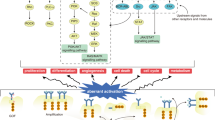
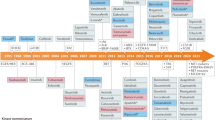
Abstract
Protein kinases are crucial targets for cancer treatment as they orchestrate important signals for oncogenesis and are often aberrantly activated owing to genomic alterations. In the past two decades, multiple kinase inhibitors have been developed, including those that are clinically effective regardless of tumour location, provided that the tumour harbours the aberrantly activated kinase. Consequently, a biomarker-based therapy model, untethered from tumour histology and organ of origin, has been established, which has led to transformative regulatory approvals of tumour-agnostic kinase inhibitors such as larotrectinib, selpercatinib, dabrafenib–trametinib and pemigatinib. However, almost all such approvals are partial in nature, as they do not include both solid and haematological cancers, even if the kinase inhibitor has shown activity in both. Moreover, clinical trials to assess these compounds are challenging because genomic sequencing of hundreds or thousands of tumours may be required to find eligible patients whose malignancy bears the targeted genetic alterations. In this Review, we describe the precision medicine paradigm that has successfully launched tumour-agnostic drug development, concentrating on small-molecule inhibitors that target kinase pathway aberrations, and we discuss the challenges in developing tumour‐agnostic agents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bhullar, K. S. et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol. Cancer 17, 48 (2018).
Ayala-Aguilera, C. C. et al. Small molecule kinase inhibitor drugs (1995–2021): medical indication, pharmacology, and synthesis. J. Med. Chem. 65, 1047–1131 (2022).
Roskoski, R. Jr Properties of FDA-approved small molecule protein kinase inhibitors: a 2021 update. Pharmacol. Res. 165, 105463 (2021).
Turski, M. L. et al. Genomically driven tumors and actionability across histologies: BRAF-mutant cancers as a paradigm. Mol. Cancer Ther. 15, 533–547 (2016).
Adashek, J. J. et al. Tissue-agnostic activity of BRAF plus MEK inhibitor in BRAF V600-mutant tumors. Mol. Cancer Ther. 21, 871–878 (2022).
Food & Drug Administration D.I.S.C.O. FDA approval of larotrectinib for advanced malignancies with NTRK gene fusion. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-fda-approval-larotrectinib-advanced-malignancies-ntrk-gene-fusion#:~:Text=1)%20larotrectinib%20is%20the%20second,for%20NTRK%20fusion%20is%20not (FDA, 2018).
Food & Drug Administration. FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc (FDA, 2019).
Food & Drug Administration. FDA approves selpercatinib for locally advanced or metastatic RET fusion-positive solid tumors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-locally-advanced-or-metastatic-ret-fusion-positive-solid-tumors (FDA, 2022).
Food & Drug Administration. FDA approves selpercatinib for locally advanced or metastatic RET fusion-positive non-small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-locally-advanced-or-metastatic-ret-fusion-positive-non-small-cell-lung (FDA, 2022).
Kurzrock, R. et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J. Clin. Oncol. 29, 2660–2666 (2011).
Thornton, K. et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 18, 3722–3730 (2012).
Nair, A. et al. FDA approval summary: lenvatinib for progressive, radio-iodine-refractory differentiated thyroid cancer. Clin. Cancer Res. 21, 5205–5208 (2015).
Yeung, K. T. & Cohen, E. E. Lenvatinib in advanced, radioactive iodine-refractory, differentiated thyroid carcinoma. Clin. Cancer Res. 21, 5420–5426 (2015).
Food & Drug Administration. FDA approves pralsetinib for non-small cell lung cancer with RET gene fusions. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pralsetinib-non-small-cell-lung-cancer-ret-gene-fusions#:~:Text=On%20August%209%2C%202023%2C%20the,by%20an%20FDA%2Dapproved%20test (FDA, 2023).
Kim, J. et al. FDA approval summary: pralsetinib for the treatment of lung and thyroid cancers with ret gene mutations or fusions. Clin. Cancer Res. 27, 5452–5456 (2021).
Subbiah, V. et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 28, 1640–1645 (2022).
Food & Drug Administration. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (FDA, 2022).
Food & Drug Administration. FDA grants regular approval to dabrafenib and trametinib combination for metastatic NSCLC with BRAF V600E mutation. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-dabrafenib-and-trametinib-combination-metastatic-nsclc-braf-v600e (FDA, 2017).
