glucose metabolism of normal human lymphocytes. Biochem. J. 110, 373–380 (1968).
Roos, D. & Loos, J. A. Changes in the carbohydrate metabolism of mitogenically stimulated human peripheral lymphocytes: II. Relative importance of glycolysis and oxidative phosphorylation on phytohaemagglutinin stimulation. Exp. Cell Res. 77, 127–135 (1973).
Wang, T., Marquardt, C. & Foker, J. Aerobic glycolysis during lymphocyte proliferation. Nature 261, 702–705 (1976).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002). This study tied, for the first time, a signalling pathway primarily involved in T cell activation to cell metabolism regulation.
Rathmell, J. C., Farkash, E. A., Gao, W. & Thompson, C. B. IL-7 enhances the survival and maintains the size of naive T cells. J. Immunol. 167, 6869–6876 (2001).
Rathmell, J. C., Elstrom, R. L., Cinalli, R. M. & Thompson, C. B. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur. J. Immunol. 33, 2223–2232 (2003).
Powell, J. D. & Delgoffe, G. M. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33, 301–311 (2010).
Tan, H. et al. Integrative proteomics and phosphoproteomics profiling reveals dynamic signaling networks and bioenergetics pathways underlying T cell activation. Immunity 46, 488–503 (2017).
Brennan, P., Babbage, J. W., Thomas, G. & Cantrell, D. p70(s6k) integrates phosphatidylinositol 3-kinase and rapamycin-regulated signals for E2F regulation in T lymphocytes. Mol. Cell. Biol. 19, 4729–4738 (1999).
Yang, K. et al. T cell exit from quiescence and differentiation into Th2 cells depend on raptor-mTORC1-mediated metabolic reprogramming. Immunity 39, 1043–1056 (2013).
Ben-Sahra, I. & Manning, B. D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 45, 72–82 (2017).
Wang, R. et al. The transcription factor myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Finlay, D. K. et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209, 2441–2453 (2012).
Fu, H. et al. The glucose transporter 2 regulates CD8+ T cell function via environment sensing. Nat. Metab. 5, 1969–1985 (2023).
Menk, A. V. et al. Early TCR signaling induces rapid aerobic glycolysis enabling distinct acute T cell effector functions. Cell Rep. 22, 1509–1521 (2018). This study describes a direct link between the proximal kinase Zap70 and the activity of PDH.
DeBerardinis, R. J. & Chandel, N. S. We need to talk about the Warburg effect. Nat. Metab. 2, 127–129 (2020).
Chang, C.-H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013). This study demonstrates that a glycolytic enzyme, GAPDH, moonlights and regulates IFNγ production, thus impacting CD8 T cell functions.
Xu, K. et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science 371, 405–410 (2021).
Bantug, G. R. et al. Mitochondria-endoplasmic reticulum contact sites function as immunometabolic hubs that orchestrate the rapid recall response of memory CD8+ T cells. Immunity 48, 542–555.e6 (2018).
Wang, Y. et al. Saturation of the mitochondrial NADH shuttles drives aerobic glycolysis in proliferating cells. Mol. Cell 82, 3270–3283.e9 (2022).
Kong, H. & Chandel, N. S. Regulation of redox balance in cancer and T cells. J. Biol. Chem. 293, 7499–7507 (2018).
Klein Geltink, R. I. et al. Mitochondrial priming by CD28. Cell 171, 385–397.e11 (2017).
Buck, M. D. et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166, 63–76 (2016).
Fischer, M. et al. Early effector maturation of naïve human CD8+ T cells requires mitochondrial biogenesis. Eur. J. Immunol. 48, 1632–1643 (2018).
Mehta, M. M., Weinberg, S. E. & Chandel, N. S. Mitochondrial control of immunity: beyond ATP. Nat. Rev. Immunol. 17, 608–620 (2017).
Birsoy, K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Ben-Sahra, I., Howell, J. J., Asara, J. M. & Manning, B. D. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328 (2013).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013). This study describes a role of mitochondria beyond ATP generation and the requirement for reactive oxygen species in T cell activation.
Reid, M. A., Dai, Z. & Locasale, J. W. The impact of cellular metabolism on chromatin dynamics and epigenetics. Nat. Cell Biol. 19, 1298–1306 (2017).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Odorizzi, P. M., Pauken, K. E., Paley, M. A., Sharpe, A. & Wherry, E. J. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1237 (2015).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Gubser, P. M. et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol. 14, 1064–1072 (2013).
