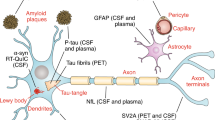
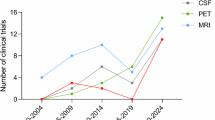
Abstract
Neurodegenerative disorders are characterized by complex neurobiological changes that are reflected in biomarker alterations detectable in blood, cerebrospinal fluid (CSF) and with brain imaging. As accessible proxies for processes that are difficult to measure, biomarkers are tools that hold increasingly important roles in drug development and clinical trial decision making. In the past few years, biomarkers have been the basis for accelerated approval of new therapies for Alzheimer disease and amyotrophic lateral sclerosis as surrogate end points reasonably likely to predict clinical benefit.Blood-based biomarkers are emerging for Alzheimer disease and other neurodegenerative disorders (for example, Parkinson disease, frontotemporal dementia), and some biomarkers may be informative across multiple disease states. Collection of CSF provides access to biomarkers not available in plasma, including markers of synaptic dysfunction and neuroinflammation. Molecular imaging is identifying an increasing array of targets, including amyloid plaques, neurofibrillary tangles, inflammation, mitochondrial dysfunction and synaptic density. In this Review, we consider how biomarkers can be implemented in clinical trials depending on their context of use, including providing information on disease risk and/or susceptibility, diagnosis, prognosis, pharmacodynamic outcomes, monitoring, prediction of response to therapy and safety. Informed choice of increasingly available biomarkers and rational deployment in clinical trials support drug development decision making and de-risk the drug development process for neurodegenerative disorders.
Key points
-
Biomarkers are drug development tools necessary for decision making in clinical trials of disease-targeting therapies.
-
Biomarkers have a specific context of use (CoU) as drug development tools. The categories of CoU include risk characterization, diagnosis, monitoring, pharmacodynamics, determination of prognosis, prediction of therapeutic effects and safety assessment.
-
Disease-targeted therapies have been approved for Alzheimer disease and amyotrophic lateral sclerosis, and appropriate use of biomarkers can assist in the development of more much-needed disease-targeting therapies for other neurodegenerative disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cummings, J., Feldman, H. H. & Scheltens, P. The “rights” of precision drug development for Alzheimer’s disease. Alzheimers Res. Ther. 11, 76 (2019).
de Lange, E. C. M., van den Brink, W., Yamamoto, Y., de Witte, W. E. A. & Wong, Y. C. Novel CNS drug discovery and development approach: model-based integration to predict neuro-pharmacokinetics and pharmacodynamics. Expert Opin. Drug Discov. 12, 1207–1218 (2017).
Srinivas, N., Maffuid, K. & Kashuba, A. D. M. Clinical pharmacokinetics and pharmacodynamics of drugs in the central nervous system. Clin. Pharmacokinet. 57, 1059–1074 (2018).
GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125 (2022).
Corriveau-Lecavalier, N. et al. Clinical criteria for a limbic-predominant amnestic neurodegenerative syndrome. Brain Commun. 6, fcae183 (2024).
Huang, Y., Li, Y., Pan, H. & Han, L. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J. Glob. Health 13, 04160 (2023).
FDA–NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource https://www.ncbi.nlm.nih.gov/books/NBK326791/ (FDA–NIH, 2021).
Ashton, N. J. et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat. Rev. Neurol. 16, 265–284 (2020).
Simren, J., Elmgren, A., Blennow, K. & Zetterberg, H. Fluid biomarkers in Alzheimer’s disease. Adv. Clin. Chem. 112, 249–281 (2023).
Hunter, D. J. et al. A pathway and approach to biomarker validation and qualification for osteoarthritis clinical trials. Curr. Drug Targets 11, 536–545 (2010).
Andreasson, U. et al. A practical guide to immunoassay method validation. Front. Neurol. 6, 179 (2015).
Stephenson, D., Badawy, R., Mathur, S., Tome, M. & Rochester, L. Digital progression biomarkers as novel endpoints in clinical trials: a multistakeholder perspective. J. Parkinsons Dis. 11, S103–S109 (2021).
Califf, R. M. Biomarker definitions and their applications. Exp. Biol. Med. 243, 213–221 (2018).
Cook, D. et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov. 13, 419–431 (2014).
Morgan, P. et al. Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat. Rev. Drug Discov. 17, 167–181 (2018).
Wong, C. H., Siah, K. W. & Lo, A. W. Estimation of clinical trial success rates and related parameters. Biostatistics 20, 273–286 (2019).
Gromova, M., Vaggelas, A., Dallmann, G. & Seimetz, D. Biomarkers: opportunities and challenges for drug development in the current regulatory landscape. Biomark Insights 15, 1177271920974652 (2020).
Cummings, J., Ortiz, A., Castellino, J. & Kinney, J. Diabetes: risk factor and translational therapeutic implications for Alzheimer’s disease. Eur. J. Neurosci. 56, 5727–5757 (2022).
Tavares, T. P. et al. Early symptoms in symptomatic and preclinical genetic frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 91, 975–984 (2020).
Lim, S. Y. & Klein, C. Parkinson’s disease is predominantly a genetic disease. J. Parkinsons Dis. 14, 467–482 (2024).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518 (2019).
Riviere, M. E. et al. Effects of the active amyloid beta immunotherapy CAD106 on PET measurements of amyloid plaque deposition in cognitively unimpaired APOE epsilon4 homozygotes. Alzheimers Dement. 20, 1839–1850 (2024).
Lopez Lopez, C. et al. The Alzheimer’s prevention initiative generation program: study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer’s disease. Alzheimers Dement. 5, 216–227 (2019).
von Linstow, C. U., Gan-Or, Z. & Brundin, P. Precision medicine in Parkinson’s disease patients with LRRK2 and GBA risk variants – let’s get even more personal. Transl. Neurodegener. 9, 39 (2020).
Brockmann, K. & Gasser, T. Prevention of Dementia in GBA-Associated Parkinson’s Disease https://www.michaeljfox.org/grant/prevention-dementia-gba-associated-parkinsons-disease (Michael J. Fox Foundation for Parkinson’s Research, 2023).
Paisán-Ruiz, C., Lewis, P. A. & Singleton, A. B. LRRK2: cause, risk, and mechanism. J. Parkinsons Dis. 3, 85–103 (2013).