Food & Administration. FDA approves dabrafenib trametinib anaplastic thyroid cancer BRAF V600E mutation. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-plus-trametinib-anaplastic-thyroid-cancer-braf-v600e-mutation (FDA, 2018).
Food & Drug Administration. FDA approves dabrafenib with trametinib for pediatric patients with low-grade glioma with a BRAF V600E mutation. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-dabrafenib-trametinib-pediatric-patients-low-grade-glioma-braf-v600e-mutation#:~:Text=On%20March%2016%2C%202023%2C%20the,mutation%20who%20require%20systemic%20therapy (FDA, 2023).
Oneal, P. A. et al. FDA approval summary: vemurafenib for the treatment of patients with erdheim-chester disease with the BRAFV600 mutation. Oncologist 23, 1520–1524 (2018).
Food & Drug Administration. FDA approves encorafenib in combination with cetuximab for metastatic colorectal cancer with a BRAF V600E mutation. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-encorafenib-combination-cetuximab-metastatic-colorectal-cancer-braf-v600e-mutation (FDA, 2020).
Saunders, I. M., Goodman, A. M. & Kurzrock, R. Real-world toxicity experience with BRAF/MEK inhibitors in patients with Erdheim–Chester disease. Oncologist 25, e386–e390 (2020).
Aaroe, A. et al. Successful treatment of non-Langerhans cell histiocytosis with the MEK inhibitor trametinib: a multicenter analysis. Blood Adv. 7, 3984–3992 (2023).
Go, R. S. et al. Histiocytic neoplasms, version 2.2021, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 19, 1277–1303 (2021).
Tiacci, E. et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 364, 2305–2315 (2011).
Wierda, W. G. et al. Hairy cell leukemia, version 2.2018, NCCN clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 15, 1414–1427 (2017).
Food & Drug Administation. FDA approves encorafenib with binimetinib for metastatic non-small cell lung cancer with a BRAF V600E mutation. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-encorafenib-binimetinib-metastatic-non-small-cell-lung-cancer-braf-v600e-mutation#:~:Text=On%20October%2011%2C%202023%2C%20the,Mektovi%2C%20Array%20BioPharma%20Inc (FDA, 2023).
Larkin, J. et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 371, 1867–1876 (2014).
Food & Drug Administration. FDA approves pemigatinib for relapsed or refractory myeloid/lymphoid neoplasms with FGFR1 rearrangement. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pemigatinib-relapsed-or-refractory-myeloidlymphoid-neoplasms-fgfr1-rearrangement#:~:Text=On%20August%2026%2C%202022%2C%20the,receptor%201%20(FGFR1)%20rearrangement (FDA, 2022).
Food & Drug Administration. FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion (FDA, 2020).
Food & Drug Administration. FDA grants accelerated approval to repotrectinib for adult and pediatric patients with NTRK gene fusion-positive solid tumors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-repotrectinib-adult-and-pediatric-patients-ntrk-gene-fusion-positive (FDA, 2024).
Food & Drug Administration. FDA approves repotrectinib for ROS1-positive non-small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-repotrectinib-ros1-positive-non-small-cell-lung-cancer (FDA, 2023).
Bower, H. et al. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J. Clin. Oncol. 34, 2851–2857 (2016).
Westin, J. R. & Kurzrock, R. It’s about time: lessons for solid tumors from chronic myelogenous leukemia therapy. Mol. Cancer Ther. 11, 2549–2555 (2012).
Call, J. W., Wang, Y., Montoya, D., Scherzer, N. J. & Heinrich, M. C. Survival in advanced GIST has improved over time and correlates with increased access to post-imatinib tyrosine kinase inhibitors: results from Life Raft Group Registry. Clin. Sarcoma Res. 9, 4 (2019).
Tateo, V. et al. Agnostic approvals in oncology: getting the right drug to the right patient with the right genomics. Pharmaceuticals 16, 614 (2023). This article provides a helpful summary of tissue-agnostic FDA approvals.
Okamura, R. et al. Analysis of NTRK alterations in pan-cancer adult and pediatric malignancies: implications for NTRK-targeted therapeutics. JCO Precis. Oncol. 2018, PO.18.00183 (2018).
Kato, S. et al. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin. Cancer Res. 23, 1988–1997 (2017).