Ma, E. H. et al. Metabolic profiling using stable isotope tracing reveals distinct patterns of glucose utilization by physiologically activated CD8+ T cells. Immunity 51, 856–870.e5 (2019). This study uses in vivo metabolic labelling to quantify the outcome of glucose-derived carbons and shows that lactate excretion is less prevalent than in vitro. The characterization of the metabolic pathways actually used in an in vivo response is continued in ref. 54.
Luda, K. M. et al. Ketolysis drives CD8+ T cell effector function through effects on histone acetylation. Immunity 56, 2021–2035.e8 (2023).
Kaymak, I. et al. Carbon source availability drives nutrient utilization in CD8+ T cells. Cell Metab. 34, 1298–1311.e6 (2022).
Cantor, J. R. et al. Physiologic medium rewires cellular metabolism and reveals uric acid as an endogenous inhibitor of UMP synthase. Cell 169, 258–272.e17 (2017). This study describes the generation of a medium recapitulating the composition of human plasma and the impact this has on a number of metabolic processes.
Sinclair, L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013).
Howden, A. J. M. et al. Quantitative analysis of T cell proteomes and environmental sensors during T cell differentiation. Nat. Immunol. 20, 1542–1554 (2019).
Huang, H. et al. In vivo CRISPR screening reveals nutrient signaling processes underpinning CD8+ T cell fate decisions. Cell 184, 1245–1261.e21 (2021).
Minogue, E. et al. Glutarate regulates T cell metabolism and anti-tumour immunity. Nat. Metab. 5, 1747–1764 (2023).
Ma, E. H. et al. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25, 345–357 (2017).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Quinn, W. J. et al. Lactate limits T cell proliferation via the NAD(H) redox state. Cell Rep. 33, 108500 (2020).
Ye, J. et al. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl Acad. Sci. USA 109, 6904–6909 (2012).
Chaneton, B. et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491, 458–462 (2012).
Gnanaprakasam, J. N. R. et al. Asparagine restriction enhances CD8+ T cell metabolic fitness and antitumoral functionality through an NRF2-dependent stress response. Nat. Metab. 5, 1423–1439 (2023).
Geiger, R. et al. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016).
Raud, B. et al. Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. Cell Metab. 28, 504–515.e7 (2018).
Pan, Y. et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–256 (2017).
Sheppard, S. et al. Fatty acid oxidation fuels natural killer cell responses against infection and cancer. Proc. Natl Acad. Sci. USA 121, e2319254121 (2024).
Raynor, J. L., Chapman, N. M. & Chi, H. Metabolic control of memory T-cell generation and stemness. Cold Spring Harb. Perspect. Biol. 13, a037770 (2021).
Hudson, W. H. et al. Proliferating transitory T cells with an effector-like transcriptional signature emerge from PD-1+ stem-like CD8+ T cells during chronic infection. Immunity 51, 1043–1058.e4 (2019).
Zhou, P. et al. Single-cell CRISPR screens in vivo map T cell fate regulomes in cancer. Nature 624, 154–163 (2023).
Ando, S. et al. mTOR regulates T cell exhaustion and PD-1 targeted immunotherapy response during chronic viral infection. J. Clin. Invest. 133, e160025 (2022).
Marçais, A. et al. High mTOR activity is a hallmark of reactive natural killer cells and amplifies early signaling through activating receptors. eLife. Sciences 6, e26423 (2017).
Keating, S. E. et al. Metabolic reprogramming supports IFN-γ production by CD56bright NK cells. J. Immunol. 196, 2552–2560 (2016).
Marçais, A. et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat. Immunol. 15, 749–757 (2014). This study describes the non-redundant role of the kinase mTOR in murine NK cells during differentiation and cytokine activation.
Keppel, M. P., Saucier, N., Mah, A. Y., Vogel, T. P. & Cooper, M. A. Activation-specific metabolic requirements for NK cell IFN-γ production. J. Immunol. 194, 1954–1962 (2015). This study dissects the fuel requirement of resting murine NK cells to sustain effector functions in response to a number of stimuli.
Picard, L. K., Littwitz-Salomon, E., Waldmann, H. & Watzl, C. Inhibition of glucose uptake blocks proliferation but not cytotoxic activity of NK cells. Cells 11, 3489 (2022).
Kern, N. et al. Pivotal role of exogenous pyruvate in human Natural Killer cell metabolism. Nat. Metab. https://doi.org/10.1038/s42255-024-01188-4 (in the press).
Romee, R. et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8, 357ra123 (2016).
Donnelly, R. P. et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J. Immunol. 93, 4477–4784 (2014).
Mah-Som, A. Y. et al. Reliance on Cox10 and oxidative metabolism for antigen-specific NK cell expansion. Cell Rep. 35, 109209 (2021).
Mah, A. Y. et al. Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight 2, e95128 (2017).