Taymans, J. M. et al. Perspective on the current state of the LRRK2 field. NPJ Parkinsons Dis. 9, 104 (2023).
Rocha, E. M., Keeney, M. T., Di Maio, R., De Miranda, B. R. & Greenamyre, J. T. LRRK2 and idiopathic Parkinson’s disease. Trends Neurosci. 45, 224–236 (2022).
Chen, Y. et al. Glucocerebrosidase as a therapeutic target for Parkinson’s disease. Expert Opin. Ther. Targets 24, 287–294 (2020).
Rizig, M. et al. Identification of genetic risk loci and causal insights associated with Parkinson’s disease in African and African admixed populations: a genome-wide association study. Lancet Neurol. 22, 1015–1025 (2023).
Jennings, D. et al. Preclinical and clinical evaluation of the LRRK2 inhibitor DNL201 for Parkinson’s disease. Sci. Transl. Med. 14, eabj2658 (2022).
Giladi, N. et al. Safety and efficacy of venglustat in GBA1-associated Parkinson’s disease: an international, multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 22, 661–671 (2023).
Greaves, C. V. & Rohrer, J. D. An update on genetic frontotemporal dementia. J. Neurol. 266, 2075–2086 (2019).
Fahed, A. C., Philippakis, A. A. & Khera, A. V. The potential of polygenic scores to improve cost and efficiency of clinical trials. Nat. Commun. 13, 2922 (2022).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Nalls, M. A. et al. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol. 14, 1002–1009 (2015).
Ballard, C. et al. Enrichment factors for clinical trials in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 5, 164–174 (2019).
Budd Haeberlein, S. et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 9, 197–210 (2022).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527 (2023).
Chapleau, M., Iaccarino, L., Soleimani-Meigooni, D. & Rabinovici, G. D. The role of amyloid PET in imaging neurodegenerative disorders: a review. J. Nucl. Med. 63, 13S–19S (2022).
Jovalekic, A. et al. Validation of quantitative assessment of florbetaben PET scans as an adjunct to the visual assessment across 15 software methods. Eur. J. Nucl. Med. Mol. Imaging 50, 3276–3289 (2023).
Hanseeuw, B. J. et al. Defining a centiloid scale threshold predicting long-term progression to dementia in patients attending the memory clinic: an [(18)F] flutemetamol amyloid PET study. Eur. J. Nucl. Med. Mol. Imaging 48, 302–310 (2021).
Leuzy, A. et al. Harmonizing tau positron emission tomography in Alzheimer’s disease: the CenTauR scale and the joint propagation model. Alzheimers Dement. 20, 5833–5848 (2024).
Shcherbinin, S. et al. Tau as a diagnostic instrument in clinical trials to predict amyloid in Alzheimer’s disease. Alzheimers Dement. 9, e12415 (2023).
Gatto, R. G. et al. Comparative assessment of regional tau distribution by Tau-PET and post-mortem neuropathology in a representative set of Alzheimer’s & frontotemporal lobar degeneration patients. PLoS ONE 18, e0284182 (2023).
Hampel, H. et al. The amyloid-beta pathway in Alzheimer’s disease. Mol. Psychiatry 26, 5481–5503 (2021).
Suarez-Calvet, M. et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer’s continuum when only subtle changes in Abeta pathology are detected. EMBO Mol. Med. 12, e12921 (2020).
Teunissen, C. E. et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 21, 66–77 (2022).
Mendes, A. J. et al. Head-to-head study of diagnostic accuracy of plasma and cerebrospinal fluid p-tau217 versus p-tau181 and p-tau231 in a memory clinic cohort. J. Neurol. 271, 2053–2066 (2024).
Therriault, J. et al. Equivalence of plasma p-tau217 with cerebrospinal fluid in the diagnosis of Alzheimer’s disease. Alzheimers Dement. 19, 4967–4977 (2023).
Ashton, N. J. et al. Diagnostic accuracy of a plasma phosphorylated tau 217 immunoassay for Alzheimer disease pathology. JAMA Neurol. 81, 255–263 (2024).
Therriault, J. et al. Comparison of two plasma p-tau217 assays to detect and monitor Alzheimer’s pathology. EBioMedicine 102, 105046 (2024).
Schindler, S. E. et al. Acceptable performance of blood biomarker tests of amyloid pathology-recommendations from the Global CEO Initiative on Alzheimer’s disease. Nat. Rev. Neurol. 20, 426–439 (2024).
Palmqvist, S. et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol. 71, 1282–1289 (2014).
Rissman, R. A. et al. Plasma Abeta42/Abeta40 and phospho-tau217 concentration ratios increase the accuracy of amyloid PET classification in preclinical Alzheimer’s disease. Alzheimers Dement. 20, 1214–1224 (2024).
Thijssen, E. H. et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 26, 387–397 (2020).
Pichet Binette, A. et al. Amyloid-associated increases in soluble tau relate to tau aggregation rates and cognitive decline in early Alzheimer’s disease. Nat. Commun. 13, 6635 (2022).
Ferreira, P. C. L. et al. Plasma p-tau231 and p-tau217 inform on tau tangles aggregation in cognitively impaired individuals. Alzheimers Dement. 19, 4463–4474 (2023).
Barthelemy, N. R., Horie, K., Sato, C. & Bateman, R. J. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J. Exp. Med. 217, e20200861 (2020).
Therriault, J. et al. Association of phosphorylated tau biomarkers with amyloid positron emission tomography vs tau positron emission tomography. JAMA Neurol. 80, 188–199 (2023).
Horie, K. et al. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer’s disease. Nat. Med. 29, 1954–1963 (2023).
Mattsson-Carlgren, N. et al. Longitudinal plasma p-tau217 is increased in early stages of Alzheimer’s disease. Brain 143, 3234–3241 (2020).
Horie, K. et al. Change in cerebrospinal fluid tau microtubule binding region detects symptom onset, cognitive decline, tangles, and atrophy in dominantly inherited Alzheimer’s disease. Ann. Neurol. 93, 1158–1172 (2023).
Jack, C. R. Jr. et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement. 20, 5143–5169 (2024).
Bateman, R. J. et al. The DIAN-TU next generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement. 13, 8–19 (2017).