Food & Drug Administration. Tissue agnostic drug development in oncology. https://www.fda.gov/media/162346/download (2022). This article reports FDA guidance on consideration for tissue-agnostic drug development in oncology.
Shreenivas, A. et al. ALK fusions in the pan-cancer setting: another tumor-agnostic target? NPJ Precis. Oncol. 7, 101 (2023).
Abdellateif, M. S., Bayoumi, A. K. & Mohammed, M. A. c-Kit receptors as a therapeutic target in cancer: current insights. Onco Targets Ther. 16, 785–799 (2023).
Abourehab, M. A. S., Alqahtani, A. M., Youssif, B. G. M. & Gouda, A. M. Globally approved EGFR inhibitors: insights into their syntheses, target kinases, biological activities, receptor interactions, and metabolism. Molecules 26, 6677 (2021).
Oh, D. Y. & Bang, Y. J. HER2-targeted therapies - a role beyond breast cancer. Nat. Rev. Clin. Oncol. 17, 33–48 (2020).
Adashek, J. J., Kato, S., Sicklick, J. K., Lippman, S. M. & Kurzrock, R. Considering molecular alterations as pan-cancer tissue-agnostic targets. Nat. Cancer 4, 1622–1626 (2023).
Essegian, D., Khurana, R., Stathias, V. & Schurer, S. C. The clinical kinase index: a method to prioritize understudied kinases as drug targets for the treatment of cancer. Cell Rep. Med. 1, 100128 (2020).
Fountzilas, E., Tsimberidou, A. M., Vo, H. H. & Kurzrock, R. Clinical trial design in the era of precision medicine. Genome Med. 14, 101 (2022).
Ceolin, L., Siqueira, D. R., Romitti, M., Ferreira, C. V. & Maia, A. L. Molecular basis of medullary thyroid carcinoma: the role of RET polymorphisms. Int. J. Mol. Sci. 13, 221–239 (2012).
Janku, F. et al. Molecular profiling of tumor tissue and plasma cell-free DNA from patients with non-langerhans cell histiocytosis. Mol. Cancer Ther. 18, 1149–1157 (2019).
Di Nunno, V., Gatto, L., Tosoni, A., Bartolini, S. & Franceschi, E. Implications of BRAF V600E mutation in gliomas: molecular considerations, prognostic value and treatment evolution. Front. Oncol. 12, 1067252 (2022).
Ascierto, P. A. et al. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 10, 85 (2012).
Salem, M. E. et al. Landscape of KRAS(G12C), associated genomic alterations, and interrelation with immuno-oncology biomarkers in KRAS-mutated cancers. JCO Precis. Oncol. 6, e2100245 (2022).
Yan, M., Parker, B. A., Schwab, R. & Kurzrock, R. HER2 aberrations in cancer: implications for therapy. Cancer Treat. Rev. 40, 770–780 (2014).
Dumbrova, E. E. I. et al. Targeting ERBB2 (HER2) amplification identified by next-generation sequencing in patients with advanced or metastatic solid tumors beyond conventional indications. JCO Precis. Oncol. 3, PO.18.00345 (2019).
Helsten, T. et al. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin. Cancer Res. 22, 259–267 (2016).
Helsten, T., Schwaederle, M. & Kurzrock, R. Fibroblast growth factor receptor signaling in hereditary and neoplastic disease: biologic and clinical implications. Cancer Metastasis Rev. 34, 479–496 (2015).
Adashek, J. J., Goloubev, A., Kato, S. & Kurzrock, R. Missing the target in cancer therapy. Nat. Cancer 2, 369–371 (2021).
Manea, C. A. et al. A review of NTRK fusions in cancer. Ann. Med. Surg. 79, 103893 (2022).
O’Haire, S. et al. Systematic review of NTRK 1/2/3 fusion prevalence pan-cancer and across solid tumours. Sci. Rep. 13, 4116 (2023).
Amatu, A., Sartore-Bianchi, A. & Siena, S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open 1, e000023 (2016).
Amatu, A. et al. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 30, viii5–viii15 (2019).
Westphalen, C. B. et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis. Oncol. 5, 69 (2021).
Hong, D. S. et al. Abstract CT062: efficacy and safety of larotrectinib in patients with cancer and NTRK gene fusions or other alterations. Cancer Res. 80, CT062–CT062 (2020).
Cocco, E., Scaltriti, M. & Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 15, 731–747 (2018).