Sheppard, S. et al. Lactate dehydrogenase A-dependent aerobic glycolysis promotes natural killer cell anti-viral and anti-tumor function. Cell Rep. 35, 109210 (2021).
Schimmer, S. et al. Fatty acids are crucial to fuel NK cells upon acute retrovirus infection. Front. Immunol. 14, 1296355 (2023).
Ganz, T. & Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 15, 500–510 (2015).
Santosa, E. K. et al. Control of nutrient uptake by IRF4 orchestrates innate immune memory. Nat. Immunol. 24, 1685–1697 (2023).
Littwitz-Salomon, E. et al. Metabolic requirements of NK cells during the acute response against retroviral infection. Nat. Commun. 12, 5376 (2021).
Assmann, N. et al. Srebp-controlled glucose metabolism is essential for NK cell functional responses. Nat. Immunol. 18, 1197–1206 (2017). This study, by making use of metabolic labelling in activated murine NK cells, demonstrates that the TCA cycle is in a specific configuration that involves a shuttle between citrate and malate and how this conditions effector functions.
Loftus, R. M. et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 9, 2341 (2018).
Li, J. H. et al. MEF2C regulates NK cell effector functions through control of lipid metabolism. Nat. Immunol. 25, 778–789 (2024).
Castro, W. et al. The transcription factor Rfx7 limits metabolism of NK cells and promotes their maintenance and immunity. Nat. Immunol. 19, 809–820 (2018).
Lee, J. E. et al. Aetyl CoA carboxylase 2 is dispensable for CD8+ T cell responses. PLoS One 10, e0137776 (2015).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Scharping, N. E. et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45, 374–388 (2016). This study is an early report of mitochondrial dysfunction in tumour-infiltrating CD8 T cells and how the correction of the mitochondrial defect restores effector potential.
Wu, H. et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat. Commun. 14, 6858 (2023).
Schurich, A. et al. Distinct metabolic requirements of exhausted and functional virus-specific CD8 T cells in the same host. Cell Rep. 16, 1243–1252 (2016).
Fisicaro, P. et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat. Med. 23, 327–336 (2017).
Vardhana, S. A. et al. Impaired mitochondrial oxidative phosphorylation limits the self-renewal of T cells exposed to persistent antigen. Nat. Immunol. 21, 1022–1033 (2020). This study shows that repeated TCR stimulation leads to impairment of OxPhos that in turn negatively affects cell proliferation. Conversely, preventing mitochondrial oxidative stress rescues proliferation.
Yu, Y.-R. et al. Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell exhaustion. Nat. Immunol. 21, 1540–1551 (2020).
Bengsch, B. et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8+ T cell exhaustion. Immunity 45, 358–373 (2016).
Taylor, C. T. & Scholz, C. C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 18, 573–587 (2022).
Miska, J. et al. HIF-1α is a metabolic switch between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression of Tregs in glioblastoma. Cell Rep. 27, 226–237.e4 (2019).
Gemta, L. F. et al. Impaired enolase 1 glycolytic activity restrains effector functions of tumor-infiltrating CD8+ T cells. Sci. Immunol. 4, eaap9520 (2019).
Siska, P. J. et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2, e93411 (2017).
Mamessier, E. et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Invest. 121, 3609–3622 (2011).
Platonova, S. et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 71, 5412–5422 (2011).
Rocca, Y. S. et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 19, 76–85 (2013).
Bi, J. & Tian, Z. NK cell exhaustion. Front. Immunol. 8, 760 (2017).
Kirschenbaum, D. et al. Time-resolved single-cell transcriptomics defines immune trajectories in glioblastoma. Cell 187, 149–165.e23 (2024).
Pouxvielh, K. et al. Tumor-induced natural killer cell dysfunction is a rapid and reversible process uncoupled from the expression of immune checkpoints. Sci. Adv. 10, eadn0164 (2024).
Merino, A. M. et al. Chronic stimulation drives human NK cell dysfunction and epigenetic reprograming. J. Clin. Invest. 129, 3770–3785 (2019).
Dean, I. et al. Rapid functional impairment of natural killer cells following tumor entry limits anti-tumor immunity. Nat. Commun. 15, 683 (2024).
Zheng, X. et al. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat. Immunol. 20, 1656–1667 (2019).
Poznanski, S. M. et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. 33, 1205–1220.e5 (2021).
Slattery, K. et al. TGFβ drives NK cell metabolic dysfunction in human metastatic breast cancer. J. Immunother. Cancer 9, e002044 (2021).
Sullivan, M. R. et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. eLife 8, e44235 (2019).
Reznik, E. et al. A landscape of metabolic variation across tumor types. Cells 6, 301–313.e3 (2018).