Wang, G. et al. Evaluation of dose-dependent treatment effects after mid-trial dose escalation in biomarker, clinical, and cognitive outcomes for gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Alzheimers Dement. 14, e12367 (2022).
Salloway, S. et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat. Med. 27, 1187–1196 (2021).
Tariot, P. N. et al. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: a study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimers Dement. 4, 150–160 (2018).
Arboleda-Velasquez, J. F. et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat. Med. 25, 1680–1683 (2019).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Rizzo, G. et al. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86, 566–576 (2016).
Dickson, D. W. Neuropathology of Parkinson disease. Parkinsonism Relat. Disord. 46, S30–s33 (2018).
Palermo, G., Giannoni, S., Bellini, G., Siciliano, G. & Ceravolo, R. Dopamine transporter imaging, current status of a potential biomarker: a comprehensive review. Int. J. Mol. Sci. 22, 11234 (2021).
Otani, R. T. V., Yamamoto, J. Y. S., Nunes, D. M., Haddad, M. S. & Parmera, J. B. Magnetic resonance and dopamine transporter imaging for the diagnosis of Parkinson´s disease: a narrative review. Arq. Neuropsiquiatr. 80, 116–125 (2022).
Bega, D. et al. Clinical utility of DaTscan in patients with suspected Parkinsonian syndrome: a systematic review and meta-analysis. NPJ Parkinsons Dis. 7, 43 (2021).
Pagano, G. et al. Trial of prasinezumab in early-stage Parkinson’s disease. N. Engl. J. Med. 387, 421–432 (2022).
Lang, A. E. et al. Trial of cinpanemab in early Parkinson’s disease. N. Engl. J. Med. 387, 408–420 (2022).
Hutchison, R. M. et al. Evaluating dopamine transporter imaging as an enrichment biomarker in a phase 2 Parkinson’s disease trial. BMC Neurol. 21, 459 (2021).
Eijsvogel, P. et al. Target engagement and immunogenicity of an active immunotherapeutic targeting pathological alpha-synuclein: a phase 1 placebo-controlled trial. Nat. Med. 30, 2631–2640 (2024).
Marek, K. et al. The Parkinson’s progression markers initiative (PPMI) – establishing a PD biomarker cohort. Ann. Clin. Transl. Neurol. 5, 1460–1477 (2018).
Stephenson, D. et al. The qualification of an enrichment biomarker for clinical trials targeting early stages of Parkinson’s disease. J. Parkinsons Dis. 9, 553–563 (2019).
Wallert, E. et al. Comparison of [18F]-FDOPA PET and [123I]-FP-CIT SPECT acquired in clinical practice for assessing nigrostriatal degeneration in patients with a clinically uncertain parkinsonian syndrome. EJNMMI Res. 12, 68 (2022).
Mollenhauer, B. et al. Longitudinal analyses of cerebrospinal fluid α-synuclein in prodromal and early Parkinson’s disease. Mov. Disord. 34, 1354–1364 (2019).
Kang, J. H. et al. CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: the Parkinson’s Progression Markers Initiative study. Acta Neuropathol. 131, 935–949 (2016).
Siderowf, A. et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 22, 407–417 (2023).
Concha-Marambio, L., Pritzkow, S., Shahnawaz, M., Farris, C. M. & Soto, C. Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nat. Protoc. 18, 1179–1196 (2023).
Grossauer, A. et al. α-synuclein seed amplification assays in the diagnosis of synucleinopathies using cerebrospinal fluid-a systematic review and meta-analysis. Mov. Disord. Clin. Pract. 10, 737–747 (2023).
Okuzumi, A. et al. Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nat. Med. 29, 1448–1455 (2023).
Hutchison, R. M. et al. Cinpanemab in early Parkinson disease: evaluation of biomarker results from the phase 2 SPARK clinical trial. Neurology 102, e209137 (2024).
Hoglinger, G. U. et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol. 23, 191–204 (2024).
Simuni, T. et al. A biological definition of neuronal alpha-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Yan, S. et al. Neuronally derived extracellular vesicle alpha-synuclein as a serum biomarker for individuals at risk of developing Parkinson disease. JAMA Neurol. 81, 59–68 (2024).
Gilboa, T. et al. Toward the quantification of alpha-synuclein aggregates with digital seed amplification assays. Proc. Natl Acad. Sci. USA 121, e2312031121 (2024).
Rutledge, J. et al. Comprehensive proteomics of CSF, plasma, and urine identify DDC and other biomarkers of early Parkinson’s disease. Acta Neuropathol. 147, 52 (2024).
Korat, S. et al. Alpha-synuclein PET tracer development-an overview about current efforts. Pharmaceuticals 14, 847 (2021).
Xiang, J. et al. Development of an alpha-synuclein positron emission tomography tracer for imaging synucleinopathies. Cell 186, 3350–3367.e3319 (2023).
Smith, R. et al. The α-synuclein PET tracer [18F]ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases. Nat. Commun. 14, 6750 (2023).
Matsuoka, K. et al. High-contrast imaging of α-synuclein pathologies in living patients with multiple system atrophy. Mov. Disord. 37, 2159–2161 (2022).
den Heijer, J. M. et al. A biomarker study in patients with GBA1-Parkinson’s disease and healthy controls. Mov. Disord. 38, 783–795 (2023).
Al Shweiki, M. R. et al. Neurofilament light chain as a blood biomarker to differentiate psychiatric disorders from behavioural variant frontotemporal dementia. J. Psychiatr. Res. 113, 137–140 (2019).
Del Campo, M. et al. New developments of biofluid-based biomarkers for routine diagnosis and disease trajectories in frontotemporal dementia. Alzheimers Dement. 18, 2292–2307 (2022).
Tsai, R. M. et al. 18F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res. Ther. 11, 13 (2019).
Aguero, C. et al. Autoradiography validation of novel tau PET tracer [F-18]-MK-6240 on human postmortem brain tissue. Acta Neuropathology. Commun. 7, 37 (2019).
Santillo, A. F. et al. [18F]RO948 tau positron emission tomography in genetic and sporadic frontotemporal dementia syndromes. Eur. J. Nucl. Med. Mol. Imaging 50, 1371–1383 (2023).
Chatterjee, M. et al. Plasma extracellular vesicle tau and TDP-43 as diagnostic biomarkers in FTD and ALS. Nat. Med. 30, 1771–1783 (2024).