Hempel, D. et al. Antitumor activity of larotrectinib in esophageal carcinoma with NTRK gene amplification. Oncologist 25, e881–e886 (2020).
Drilon, A. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med. 378, 731–739 (2018).
Laetsch, T. W. et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 19, 705–714 (2018).
Hong, D. S. et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 21, 531–540 (2020).
De Braud, F. G. et al. Alka-372-001: first-in-human, phase I study of entrectinib – an oral pan-trk, ROS1, and ALK inhibitor – in patients with advanced solid tumors with relevant molecular alterations. J. Clin. Oncol. 33, 2517–2517 (2015).
Patel, M. R. et al. STARTRK-1: phase 1/2a study of entrectinib, an oral Pan-Trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations. J. Clin. Oncol. 33, 2596–2596 (2015).
Doebele, R. C. et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 21, 271–282 (2020).
Xiang, S. & Lu, X. Selective type II TRK inhibitors overcome xDFG mutation mediated acquired resistance to the second-generation inhibitors selitrectinib and repotrectinib. Acta Pharm. Sin. B 14, 517–532 (2024).
Takahashi, M. RET receptor signaling: function in development, metabolic disease, and cancer. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 98, 112–125 (2022).
Adashek, J. J. et al. Hallmarks of RET and co-occuring genomic alterations in RET-aberrant cancers. Mol. Cancer Ther. 20, 1769–1776 (2021).
Desilets, A., Repetto, M., Yang, S. R., Sherman, E. J. & Drilon, A. RET-altered cancers—A tumor-agnostic review of biology, diagnosis and targeted therapy activity. Cancers 15, 4146 (2023).
Iwashita, T., Murakami, H., Asai, N. & Takahashi, M. Mechanism of ret dysfunction by Hirschsprung mutations affecting its extracellular domain. Hum. Mol. Genet. 5, 1577–1580 (1996).
Eng, C. & Plitt, G. Multiple endocrine neoplasia type 2. GeneReviews https://www.ncbi.nlm.nih.gov/books/NBK1257/ (1999).
Parimi, V. et al. Genomic landscape of 891 RET fusions detected across diverse solid tumor types. NPJ Precis. Oncol. 7, 10 (2023).
Elisei, R. et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 31, 3639–3646 (2013).
Brose, M. S. et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 22, 1126–1138 (2021).
Subbiah, V. et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 23, 1261–1273 (2022).
Kurzrock, R. Selpercatinib aimed at RET-altered cancers. N. Engl. J. Med. 383, 868–869 (2020).
Griesinger, F. et al. Safety and efficacy of pralsetinib in RET fusion-positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann. Oncol. 33, 1168–1178 (2022).
Subbiah, V. et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 9, 491–501 (2021).
Duke, E. S. et al. FDA approval summary: selpercatinib for the treatment of advanced RET fusion-positive solid tumors. Clin. Cancer Res. 29, 3573–3578 (2023).
Ullah, R., Yin, Q., Snell, A. H. & Wan, L. RAF-MEK-ERK pathway in cancer evolution and treatment. Semin. Cancer Biol. 85, 123–154 (2022).
Maloney, R. C., Zhang, M., Jang, H. & Nussinov, R. The mechanism of activation of monomeric B-Raf V600E. Comput. Struct. Biotechnol. J. 19, 3349–3363 (2021).
Munoz, J., Schlette, E. & Kurzrock, R. Rapid response to vemurafenib in a heavily pretreated patient with hairy cell leukemia and a BRAF mutation. J. Clin. Oncol. 31, e351–e352 (2013).
Tiacci, E. et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N. Engl. J. Med. 373, 1733–1747 (2015).
Chen, L. et al. Fibroblast growth factor receptor fusions in cancer: opportunities and challenges. J. Exp. Clin. Cancer Res. 40, 345 (2021).
Du, S., Zhang, Y. & Xu, J. Current progress in cancer treatment by targeting FGFR signaling. Cancer Biol. Med. 20, 490–499 (2023).
Abou-Alfa, G. K. et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 21, 671–684 (2020).
Marinelli, L. M. et al. Myeloid/lymphoid neoplasm with FGFR1 rearrangement presenting with polycythemia vera and t-cell acute lymphoblastic leukemia. Cancer Genet. 276-277, 43–47 (2023).
Strati, P. et al. Myeloid/lymphoid neoplasms with FGFR1 rearrangement. Leuk. Lymphoma 59, 1672–1676 (2018).