Xiao, Z., Dai, Z. & Locasale, J. W. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat. Commun. 10, 3763 (2019).
Liu, X., Hoft, D. F. & Peng, G. Tumor microenvironment metabolites directing T cell differentiation and function. Trends Immunol. 43, 132–147 (2022).
Raynor, J. L. & Chi, H. Nutrients: signal 4 in T cell immunity. J. Exp. Med. 221, e20221839 (2024).
Chang, C.-H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015). This study describes the metabolic competition taking place in the tumour environment and how this is linked to tumour progression.
Ho, P.-C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015). This study describes one example of a bioenergetic metabolite also acting as an immune modulator.
Mazzoni, A. et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695 (2002).
Terness, P. et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 196, 447–457 (2002).
Gropper, Y. et al. Culturing CTLs under hypoxic conditions enhances their cytolysis and improves their anti-tumor function. Cell Rep. 20, 2547–2555 (2017).
Scharping, N. E. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat. Immunol. 22, 205–215 (2021).
Yang, Y. et al. Thioredoxin activity confers resistance against oxidative stress in tumor-infiltrating NK cells. J. Clin. Invest. 130, 5508–5522 (2020).
Corcoran, S. E. & O’Neill, L. A. J. HIF1α and metabolic reprogramming in inflammation. J. Clin. Invest. 126, 3699–3707 (2016).
Hamanaka, R. B. & Chandel, N. S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 35, 505–513 (2010).
Ni, J. et al. Single-cell RNA sequencing of tumor-infiltrating NK cells reveals that inhibition of transcription factor HIF-1α unleashes NK cell activity. Immunity 52, 1075–1087.e8 (2020). This study describes the counter-intuitive finding that HIF1α is detrimental in NK cells infiltrating solid tumours.
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Elia, I. et al. Tumor cells dictate anti-tumor immune responses by altering pyruvate utilization and succinate signaling in CD8+ T cells. Cell Metab. 34, 1137–1150.e6 (2022).
Kumagai, S. et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell 40, 201–218.e9 (2022).
Certo, M., Tsai, C.-H., Pucino, V., Ho, P.-C. & Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021).
Haas, R. et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 13, e1002202 (2015).
Fischer, K. et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 (2007).
Calcinotto, A. et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 72, 2746–2756 (2012).
Tsai, Y.-L. et al. TCR signaling promotes formation of an STS1-Cbl-b complex with pH-sensitive phosphatase activity that suppresses T cell function in acidic environments. Immunity 56, 2682–2698.e9 (2023).
Tsai, C.-H. et al. Immunoediting instructs tumor metabolic reprogramming to support immune evasion. Cell Metab. 35, 118–133.e7 (2023).
Harmon, C. et al. Lactate-mediated acidification of tumor microenvironment induces apoptosis of liver-resident NK cells in colorectal liver metastasis. Cancer Immunol. Res. 7, 335–346 (2019).
Dodard, G. et al. Inflammation-induced lactate leads to rapid loss of hepatic tissue-resident NK cells. Cell Rep. 32, 107855 (2020).
Hui, S. et al. Glucose feeds the TCA cycle via circulating lactate. Nature 551, 115–118 (2017).
Watson, M. J. et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature 591, 645–651 (2021).
Angelin, A. et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293.e7 (2017).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017).
Feng, Q. et al. Lactate increases stemness of CD8+ T cells to augment anti-tumor immunity. Nat. Commun. 13, 4981 (2022). This study describes the positive role of lactate on CD8 T cells by disentangling the impact of acidification and lactate itself.
Parks, S. K., Mueller-Klieser, W. & Pouysségur, J. Lactate and acidity in the cancer microenvironment. Annu. Rev. Cancer Biol. 4, 141–158 (2020).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Figueroa, M. E. et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 18, 553–567 (2010).
Bunse, L. et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 24, 1192–1203 (2018).
Notarangelo, G. et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science 377, 1519–1529 (2022).
Tyrakis, P. A. et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature 540, 236–241 (2016).
Allard, B., Longhi, M. S., Robson, S. C. & Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 276, 121–144 (2017).
Schneider, E. et al. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat. Commun. 12, 5911 (2021).
Neo, S. Y. et al. CD73 immune checkpoint defines regulatory NK cells within the tumor microenvironment. J. Clin. Invest. 130, 1185–1198 (2020).
Allard, B., Allard, D., Buisseret, L. & Stagg, J. The adenosine pathway in immuno-oncology. Nat. Rev. Clin. Oncol. 17, 611–629 (2020).