Scialo, C. et al. TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2, fcaa142 (2020).
Fontana, E. et al. Detection of TDP-43 seeding activity in the olfactory mucosa from patients with frontotemporal dementia. Alzheimers Dement. 20, 1156–1165 (2023).
Mehta, P. R., Brown, A. L., Ward, M. E. & Fratta, P. The era of cryptic exons: implications for ALS-FTD. Mol. Neurodegener. 18, 16 (2023).
Sellami, L. et al. Plasma progranulin levels for frontotemporal dementia in clinical practice: a 10-year French experience. Neurobiol. Aging 91, 167.e161–167.e169 (2020).
Swift, I. J. et al. A systematic review of progranulin concentrations in biofluids in over 7,000 people-assessing the pathogenicity of GRN mutations and other influencing factors. Alzheimers Res. Ther. 16, 66 (2024).
Miller, T. M. et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 387, 1099–1110 (2022).
Tabrizi, S. J. et al. Targeting huntingtin expression in patients with Huntington’s disease. N. Engl. J. Med. 380, 2307–2316 (2019).
Karikari, T. K. et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433 (2020).
Mielke, M. M. et al. Comparison of CSF phosphorylated tau 181 and 217 for cognitive decline. Alzheimers Dement. 18, 602–611 (2022).
Ashton, N. J. et al. Differential roles of Abeta42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat. Med. 28, 2555–2562 (2022).
Lagarde, J. et al. Tau-PET imaging predicts cognitive decline and brain atrophy progression in early Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 93, 459–467 (2022).
Wilson, R. S. et al. TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol. 70, 1418–1424 (2013).
Tosun, D. et al. A cross-sectional study of alpha-synuclein seed amplification assay in Alzheimer’s disease neuroimaging initiative: prevalence and associations with Alzheimer’s disease biomarkers and cognitive function. Alzheimers Dement. 20, 5114–5131 (2024).
Sperling, R. A. et al. Amyloid and tau prediction of cognitive and functional decline in unimpaired older individuals: longitudinal data from the A4 and LEARN studies. J. Prev. Alzheimers Dis. 11, 802–813 (2024).
Papp, K. V., Rentz, D. M., Orlovsky, I., Sperling, R. A. & Mormino, E. C. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: the PACC5. Alzheimers Dement. 3, 668–677 (2017).
Ayutyanont, N. et al. The Alzheimer’s prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer’s disease treatments in presenilin 1 E280A mutation carriers. J. Clin. Psychiatry 75, 652–660 (2014).
Mata, I. F. et al. GBA Variants are associated with a distinct pattern of cognitive deficits in Parkinson’s disease. Mov. Disord. 31, 95–102 (2016).
Davis, M. Y. et al. Association of GBA mutations and the E326K polymorphism with motor and cognitive progression in Parkinson disease. JAMA Neurol. 73, 1217–1224 (2016).
Shahid, M. et al. An increased rate of longitudinal cognitive decline is observed in Parkinson’s disease patients with low CSF Aß42 and an APOE ε4 allele. Neurobiol. Dis. 127, 278–286 (2019).
Batzu, L. et al. Plasma p-tau181, neurofilament light chain and association with cognition in Parkinson’s disease. NPJ Parkinsons Dis. 8, 154 (2022).
Liu, Y., Dou, K., Xue, L., Li, X. & Xie, A. Neurofilament light as a biomarker for motor decline in Parkinson’s disease. Front. Neurosci. 16, 959261 (2022).
Urso, D., Batzu, L., Logroscino, G., Ray Chaudhuri, K. & Pereira, J. B. Neurofilament light predicts worse nonmotor symptoms and depression in Parkinson’s disease. Neurobiol. Dis. 185, 106237 (2023).
Poos, J. M. et al. Longitudinal cognitive changes in genetic frontotemporal dementia within the GENFI cohort. Neurology 99, e281–e295 (2022).
Asken, B. M. et al. Plasma inflammation for predicting phenotypic conversion and clinical progression of autosomal dominant frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 94, 541–549 (2023).
Saracino, D. et al. Plasma NfL levels and longitudinal change rates in C9orf72 and GRN-associated diseases: from tailored references to clinical applications. J. Neurol. Neurosurg. Psychiatry 92, 1278–1288 (2021).
Rojas, J. C. et al. Plasma neurofilament light for prediction of disease progression in familial frontotemporal lobar degeneration. Neurology 96, e2296–e2312 (2021).
Zetterberg, H. et al. The role of neurofilament light in genetic frontotemporal lobar degeneration. Brain Commun. 5, fcac310 (2023).
Giannini, L. A. A. et al. Clinical value of longitudinal serum neurofilament light chain in prodromal genetic frontotemporal dementia. Neurology 101, e1069–e1082 (2023).
Staffaroni, A. M. et al. Temporal order of clinical and biomarker changes in familial frontotemporal dementia. Nat. Med. 28, 2194–2206 (2022).
Heller, C. et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 91, 263–270 (2020).
van der Ende, E. L. et al. Neuronal pentraxin 2: a synapse-derived CSF biomarker in genetic frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 91, 612–621 (2020).
Poos, J. M. et al. Longitudinal brain atrophy rates in presymptomatic carriers of genetic frontotemporal dementia. Neurology 99, e2661–e2671 (2022).
Scheerens, H. et al. Current status of companion and complementary diagnostics: strategic considerations for development and launch. Clin. Transl. Sci. 10, 84–92 (2017).
Dunn, B., Stein, P., Temple, R. & Cavazzoni, P. An appropriate use of accelerated approval – aducanumab for Alzheimer’s disease. N. Engl. J. Med. 385, 856–857 (2021).
Mintun, M. A. et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med. 384, 1691–1704 (2021).
Salloway, S. et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 79, 13–21 (2022).
Tijms, B. M. et al. Cerebrospinal fluid proteomics in patients with Alzheimer’s disease reveals five molecular subtypes with distinct genetic risk profiles. Nat. Aging 4, 33–47 (2024).
Dammer, E. B. et al. Proteomic analysis of Alzheimer’s disease cerebrospinal fluid reveals alterations associated with APOE epsilon4 and atomoxetine treatment. Sci. Transl. Med. 16, eadn3504 (2024).