Verstovsek, S. et al. FIGHT-203, an ongoing phase 2 study of pemigatinib in patients with myeloid/lymphoid neoplasms (MLNs) with fibroblast growth factor receptor 1 (FGFR1) rearrangement (MLNFGFR1): a focus on centrally reviewed clinical and cytogenetic responses in previously treated patients. Blood 140, 3980–3982 (2022).
Solomon, B., Varella-Garcia, M. & Camidge, D. R. ALK gene rearrangements: a new therapeutic target in a molecularly defined subset of non-small cell lung cancer. J. Thorac. Oncol. 4, 1450–1454 (2009).
AACR Project Genie Consortium. AACR project GENIE: powering precision medicine through an international consortium. Cancer Discov. 7, 818–831 (2017).
Ross, J. S. et al. ALK fusions in a wide variety of tumor types respond to anti-ALK targeted therapy. Oncologist 22, 1444–1450 (2017).
Nikanjam, M., Okamura, R., Barkauskas, D. A. & Kurzrock, R. Targeting fusions for improved outcomes in oncology treatment. Cancer 126, 1315–1321 (2020).
Adashek, J. J., Sapkota, S., de Castro Luna, R. & Seiwert, T. Y. Complete response to alectinib in ALK-fusion metastatic salivary ductal carcinoma. NPJ Precis. Oncol. 7, 36 (2023).
Pal, S. K. et al. Responses to alectinib in ALK-rearranged papillary renal cell carcinoma. Eur. Urol. 74, 124–128 (2018).
Shaw, A. T. et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann. Oncol. 30, 1121–1126 (2019).
Shaw, A. T. et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 371, 1963–1971 (2014).
Food & Drug Administration. FDA approves crizotinib capsules. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-capsules (FDA, 2016).
Jakubowski, C. D., Mohan, A. A., Kamel, I. R. & Yarchoan, M. Response to crizotinib in ros1 fusion-positive intrahepatic cholangiocarcinoma. JCO Precis. Oncol. 4, 825–828 (2020).
Wang, T. & Shen, Y. Y. Rare ROS1-CENPW gene in pancreatic acinar cell carcinoma and the effect of crizotinib plus AG chemotherapy: a case report. World J. Clin. Cases 11, 5823–5829 (2023).
Addeo, A., Banna, G. L. & Friedlaender, A. KRAS G12C mutations in NSCLC: from target to resistance. Cancers 13, 2541 (2021).
Zhu, C. et al. Targeting KRAS mutant cancers: from druggable therapy to drug resistance. Mol. Cancer 21, 159 (2022).
Sacher, A. et al. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N. Engl. J. Med. 389, 710–721 (2023).
Lu, S., Jang, H., Nussinov, R. & Zhang, J. The structural basis of oncogenic mutations G12, G13 and Q61 in small GTPase K-Ras4B. Sci. Rep. 6, 21949 (2016).
Huang, L., Guo, Z., Wang, F. & Fu, L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct. Target. Ther. 6, 386 (2021).
Qunaj, L., May, M. S., Neugut, A. I. & Herzberg, B. O. Prognostic and therapeutic impact of the KRAS G12C mutation in colorectal cancer. Front. Oncol. 13, 1252516 (2023).
Al-Tassan, N. et al. Inherited variants of MYH associated with somatic G:C–>T:A mutations in colorectal tumors. Nat. Genet. 30, 227–232 (2002).
Kim, C. J. et al. Genetic alterations of the MYH gene in gastric cancer. Oncogene 23, 6820–6822 (2004).
Disel, U. et al. Increased KRAS G12C prevalence, high tumor mutational burden, and specific mutational signatures are associated with MUTYH mutations: a pan-cancer analysis. Oncologist 29, e213–e223 (2023).
Kim, H. J., Lee, H. N., Jeong, J. S. & Jang, S. B. Oncogenic KRAS: signaling and drug resistance. Cancers 13, 5599 (2021).
Kwan, A. K., Piazza, G. A., Keeton, A. B. & Leite, C. A. The path to the clinic: a comprehensive review on direct KRAS(G12C) inhibitors. J. Exp. Clin. Cancer Res. 41, 27 (2022).
Vasta, J. D. et al. KRAS is vulnerable to reversible switch-II pocket engagement in cells. Nat. Chem. Biol. 18, 596–604 (2022).