Todd, K. L. et al. A2AR eGFP reporter mouse enables elucidation of A2AR expression dynamics during anti-tumor immune responses. Nat. Commun. 14, 6990 (2023).
Vigano, S. et al. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front. Immunol. 10, 925 (2019).
Young, A. et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 78, 1003–1016 (2018).
Perrot, I. et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 27, 2411–2425.e9 (2019).
McPhedran, S. J., Carleton, G. A. & Lum, J. J. Metabolic engineering for optimized CAR-T cell therapy. Nat. Metab. 6, 396–408 (2024).
Liu, X., Hoft, D. F. & Peng, G. Tumor microenvironment metabolites directing T cell differentiation and function. Trends Immunol. 43, 132–147 (2021).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018).
Manzo, T. et al. Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J. Exp. Med. 217, e20191920 (2020).
Ma, X. et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 33, 1001–1012.e5 (2021).
Nava Lauson, C. B. et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 35, 633–650.e9 (2023).
Ma, X. et al. Cholesterol induces CD8+ T cell exhaustion in the tumor microenvironment. Cell Metab. 30, 143–156.e5 (2019).
Xu, S. et al. Uptake of oxidized lipids by the scavenger receptor CD36 promotes lipid peroxidation and dysfunction in CD8+ T cells in tumors. Immunity 54, 1561–1577.e7 (2021).
Rostamian, H. et al. Restricting tumor lactic acid metabolism using dichloroacetate improves T cell functions. BMC Cancer 22, 39 (2022).
Belkahla, S. et al. The metabolism of cells regulates their sensitivity to NK cells depending on p53 status. Sci. Rep. 12, 3234 (2022).
Mangalhara, K. C. et al. Manipulating mitochondrial electron flow enhances tumor immunogenicity. Science 381, 1316–1323 (2023).
Triplett, T. A. et al. Reversal of indoleamine 2,3-dioxygenase-mediated cancer immune suppression by systemic kynurenine depletion with a therapeutic enzyme. Nat. Biotechnol. 36, 758–764 (2018).
Platten, M., Nollen, E. A. A., Röhrig, U. F., Fallarino, F. & Opitz, C. A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 18, 379–401 (2019).
Beloueche-Babari, M. et al. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br. J. Cancer 122, 895–903 (2020).
Klysz, D. D. et al. Inosine induces stemness features in CAR-T cells and enhances potency. Cancer Cell 42, 266–282.e8 (2024).
Giuffrida, L. et al. CRISPR/Cas9 mediated deletion of the adenosine A2A receptor enhances CAR T cell efficacy. Nat. Commun. 12, 3236 (2021).
Tieu, V. et al. A versatile CRISPR-Cas13d platform for multiplexed transcriptomic regulation and metabolic engineering in primary human T cells. Cell https://doi.org/10.1016/j.cell.2024.01.035 (2024). This study demonstrates that metabolic engineering of CAR T cells using a cutting-edge CRISPR-based platform leads to better antitumour functions.
Chambers, A. M. et al. Engineered natural killer cells impede the immunometabolic CD73-adenosine axis in solid tumors. eLife 11, e73699 (2022).
Ma, J. et al. Lithium carbonate revitalizes tumor-reactive CD8+ T cells by shunting lactic acid into mitochondria. Nat. Immunol. 25, 552–561 (2024).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019). This study shows that tumour cells are more dependent on glutamine than effector T cells in a mouse model of colon cancer upon in vivo targeting of glutamine usage; a previously undefined specificity of tumour cells.
Oh, M.-H. et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J. Clin. Invest. 130, 3865–3884 (2020).
Edwards, D. N. et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Invest. 131, e140100 (2021).
Byun, J.-K. et al. Inhibition of glutamine utilization synergizes with immune checkpoint inhibitor to promote antitumor immunity. Mol. Cell 80, 592–606.e8 (2020).
Nabe, S. et al. Reinforce the antitumor activity of CD8+ T cells via glutamine restriction. Cancer Sci. 109, 3737–3750 (2018).
Maia, A., Tarannum, M. & Romee, R. Genetic manipulation approaches to enhance the clinical application of NK cell-based immunotherapy. Stem Cell Transl. Med. 13, 230–242 (2024).
Sadelain, M., Rivière, I. & Riddell, S. Therapeutic T cell engineering. Nature 545, 423–431 (2017).
Felices, M. et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 3, e96219 (2018).
Klebanoff, C. A. et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight 2, e95103 (2017). This study presents the unexpected finding that inhibition of the PI3K–AKT pathway in in vitro expanded T or CAR T cells, leads to a better in vivo anti-tumour response upon reinfusion without affecting cell yield.
Crompton, J. G. et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 75, 296–305 (2015).