Jennings, D. et al. LRRK2 inhibition by BIIB122 in healthy participants and patients with Parkinson’s disease. Mov. Disord. 38, 386–398 (2023).
den Heijer, J. M. et al. A phase 1B trial in GBA1-associated Parkinson’s disease of BIA-28-6156, a glucocerebrosidase activator. Mov. Disord. 38, 1197–1208 (2023).
Vissers, M., Heuberger, J. & Groeneveld, G. J. Targeting for success: demonstrating proof-of-concept with mechanistic early phase clinical pharmacology studies for disease-modification in neurodegenerative disorders. Int. J. Mol. Sci. 22, 1615 (2021).
Potter, W. Z. Optimizing early Go/No Go decisions in CNS drug development. Expert Rev. Clin. Pharmacol. 8, 155–157 (2015).
Kennedy, M. E. et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS beta-amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl. Med. 8, 363ra150 (2016).
Bateman, R. J. et al. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann. Neurol. 66, 48–54 (2009).
Doody, R. S. et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 369, 341–350 (2013).
Egan, M. F. et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med. 380, 1408–1420 (2019).
Pontecorvo, M. J. et al. Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 79, 1250–1259 (2022).
Novak, P. et al. ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat. Aging 1, 521–534 (2021).
Mummery, C. J. et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer’s disease: a phase 1b, randomized, placebo-controlled trial. Nat. Med. 29, 1437–1447 (2023).
Jankovic, J. et al. Safety and tolerability of multiple ascending doses of PRX002/RG7935, an anti-α-synuclein monoclonal antibody, in patients with Parkinson disease: a randomized clinical trial. JAMA Neurol. 75, 1206–1214 (2018).
Padmanabhan, S. et al. An assessment of LRRK2 serine 935 phosphorylation in human peripheral blood mononuclear cells in idiopathic Parkinson’s disease and G2019S LRRK2 cohorts. J. Parkinsons Dis. 10, 623–629 (2020).
Steger, M. et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 5, e12813 (2016).
Vissers, M. et al. A leucine-rich repeat kinase 2 (LRRK2) pathway biomarker characterization study in patients with Parkinson’s disease with and without LRRK2 mutations and healthy controls. Clin. Transl. Sci. 16, 1408–1420 (2023).
Gomes, S. et al. Elevated urine BMP phospholipids in LRRK2 and VPS35 mutation carriers with and without Parkinson’s disease. NPJ Parkinsons Dis. 9, 52 (2023).
Merchant, K. M. et al. LRRK2 and GBA1 variant carriers have higher urinary bis(monacylglycerol) phosphate concentrations in PPMI cohorts. NPJ Parkinsons Dis. 9, 30 (2023).
Peterschmitt, M. J. et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of oral venglustat in healthy volunteers. Clin. Pharmacol. Drug Dev. 10, 86–98 (2021).
Ljubenkov, P. A. et al. Effect of the histone deacetylase inhibitor FRM-0334 on progranulin levels in patients with progranulin gene haploinsufficiency: a randomized clinical trial. JAMA Netw. Open 4, e2125584 (2021).
Kurnellas, M. et al. Latozinemab, a novel progranulin-elevating therapy for frontotemporal dementia. J. Transl. Med. 21, 387 (2023).
Lehmer, C. et al. Poly-GP in cerebrospinal fluid links C9orf72-associated dipeptide repeat expression to the asymptomatic phase of ALS/FTD. EMBO Mol. Med. 9, 859–868 (2017).
Gendron, T. F. et al. Poly(GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci. Transl. Med. 9, eaai7866 (2017).
Wilson, K. M. et al. Development of a sensitive trial-ready poly(GP) CSF biomarker assay for C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 93, 761–771 (2022).
Tran, H. et al. Suppression of mutant C9orf72 expression by a potent mixed backbone antisense oligonucleotide. Nat. Med. 28, 117–124 (2022).
Krishnan, G. et al. Poly(GR) and poly(GA) in cerebrospinal fluid as potential biomarkers for C9ORF72-ALS/FTD. Nat. Commun. 13, 2799 (2022).
Wang, Q. et al. Investigating white matter neuroinflammation in Alzheimer disease using diffusion-based neuroinflammation imaging. Neurology 102, e208013 (2024).
Masdeu, J. C., Pascual, B. & Fujita, M. Imaging neuroinflammation in neurodegenerative disorders. J. Nucl. Med. 63, 45S–52S (2022).
Zhou, R. et al. PET imaging of neuroinflammation in Alzheimer’s disease. Front. Immunol. 12, 739130 (2021).
Arnold, S. E. et al. Biological effects of sodium phenylbutyrate and taurursodiol in Alzheimer’s disease. Alzheimers Dement. 10, e12487 (2024).
Ulland, T. K. et al. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170, 649–663.e613 (2017).
Schlepckow, K., Morenas-Rodriguez, E., Hong, S. & Haass, C. Stimulation of TREM2 with agonistic antibodies-an emerging therapeutic option for Alzheimer’s disease. Lancet Neurol. 22, 1048–1060 (2023).
Zhao, A. et al. Soluble TREM2 levels associate with conversion from mild cognitive impairment to Alzheimer’s disease. J Clin Invest 132, e158708 (2022).
Lista, S. et al. Tracking neuroinflammatory biomarkers in Alzheimer’s disease: a strategy for individualized therapeutic approaches? J. Neuroinflammation 21, 187 (2024).
Cummings, J. Disease modification and neuroprotection in neurodegenerative disorders. Transl. Neurodegener. 6, 25 (2017).
Food and Drug Administration. Early Alzheimer’s Disease: Developing Drugs for Treatment https://www.fda.gov/regulatory-information/search-fda-guidance-documents/early-alzheimers-disease-developing-drugs-treatment (FDA, 2024).
Bodick, N. et al. Protocols to demonstrate slowing of Alzheimer disease progression. Position paper from the International Working Group on Harmonization of Dementia Drug Guidelines. Alzheimer Dis. Assoc. Disord. 11, 50–53 (1997).
Bhattaram, V. A., Siddiqui, O., Kapcala, L. P. & Gobburu, J. V. Endpoints and analyses to discern disease-modifying drug effects in early Parkinson’s disease. AAPS J. 11, 456–464 (2009).