Broker, J. et al. Fragment optimization of reversible binding to the switch II pocket on KRAS leads to a potent, in vivo active KRAS(G12C) inhibitor. J. Med. Chem. 65, 14614–14629 (2022).
Desai, J. et al. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial. Nat. Med. 30, 271–278 (2024).
Food & Drug Administration. FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sotorasib-kras-g12c-mutated-nsclc (FDA, 2021).
Food & Drug Administration. FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSCLC. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-adagrasib-kras-g12c-mutated-nsclc (FDA, 2022).
Skoulidis, F. et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 384, 2371–2381 (2021).
Mausey, N. & Halford, Z. Targeted therapies for previously “Undruggable” KRAS-mutated non-small cell lung cancer: a review of sotorasib and adagrasib. Ann. Pharmacother. 58, 622–635 (2023).
Janne, P. A. et al. Adagrasib in non-small-cell lung cancer harboring a KRAS(G12C) mutation. N. Engl. J. Med. 387, 120–131 (2022).
Hong, D. S. et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 383, 1207–1217 (2020).
Bekaii-Saab, T. S. et al. Adagrasib in advanced solid tumors harboring a KRAS(G12C) mutation. J. Clin. Oncol. 41, 4097–4106 (2023).
Iqbal, N. & Iqbal, N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol. Biol. Int. 2014, 852748 (2014).
Shayeb, A. M., Kurzrock, R., Adashek, J. J. & Kato, S. Comprehensive analysis of human epidermal growth factor receptor 2 through DNA, mRNA, and protein in diverse malignancies. JCO Precis. Oncol. 7, e2200604 (2023).
Wang, D. et al. Associations of HER2 mutation with immune-related features and immunotherapy outcomes in solid tumors. Front. Immunol. 13, 799988 (2022).
Yan, M. et al. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 34, 157–164 (2015).
Food & Drug Administration. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant non-small cell lung cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-her2-mutant-non-small-cell-lung. (FDA, 2022).
Meric-Bernstam, F. et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J. Clin. Oncol. 42, JCO2302005 (2023).
Food & Drug Administration. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive solid tumors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2 (FDA, 2024).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Food & Drug Administration. FDA approves fam-trastuzumab deruxtecan-nxki for HER2-low breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer (FDA, 2022).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020).
Wang, Y., Xu, H., Han, Y., Wu, Y. & Wang, J. Comparative efficacy of tyrosine kinase inhibitors and antibody-drug conjugates in HER2-positive metastatic breast cancer patients with brain metastases: a systematic review and network meta-analysis. Cancers 14, 3372 (2022).
Cortes, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022).
Food & Drug Administration. FDA approves tucatinib for patients with HER2-positive metastatic breast cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tucatinib-patients-her2-positive-metastatic-breast-cancer (FDA, 2020).
Food & Drug Administration. FDA grants accelerated approval to tucatinib with trastuzumab for colorectal cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tucatinib-trastuzumab-colorectal-cancer (FDA, 2023).
Robichaux, J. P. et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 36, 444–457.e447 (2019).
Imatinib package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021588s047lbl.pdf (Novartis, 2016).
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001).
Kurzrock, R., Gutterman, J. U. & Talpaz, M. The molecular genetics of Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 319, 990–998 (1988).
Kloetzer, W. et al. The human cellular abl gene product in the chronic myelogenous leukemia cell line K562 has an associated tyrosine protein kinase activity. Virology 140, 230–238 (1985).
Kurzrock, R. et al. A novel c-abl protein product in Philadelphia-positive acute lymphoblastic leukaemia. Nature 325, 631–635 (1987).
Dagher, R. et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin. Cancer Res. 8, 3034–3038 (2002).
Wahida, A. et al. The coming decade in precision oncology: six riddles. Nat. Rev. Cancer 23, 43–54 (2023).
Flynn, J. P. & Gerriets, V. Imatinib StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK551676/ (2023).
Cheah, C. Y. et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood 123, 3574–3577 (2014).
Apperley, J. F. et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N. Engl. J. Med. 347, 481–487 (2002).
David, M. et al. Durable responses to imatinib in patients with PDGFRB fusion gene-positive and BCR-ABL-negative chronic myeloproliferative disorders. Blood 109, 61–64 (2007).
Magnusson, M. K., Meade, K. E., Nakamura, R., Barrett, J. & Dunbar, C. E. Activity of STI571 in chronic myelomonocytic leukemia with a platelet-derived growth factor beta receptor fusion oncogene. Blood 100, 1088–1091 (2002).