Langdon, S. et al. Combination of dual mTORC1/2 inhibition and immune-checkpoint blockade potentiates anti-tumour immunity. OncoImmunology 7, e1458810 (2018).
Carnevalli, L. S. et al. PI3Kα/δ inhibition promotes anti-tumor immunity through direct enhancement of effector CD8+ T-cell activity. J. Immunother. Cancer 6, 158 (2018).
Urak, R. et al. Ex vivo Akt inhibition promotes the generation of potent CD19CAR T cells for adoptive immunotherapy. J. Immunother. Cancer 5, 26 (2017).
Zheng, W. et al. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia 32, 1157–1167 (2018).
Suzuki, J. et al. The tumor suppressor menin prevents effector CD8 T-cell dysfunction by targeting mTORC1-dependent metabolic activation. Nat. Commun. 9, 3296 (2018).
Besson, L. et al. Cutting edge: mTORC1 inhibition in metastatic breast cancer patients negatively affects peripheral NK cell maturation and number. J. Immunol. 206, 2265–2270 (2021).
Crist, M. et al. Metformin increases natural killer cell functions in head and neck squamous cell carcinoma through CXCL1 inhibition. J. Immunother. Cancer 10, e005632 (2022).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372, eaba1786 (2021).
Li, Y. et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 28, 2133–2144 (2022).
Hu-Lieskovan, S. et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci. Transl. Med. 7, 279ra41 (2015).
Ebert, P. J. R. et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44, 609–621 (2016).
Liu, L. et al. The BRAF and MEK inhibitors dabrafenib and trametinib: effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin. Cancer Res. 21, 1639–1651 (2015).
Verma, V. et al. MEK inhibition reprograms CD8+ T lymphocytes into memory stem cells with potent antitumor effects. Nat. Immunol. 22, 53–66 (2020).
Sukumar, M. et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 123, 4479–4488 (2013).
Klein Geltink, R. I. et al. Metabolic conditioning of CD8+ effector T cells for adoptive cell therapy. Nat. Metab. 2, 703–716 (2020). This study shows that a transient glucose restriction in the culture medium metabolically primes their effector functions and enhances tumour clearance upon in vivo reinfusion.
MacPherson, S. et al. Clinically relevant T cell expansion media activate distinct metabolic programs uncoupled from cellular function. Mol. Ther. Methods Clin. Dev. 24, 380–393 (2022).
Hermans, D. et al. Lactate dehydrogenase inhibition synergizes with IL-21 to promote CD8+ T cell stemness and antitumor immunity. Proc. Natl Acad. Sci. USA 117, 6047–6055 (2020).
Li, C. et al. The transcription factor Bhlhe40 programs mitochondrial regulation of resident CD8+ T cell fitness and functionality. Immunity 51, 491–507.e7 (2019).
Lontos, K. et al. Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors. J. Immunother. Cancer 11, e006522 (2023).
Dumauthioz, N. et al. Enforced PGC-1α expression promotes CD8 T cell fitness, memory formation and antitumor immunity. Cell Mol. Immunol. 18, 1761–1771 (2021).
Wu, M.-H. et al. Deleting the mitochondrial respiration negative regulator MCJ enhances the efficacy of CD8+ T cell adoptive therapies in pre-clinical studies. Nat. Commun. 15, 4444 (2024).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Menk, A. V. et al. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J. Exp. Med. 215, 1091–1100 (2018).
Frisch, A., Wang, Y., Wang, Y., Lontos, K. & Delgoffe, G. 330 Redirecting glucose usage during in vitro expansion improves the in vivo persistence and function of adoptive T cell therapies for cancer. J. Immunother. Cancer 10, A347 (2022).
Zhang, Y. et al. Enhancing CD8+ T cell fatty acid catabolism within a metabolically challenging tumor microenvironment increases the efficacy of melanoma immunotherapy. Cancer Cell 32, 377–391.e9 (2017). This study shows that hypoxia and glucose restriction induce a metabolic rewiring of CD8 T cells towards FAO to preserve functions; mimicking this rewiring using a PPARα agonist improves response to PD1 blockade.
Wenes, M. et al. The mitochondrial pyruvate carrier regulates memory T cell differentiation and antitumor function. Cell Metab. 34, 731–746.e9 (2022).
Chamoto, K. et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc. Natl Acad. Sci. USA 114, E761–E770 (2017).
Kondo, T. et al. The NOTCH-FOXM1 axis plays a key role in mitochondrial biogenesis in the induction of human stem cell memory-like CAR-T cells. Cancer Res. 80, 471–483 (2020).