Cummings, J., Fox, N., Vellas, B., Aisen, P. & Shan, G. Biomarker and clinical trial design support for disease-modifying therapies: report of a survey of the EU/US: Alzheimer’s disease task force. J. Prev. Alzheimers Dis. 5, 103–109 (2018).
Liu-Seifert, H. et al. Delayed-start analyses in the phase 3 solanezumab EXPEDITION3 study in mild Alzheimer’s disease. J. Prev. Alzheimers Dis. 5, 8–14 (2018).
Liu-Seifert, H., Andersen, S. W., Lipkovich, I., Holdridge, K. C. & Siemers, E. A novel approach to delayed-start analyses for demonstrating disease-modifying effects in Alzheimer’s disease. PLoS ONE 10, e0119632 (2015).
Elhage, A. et al. Defining benefit: clinically and biologically meaningful outcomes in the next-generation Alzheimer’s disease clinical care pathway. Alzheimers Dement. 21, e14425 (2025).
Dickson, S. P. et al. ‘Time Saved’ as a demonstration of clinical meaningfulness and illustrated using the donanemab TRAILBLAZER-ALZ study findings. J. Prev. Alzheimers Dis. 10, 595–599 (2023).
McGhee, D. J., Ritchie, C. W., Zajicek, J. P. & Counsell, C. E. A review of clinical trial designs used to detect a disease-modifying effect of drug therapy in Alzheimer’s disease and Parkinson’s disease. BMC Neurol. 16, 92 (2016).
Bateman, R. J. et al. Two phase 3 trials of gantenerumab in early Alzheimer’s disease. N. Engl. J. Med. 389, 1862–1876 (2023).
Cummings, J. Anti-amyloid monoclonal antibodies are transformative treatments that redefine Alzheimer’s disease therapeutics. Drugs 83, 569–576 (2023).
Mila-Aloma, M. et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-beta pathology in preclinical Alzheimer’s disease. Nat. Med. 28, 1797–1801 (2022).
Dore, V. et al. Relationship between amyloid and tau levels and its impact on tau spreading. Eur. J. Nucl. Med. Mol. Imaging 48, 2225–2232 (2021).
Gueorguieva, I. et al. Donanemab exposure and efficacy relationship using modeling in Alzheimer’s disease. Alzheimers Dement. 9, e12404 (2023).
Roveta, F. et al. Synaptic proteins as fluid biomarkers in Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis. 90, 1381–1393 (2022).
Warpechowski, M., Warpechowski, J., Kulczynska-Przybik, A. & Mroczko, B. Biomarkers of activity-dependent plasticity and persistent enhancement of synaptic transmission in Alzheimer disease: a review of the current status. Med. Sci. Monit. 29, e938826 (2023).
Swanson, C. J. et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimers Res. Ther. 13, 80–94 (2021).
Walker, Z. et al. Clinical utility of FDG PET in Parkinson’s disease and atypical parkinsonism associated with dementia. Eur. J. Nucl. Med. Mol. Imaging 45, 1534–1545 (2018).
Nestor, P. J. et al. Clinical utility of FDG-PET for the differential diagnosis among the main forms of dementia. Eur. J. Nucl. Med. Mol. Imaging 45, 1509–1525 (2018).
Simuni, T. et al. Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s Progression Markers Initiative Cohort. Mov. Disord. 33, 771–782 (2018).
Whone, A. et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 142, 512–525 (2019).
Katz, R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx 1, 189–195 (2004).
Food and Drug Administration. Expedited Programs for Serious Conditions – Drugs and Biologics https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expedited-programs-serious-conditions-drugs-and-biologics (FDA, 2014).
Sevigny, J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016).
Assuncao, S. S. et al. Meaningful benefits: a framework to assess disease-modifying therapies in preclinical and early Alzheimer’s disease. Alzheimers Res. Ther. 14, 54 (2022).
Reiman, E. M., Cummings, J. L., Langbaum, J. B., Mattke, S. & Alexander, R. C. A chance to prevent Alzheimer’s disease sooner than you think. Lancet Neurol. 23, 144–145 (2024).
Aisen, P. et al. The case for regulatory approval of amyloid-lowering immunotherapies in Alzheimer’s disease based on clearcut biomarker evidence. Alzheimers Dement. 21, e14342 (2024).
Therriault, J. et al. Diagnosis of Alzheimer’s disease using plasma biomarkers adjusted to clinical probability. Nat. Aging 4, 1529–1537 (2024).
McDade, E. et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res. Ther. 14, 191 (2022).
Heiss, J. D. et al. Trial of magnetic resonance-guided putaminal gene therapy for advanced Parkinson’s disease. Mov. Disord. 34, 1073–1078 (2019).
Alcalay, R. N. et al. Longitudinal measurements of glucocerebrosidase activity in Parkinson’s patients. Ann. Clin. Transl. Neurol. 7, 1816–1830 (2020).
Lerche, S. et al. The mutation matters: CSF profiles of GCase, sphingolipids, α-synuclein in PD(GBA). Mov. Disord. 36, 1216–1228 (2021).
Meeter, L. H. et al. Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Ann. Clin. Transl. Neurol. 3, 623–636 (2016).
Oeckl, P. et al. Serum GFAP differentiates Alzheimer’s disease from frontotemporal dementia and predicts MCI-to-dementia conversion. J. Neurol. Neurosurg. Psychiatry 93, 659–667 (2022).
Whitwell, J. L. et al. Brain atrophy over time in genetic and sporadic frontotemporal dementia: a study of 198 serial magnetic resonance images. Eur. J. Neurol. 22, 745–752 (2015).
Muller, H. P. et al. Combined cerebral atrophy score in Huntington’s disease based on atlas-based MRI volumetry: sample size calculations for clinical trials. Parkinsonism Relat. Disord. 63, 179–184 (2019).
Tabrizi, S. J. et al. Potential disease-modifying therapies for Huntington’s disease: lessons learned and future opportunities. Lancet Neurol. 21, 645–658 (2022).
Bejanin, A. et al. Longitudinal structural and metabolic changes in frontotemporal dementia. Neurology 95, e140–e154 (2020).
Gordon, E., Bocchetta, M., Nicholas, J., Cash, D. M. & Rohrer, J. D. A comparison of automated atrophy measures across the frontotemporal dementia spectrum: implications for trials. Neuroimage Clin. 32, 102842 (2021).