Vega-Ruiz, A. et al. Phase II study of imatinib mesylate as therapy for patients with systemic mastocytosis. Leuk. Res. 33, 1481–1484 (2009).
Alvarez-Twose, I. et al. Imatinib in systemic mastocytosis: a phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget 8, 68950–68963 (2017).
McClure, R. F. et al. Clinical significance of DNA variants in chronic myeloid neoplasms: a report of the association for molecular pathology. J. Mol. Diagn. 20, 717–737 (2018).
Jain, N. et al. Imatinib has limited therapeutic activity for hypereosinophilic syndrome patients with unknown or negative PDGFRalpha mutation status. Leuk. Res. 33, 837–839 (2009).
Kantarjian, H. M. et al. Significance of the P210 versus P190 molecular abnormalities in adults with Philadelphia chromosome-positive acute leukemia. Blood 78, 2411–2418 (1991).
Ravandi, F. Managing philadelphia chromosome-positive acute lymphoblastic leukemia: role of tyrosine kinase inhibitors. Clin. Lymphoma Myeloma Leuk. 11, 198–203 (2011).
Jabbour, E., Haddad, F. G., Short, N. J. & Kantarjian, H. Treatment of adults with philadelphia chromosome-positive acute lymphoblastic leukemia-from intensive chemotherapy combinations to chemotherapy-free regimens: a review. JAMA Oncol. 8, 1340–1348 (2022).
Navarrete-Dechent, C., Mori, S., Barker, C. A., Dickson, M. A. & Nehal, K. S. Imatinib treatment for locally advanced or metastatic dermatofibrosarcoma protuberans: a systematic review. JAMA Dermatol. 155, 361–369, (2019).
Parab, T. M. et al. Gastrointestinal stromal tumors: a comprehensive review. J. Gastrointest. Oncol. 10, 144–154 (2019).
Raoof, S. & Kurzrock, R. For insights into the real world, consider real-world data. Sci. Transl. Med. 14, eabn6911 (2022).
Dickson, D. et al. The master observational trial: a new class of master protocol to advance precision medicine. Cell 180, 9–14 (2020).
Adashek, J. J., Subbiah, V. & Kurzrock, R. From tissue-agnostic to N-of-One therapies: (R)evolution of the precision paradigm. Trends Cancer 7, 15–28 (2021).
Sicklick, J. K. et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 25, 744–750 (2019).
Sicklick, J. K. et al. Molecular profiling of advanced malignancies guides first-line N-of-1 treatments in the I-PREDICT treatment-naive study. Genome Med. 13, 155 (2021). References 167 and 168 report positive results from a N-of-1 combination therapy clinical trial with a tissue-agnostic approach to treatment.
Kato, S. et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 11, 4965 (2020).
Subbiah, V., Burris, H. A. 3rd & Kurzrock, R. Revolutionizing cancer drug development: harnessing the potential of basket trials. Cancer 130, 186–200 (2023). This Review describes the optimal clinical trial design for tissue-agnostic drug development.
NIH National Cancer Institute. ComboMATCH precision medicine clinical trials. https://www.cancer.gov/research/infrastructure/clinical-trials/nci-supported/combomatch#about-combomatch (NCI, 2024).
Rodon, J. et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat. Med. 25, 751–758 (2019).
Gabay, M. 21st century cures act. Hosp. Pharm. 52, 264–265 (2017).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Wisinski, K. B. et al. Trametinib in patients with NF1-, GNAQ-, or GNA11-mutant tumors: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocols S1 and S2. JCO Precis. Oncol. 7, e2200421 (2023).
Casey, D. et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin. Cancer Res. 27, 4142–4146 (2021).
Krop, I. E. et al. Phase II study of taselisib in PIK3CA-mutated solid tumors other than breast and squamous lung cancer: results from the NCI-MATCH ECOG-ACRIN trial (EAY131) subprotocol I. JCO Precis. Oncol. 6, e2100424 (2022).
Narayan, P. et al. FDA approval summary: alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin. Cancer Res. 27, 1842–1849 (2021).
Quintanal-Villalonga, A. et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat. Rev. Clin. Oncol. 17, 360–371 (2020).