Alizadeh, D. et al. IL15 enhances CAR-T cell antitumor activity by reducing mTORC1 activity and preserving their stem cell memory phenotype. Cancer Immunol. Res. 7, 759–772 (2019).
Shenoy, A. R., Kirschnek, S. & Häcker, G. IL-15 regulates Bcl-2 family members Bim and Mcl-1 through JAK/STAT and PI3K/AKT pathways in T cells. Eur. J. Immunol. 44, 2500–2507 (2014).
Huntington, N. D. et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat. Immunol. 8, 856–863 (2007).
Wright, T. et al. Anti-apoptotic MCL-1 promotes long-chain fatty acid oxidation through interaction with ACSL1. Mol. Cell 84, 1338–1353.e8 (2024).
Hurton, L. V. et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc. Natl Acad. Sci. USA 113, E7788–E7797 (2016).
Chen, Y. et al. Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin. Cancer Res. 25, 2915–2924 (2019).
Krenciute, G. et al. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res. 5, 571–581 (2017).
Li, L. et al. Loss of metabolic fitness drives tumor resistance after CAR-NK cell therapy and can be overcome by cytokine engineering. Sci. Adv. 9, eadd6997 (2023).
Guo, Y. et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 22, 746–756 (2021).
Sperling, A. S. et al. Updated phase I study results of PHE885, a T-Charge manufactured BCMA-directed CAR-T cell therapy, for patients (pts) with r/r multiple myeloma (RRMM). J. Clin. Oncol. 41, 8004–8004 (2023).
Ghassemi, S. et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol. Res. 6, 1100–1109 (2018).
Ghassemi, S. et al. Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 6, 118–128 (2022).
Chan, J. D. et al. FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy. Nature 629, 201–210 (2024).
Doan, A. E. et al. FOXO1 is a master regulator of memory programming in CAR T cells. Nature 629, 211–218 (2024).
Sukumar, M. et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 23, 63–76 (2016). This study shows that CD8 T cells with enhanced stemness capacities can be identified in a population and sorted using a common mitochondrial potential measure; these cells have enhanced anti-tumour capacities.
Cooper, M. A. et al. Cytokine-induced memory-like natural killer cells. Proc. Natl Acad. Sci. USA 106, 1915–1919 (2009).
Terrén, I. et al. Metabolic changes of Interleukin-12/15/18-stimulated human NK cells. Sci. Rep. 11, 6472 (2021).
Dong, H. et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl Acad. Sci. USA 119, e2122379119 (2022).
Gang, M. et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 136, 2308–2318 (2020).
Gabriel, S. S. et al. Transforming growth factor-β-regulated mTOR activity preserves cellular metabolism to maintain long-term T cell responses in chronic infection. Immunity 54, 1698–1714.e5 (2021).
Viel, S. et al. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 9, ra19 (2016).
Kloss, C. C. et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Chang, Z. L. et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 14, 317–324 (2018).
Wei, J. et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 576, 471–476 (2019).
Sun, X. et al. Deletion of the mRNA endonuclease Regnase-1 promotes NK cell anti-tumor activity via OCT2-dependent transcription of Ifng. Immunity 57, 1360–1377.e13 (2024).
Mino, T. et al. Regnase-1 and roquin regulate a common element in inflammatory mRNAs by spatiotemporally distinct mechanisms. Cell 161, 1058–1073 (2015).
Nagahama, Y. et al. Regnase-1 controls colon epithelial regeneration via regulation of mTOR and purine metabolism. Proc. Natl Acad. Sci. USA 115, 11036–11041 (2018).
Zheng, W. et al. Regnase-1 suppresses TCF-1+ precursor exhausted T-cell formation to limit CAR–T-cell responses against ALL. Blood 138, 122–135 (2021).
Wang, L. et al. Induction of immortal-like and functional CAR T cells by defined factors. J. Exp. Med. 221, e20232368 (2024).
Mai, D. et al. Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells. Proc. Natl Acad. Sci. USA 120, e2218632120 (2023).
Ye, L. et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 34, 595–614.e14 (2022).
Si, X. et al. Mitochondrial isocitrate dehydrogenase impedes CAR T cell function by restraining antioxidant metabolism and histone acetylation. Cell Metab. 36, 176–192.e10 (2024).
Demaria, O. et al. A tetraspecific engager armed with a non-alpha IL-2 variant harnesses natural killer cells against B cell non-Hodgkin lymphoma. Sci. Immunol. 9, eadp3720 2024).
Böttcher, J. P. et al. NK cells stimulate recruitment of cdc1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018).
Walzer, T., Dalod, M., Robbins, S. H., Zitvogel, L. & Vivier, E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood 106, 2252–2258 (2005).