Reiman, E. M. & Alzheimer’s Disease Biomarkers Working Group for the Alliance for Aging, R. Fluorodeoxyglucose positron emission tomography: emerging roles in the evaluation of putative Alzheimer’s disease-modifying treatments. Neurobiol. Aging 32, S44–S47 (2011).
Sun, D., Gao, W., Hu, H. & Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 12, 3049–3062 (2022).
Meunier, L. & Larrey, D. Drug-induced liver injury: biomarkers, requirements, candidates, and validation. Front. Pharmacol. 10, 1482 (2019).
Cummings, J. et al. Aducanumab: appropriate use recommendations update. J. Prev. Alzheimers Dis. 9, 221–230 (2022).
Cummings, J. et al. Lecanemab: appropriate use recommendations. J. Prev. Alzheimers Dis. 10, 362–377 (2023).
Honig, L. S. et al. Updated safety results from phase 3 lecanemab study in early Alzheimer’s disease. Alzheimers Res. Ther. 16, 105 (2024).
Alves, F., Kalinowski, P. & Ayton, S. Accelerated brain volume loss caused by anti-beta-amyloid drugs: a systematic review and meta-analysis. Neurology 100, e2114–e2124 (2023).
Belder, C. R. S. et al. Brain volume change following anti-amyloid beta immunotherapy for Alzheimer’s disease: amyloid-removal-related pseudo-atrophy. Lancet Neurol. 23, 1025–1034 (2024).
Baptista, M. A. et al. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS ONE 8, e80705 (2013).
Baptista, M. A. S. et al. LRRK2 inhibitors induce reversible changes in nonhuman primate lungs without measurable pulmonary deficits. Sci. Transl. Med. 12, eaav0820 (2020).
Sevigny, J. et al. Progranulin AAV gene therapy for frontotemporal dementia: translational studies and phase 1/2 trial interim results. Nat. Med. 30, 1406–1415 (2024).
Woodcock, J. & LaVange, L. M. Master protocols to study multiple therapies, multiple diseases, or both. N. Engl. J. Med. 377, 62–70 (2017).
Roustit, M. et al. Platform trials. Therapie 78, 29–38 (2023).
Adaptive Platform Trials Coalition. Adaptive platform trials: definition, design, conduct and reporting considerations. Nat. Rev. Drug Discov. 18, 797–807 (2019).
Berry, S. M., Connor, J. T. & Lewis, R. J. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA 313, 1619–1620 (2015).
Quintana, M. et al. Design and statistical innovations in a platform trial for amyotrophic lateral sclerosis. Ann. Neurol. 94, 547–560 (2023).
Foltynie, T. et al. Towards a multi-arm multi-stage platform trial of disease modifying approaches in Parkinson’s disease. Brain 146, 2717–2722 (2023).
Cummings, J., Montes, A., Kamboj, S. & Cacho, J. F. The role of basket trials in drug development for neurodegenerative disorders. Alzheimers Res. Ther. 14, 73 (2022).
Vassar, R. Editorial: implications for BACE1 inhibitor clinical trials: adult conditional BACE1 knockout mice exhibit axonal organization defects in the hippocampus. J. Prev. Alzheimers Dis. 6, 78–84 (2019).
Mattsson-Carlgren, N. et al. Cerebrospinal fluid biomarkers in autopsy-confirmed Alzheimer disease and frontotemporal lobar degeneration. Neurology 98, e1137–e1150 (2022).
Soleimani-Meigooni, D. N. et al. 18F-flortaucipir PET to autopsy comparisons in Alzheimer’s disease and other neurodegenerative diseases. Brain 143, 3477–3494 (2020).
Teipel, S. J. et al. Association of PET-based stages of amyloid deposition with neuropathological markers of Abeta pathology. Ann. Clin. Transl. Neurol. 8, 29–42 (2021).
Syrjanen, J. A. et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 18, 1128–1140 (2022).
Brum, W. S. et al. Effect of neprilysin inhibition on Alzheimer disease plasma biomarkers: a secondary analysis of a randomized clinical trial. JAMA Neurol. 81, 197–200 (2024).
Verberk, I. M. W. et al. Characterization of pre-analytical sample handling effects on a panel of Alzheimer’s disease-related blood-based biomarkers: results from the Standardization of Alzheimer’s Blood Biomarkers (SABB) working group. Alzheimers Dement. 18, 1484–1497 (2022).
Grill, J. D. et al. Eligibility rates among racially and ethnically diverse US participants in phase 2 and phase 3 placebo-controlled, double-blind, randomized trials of lecanemab and elenbecestat in early Alzheimer disease. Ann. Neurol. 95, 288–298 (2024).
Food and Drug Administration. Diversity action plans to improve enrollment of participants from underrepresented populations in clinical studies. Draft Guidance for Industry https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-action-plans-improve-enrollment-participants-underrepresented-populations-clinical-studies (FDA, 2024).
Jutten, R. J. et al. Why a clinical trial is as good as its outcome measure: a framework for the selection and use of cognitive outcome measures for clinical trials of Alzheimer’s disease. Alzheimers Dement. 19, 708–720 (2023).
Feldman, H. H. et al. A framework for translating tauopathy therapeutics: drug discovery to clinical trials. Alzheimers Dement. 20, 8129–8152 (2024).
Wilson, D. M. et al. Hallmarks of neurodegenerative diseases. Cell 186, 693–714 (2023).
Robinson, J. L. et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141, 2181–2193 (2018).
Toledo, J. B. et al. Dementia with Lewy bodies: impact of co-pathologies and implications for clinical trial design. Alzheimers Dement. 19, 318–332 (2023).
Smith, C. et al. Neuropathology of dementia in patients with Parkinson’s disease: a systematic review of autopsy studies. J. Neurol. Neurosurg. Psychiatry 90, 1234–1243 (2019).
Nichols, E. et al. The prevalence, correlation, and co-occurrence of neuropathology in old age: harmonisation of 12 measures across six community-based autopsy studies of dementia. Lancet Healthy Longev. 4, e115–e125 (2023).
Ashton, N. J. et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 12, 3400 (2021).
Bridel, C. et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 76, 1035–1048 (2019).
Cummings, J. L. et al. The therapeutic landscape of tauopathies: challenges and prospects. Alzheimers Res. Ther. 15, 168 (2023).