Casak, S. J. et al. FDA approval summary: ivosidenib for the treatment of patients with advanced unresectable or metastatic, chemotherapy refractory cholangiocarcinoma with an IDH1 mutation. Clin. Cancer Res. 28, 2733–2737 (2022).
Mullard, A. FDA approves IDH1 and IDH2 inhibitor for brain cancer. Nat. Rev. Drug Discov. 23, 731 (2024).
Myers, R. A., Wirth, S., Williams, S. & Kiel, P. J. Enasidenib: an oral IDH2 inhibitor for the treatment of acute myeloid leukemia. J. Adv. Pract. Oncol. 9, 435–440 (2018).
Szeto, C. W. et al. Association of differential expression of immunoregulatory molecules and presence of targetable mutations may inform rational design of clinical trials. ESMO Open 7, 100396 (2022).
Kondrashov, A. et al. Antibody-drug conjugates in solid tumor oncology: an effectiveness payday with a targeted payload. Pharmaceutics 15, 2160 (2023).
Heyer, E. E. et al. Diagnosis of fusion genes using targeted RNA sequencing. Nat. Commun. 10, 1388 (2019).
Adashek, J. J., Kato, S., Sicklick, J. K., Lippman, S. M. & Kurzrock, R. If it’s a target, it’s a pan-cancer target: tissue is not the issue. Cancer Treat. Rev. 125, 102721 (2024).
Subbiah, V. & Kurzrock, R. Universal germline and tumor genomic testing needed to win the war against cancer: genomics is the diagnosis. J. Clin. Oncol. 41, 3100–3103 (2023).
Subbiah, V. & Kurzrock, R. Universal genomic testing needed to win the war against cancer: genomics is the diagnosis. JAMA Oncol. 2, 719–720 (2016). This article describes the importance of genomic and germline testing to improve cancer therapies.
Acknowledgements
R.K. is funded in part by 5U01CA180888-08 and 5UG1CA233198-05.
Author information
Authors and Affiliations
Contributions
R.K. and J.J.A. researched data for the article. All authors contributed substantially to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
J.J.A. serves on the advisory board of CureMatch and is a consultant for datma. R.K. has received research funding from Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, MedImmune, Merck Serono, Omniseq, Pfizer, Sequenom, Sysmex, Takeda and TopAlliance and from the NCI; as well as consultant and/or speaker fees and/or advisory board/consultant for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Inc., Biological Dynamics, Caris, Daiichi, Datar Cancer Genetics, EISAI, EMD Serono, EOM Pharmaceuticals, Iylon, Jackson Laboratories, LabCorp, Lanauria Therapeutics, Merck, NeoGenomics, Neomed, Pfizer, Precirix, Prosperdtx, Quanta Therapeutics, Recordati, Regeneron, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch, Inc.; serves on the Board of CureMatch and CureMetrix and XZOM, and is a co-founder of CureMatch. M.N. declares no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Axel Hoos and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
AACR PROJECT GENIE: https://www.aacr.org/professionals/research/aacr-project-genie/
Glossary
- Basket studies
-
Trials that include various cancers based on genomic characteristics instead of tumour of origin and in which patients are pooled irrespective of cancer type.
- Cytogenetic response
-
For chronic myelogenous leukaemia, a decrease in the percentage of bone marrow cells containing the disease-defining Philadelphia chromosome.
- Gatekeeper mutations
-
Mutation that occur in the gatekeeper residue of a kinase, which controls access to the pocket to which drugs bind and can lead to drug resistance.
- Haematological response
-
Improvement in blood cell levels (white blood cells, red blood cells and platelets) as a result of treatment.
- Marfanoid habitus
-
Signs resembling those of Marfan syndrome, including long limbs, slender fingers and toes, and extremely flexible joints.
- NCCN guidelines
-
The NCCN clinical guidelines comprise recommendations for the prevention, diagnosis and management of malignancies across the continuum of care. The NCCN guidelines currently apply to more than 97% of cancers affecting patients in the United States.
- Solvent-front mutations
-
Mutation that affect the conserved surface-exposed amino acid sequence that is close to the ATP-binding site of the kinase. They can reorient the surrounding residues thereby affecting ATP-binding and kinase activity, thus leading to resistance to kinase inhibitors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adashek, J.J., Nikanjam, M. & Kurzrock, R. Tumour-agnostic kinase inhibitors. Nat Rev Drug Discov 24, 504–520 (2025). https://doi.org/10.1038/s41573-025-01147-y
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41573-025-01147-y