Ferlazzo, G. & Moretta, L. Dendritic cell editing by natural killer cells. Crit. Rev. Oncog. 19, 67–75 (2014).
Waggoner, S. N., Cornberg, M., Selin, L. K. & Welsh, R. M. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481, 394–398 (2012).
Narni-Mancinelli, E. et al. Tuning of natural killer cell reactivity by NKp46 and helios calibrates T cell responses. Science 335, 344–348 (2012).
Kilian, M. et al. The immunoglobulin superfamily ligand B7H6 subjects T cell responses to NK cell surveillance. Sci. Immunol. 9, eadj7970 (2024).
Buescher, J. M. et al. A roadmap for interpreting 13C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. 34, 189–201 (2015).
Bartman, C. R., TeSlaa, T. & Rabinowitz, J. D. Quantitative flux analysis in mammals. Nat. Metab. 3, 896–908 (2021).
Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and isotope tracing. Cell 173, 822–837 (2018).
van der Windt, G. J. W., Chang, C.-H. & Pearce, E. L. Measuring bioenergetics in T cells using a seahorse extracellular flux analyzer. Curr. Protoc. Immunol. 113, 3.16B.1–3.16B.14 (2016).
Argüello, R. J. et al. SCENITH: a flow cytometry-based method to functionally profile energy metabolism with single-cell resolution. Cell Metab. 32, 1063–1075.e7 (2020).
Hartmann, F. J. et al. Single-cell metabolic profiling of human cytotoxic T cells. Nat. Biotechnol. 39, 186–197 (2020).
Ahl, P. J. et al. Met-Flow, a strategy for single-cell metabolic analysis highlights dynamic changes in immune subpopulations. Commun. Biol. 3, 305 (2020).
Sinclair, L. V., Neyens, D., Ramsay, G., Taylor, P. M. & Cantrell, D. A. Single cell analysis of kynurenine and System L amino acid transport in T cells. Nat. Commun. 9, 1981 (2018).
Pelgrom, L. R. et al. QUAS-R: an SLC1A5-mediated glutamine uptake assay with single-cell resolution reveals metabolic heterogeneity with immune populations. Cell Rep. 42, 112828 (2023).
Russo, E. et al. SPICE-Met: profiling and imaging energy metabolism at the single-cell level using a fluorescent reporter mouse. EMBO J. 41, e111528 (2022).
Zhang, Z., Cheng, X., Zhao, Y. & Yang, Y. Lighting up live-cell and in vivo central carbon metabolism with genetically encoded fluorescent sensors. Annu. Rev. Anal. Chem. 13, 293–314 (2020).
Perry, S. W., Norman, J. P., Barbieri, J., Brown, E. B. & Gelbard, H. A. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques 50, 98–115 (2011).
Cosgrove, J. et al. A call for accessible tools to unlock single-cell immunometabolism research. Nat. Metab. 6, 779–782 (2024).
Acknowledgements
The T.W.–A.M. laboratory at the Centre International de Recherche en Infectiologie is supported by funding from the Agence Nationale de la Recherche, the Institut National du Cancer, the Ligue nationale contre le cancer, the Fondation ARC pour la recherche sur le cancer and institutional grants awarded to the Centre International de Recherche en Infectiologie. The E.V. laboratory at CIML is funded by the European Research Council under the European Union’s Horizon (Treatlivmets, grant agreement no. 101118936), MSDAvenir, Innate Pharma, and institutional grants awarded to the CIML (INSERM, CNRS and Aix-Marseille University). E.V. is “Natural Killer Cells” Chair, Fondation Gustave Roussy.
Author information
Authors and Affiliations
Contributions
A.M., T.W. and S.V. wrote the manuscript with the help of E.V. S.V. designed the figures. All authors contributed to the revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.V., T.W. and A.M. declare no competing interest. E.V. is a founder and employee of Innate Pharma.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Bruce McCreedy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Viel, S., Vivier, E., Walzer, T. et al. Targeting metabolic dysfunction of CD8 T cells and natural killer cells in cancer. Nat Rev Drug Discov 24, 190–208 (2025). https://doi.org/10.1038/s41573-024-01098-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-024-01098-w
This article is cited by
-
Advances and obstacles of T cell-based immunotherapy in gynecological malignancies
Molecular Cancer (2025)
-
Enhancing radiotherapy-induced anti-tumor immunity via nanoparticle-mediated STING agonist synergy
Molecular Cancer (2025)
-
Tumoral ALOX5 mediated arachidonic acid metabolism regulates immune response in non-small cell lung cancer
Cellular Oncology (2025)
-
Current advancement of immune function paradox of tumour-infiltrating cells and their immunotherapeutic targets: a mini-review
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)