Writing Group; Edavarone (MCI-186) ALS 19 Study Group.Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16, 505–512 (2017).
Abe, K. et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Frontotemporal Degener. 15, 610–617 (2014).
Food and Drug Administration. Qualification Process for Drug Development Tools Guidance for Industry and FDA Staff https://www.fda.gov/regulatory-information/search-fda-guidance-documents/qualification-process-drug-development-tools-guidance-industry-and-fda-staff (2020).
Food and Drug Administration. Biomarker qualification: evidentiary framework. Guidance for Industry and FDA staff. DRAFT GUIDANCE. https://www.fda.gov/media/119271/download (FDA, 2018).
Gaetani, L. et al. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 90, 870–881 (2019).
European Medicines Agency. Qualification Opinion of Alzheimer’s Disease Novel Methodologies/Biomarkers for PET Amyloid Imaging as a Biomarker for Enrichment for use in Regulatory Trials in Predementia Alzheimer’s Disease https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/qualification-opinion-alzheimers-disease-novel-methodologies-biomarkers-use-cerebrospinal-fluid-amyloid-beta-1-42-and-t-tau-and-or-positron-emission-tomography-amyloid-imaging-positive-negative_en.pdf (EMA, 2012).
European Medicine Agencies. Qualification Opinion on Dopamine Transporter Imaging as an Enrichment Biomarker for Parkinson’s Disease Clinical Trials in Patients with Early Parkinsonian Symptoms. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/qualification-opinion-dopamine-transporter-imaging-enrichment-biomarker-parkinsons-disease-clinical-trials-patients-early-parkinsonian-symptoms_en.pdf (EMA, 2018).
Ferreira, G. S., Veening-Griffioen, D. H., Boon, W. P. C., Moors, E. H. M. & van Meer, P. J. K. Levelling the translational gap for animal to human efficacy data. Animals 10, 1199 (2020).
Sabbagh, J. J., Kinney, J. W. & Cummings, J. L. Alzheimer’s disease biomarkers in animal models: closing the translational gap. Am. J. Neurodegener. Dis. 2, 108–120 (2013).
Liu, X. et al. Mechanistic pharmacokinetic-pharmacodynamic modeling of BACE1 inhibition in monkeys: development of a predictive model for amyloid precursor protein processing. Drug Metab. Dispos. 41, 1319–1328 (2013).
Yu, Y. J. et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci. Transl. Med. 6, 261ra154 (2014).
Wang, S. et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 217, e20200785 (2020).
Schauer, S. P. et al. Primate cerebrospinal fluid CHI3L1 reflects brain TREM2 agonism. Alzheimers Dement. 20, 5861–5888 (2024).
Beckman, D. et al. A novel tau-based rhesus monkey model of Alzheimer’s pathogenesis. Alzheimers Dement. 17, 933–945 (2021).
Beckman, D. et al. Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proc. Natl Acad. Sci. USA 116, 26239–26246 (2019).
Sukoff Rizzo, S. J. et al. Bridging the rodent to human translational gap: marmosets as model systems for the study of Alzheimer’s disease. Alzheimers Dement. 9, e12417 (2023).
Cummings, J. L. Translational scoring of candidate treatments for Alzheimer’s disease: a systematic approach. Dement. Geriatr. Cogn. Disord. 49, 22–37 (2020).
Boxer, A. L. & Sperling, R. Accelerating Alzheimer’s therapeutic development: the past and future of clinical trials. Cell 186, 4757–4772 (2023).
Sanchez, E. et al. Association of plasma biomarkers with cognition, cognitive decline, and daily function across and within neurodegenerative diseases: results from the Ontario Neurodegenerative Disease Research Initiative. Alzheimers Dement. 20, 1753–1770 (2024).
Friedman, L. G. et al. Value-generating exploratory trials in neurodegenerative dementias. Neurology 96, 944–954 (2021).
Iturria-Medina, Y. et al. Unified epigenomic, transcriptomic, proteomic, and metabolomic taxonomy of Alzheimer’s disease progression and heterogeneity. Sci. Adv. 8, eabo6764 (2022).
Vromen, E. M. et al. CSF proteomic signature predicts progression to Alzheimer’s disease dementia. Alzheimers Dement. 8, e12240 (2022).
Cummings, J. & Fox, N. Defining disease modifying therapy for Alzheimer’s disease. J. Prev. Alzheimers Dis. 4, 109–115 (2017).
Vijiaratnam, N. & Foltynie, T. How should we be using biomarkers in trials of disease modification in Parkinson’s disease? Brain 146, 4845–4869 (2023).
Hu, Y. et al. Fluid biomarkers in the context of amyloid-targeting disease-modifying treatments in Alzheimer’s disease. Med 5, 1206–1226 (2024).
Swift, I. J. et al. Fluid biomarkers in frontotemporal dementia: past, present and future. J. Neurol. Neurosurg. Psychiatry 92, 204–215 (2021).
Morant, A. V., Jagalski, V. & Vestergaard, H. T. Labeling of disease-modifying therapies for neurodegenerative disorders. Front. Med. 6, 223 (2019).
Food and Drug Administration. Clinical Studies Section of Labeling for Human Prescription Drug and Biological Products — Content and Format. Guidance for Industry https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-studies-section-labeling-human-prescription-drug-and-biological-products-content-and-format (FDA, 2006).
Food and Drug Administration. Developing Targeted Therapies in Low-Frequency Molecular Subsets of a Disease. Guidance for Industry. https://www.fda.gov/media/117173/download (FDA, 2018).
Acknowledgements
J.L.C. is supported by NIGMS grant P20GM109025; NINDS grant U01NS093334; NIA grant R01AG053798; NIA grant P30AG072959; NIA grant R35AG71476; NIA R25 AG083721-01; Alzheimer’s Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; Joy Chambers-Grundy Endowment. The authors thank J. Silva-Rodríguez (Reina Sofia Alzheimer Centre, CIEN Foundation, ISCIII, Madrid, Spain), M. Malpetti (University of Cambridge, UK), E. Westman (Karolinska Institutet, Sweden) and A. Moscoso (University of Gothenburg, Sweden) for their generous support creating the imaging figures.
Author information
Authors and Affiliations
Contributions
J.L.C., C.E.T., B.K.F., I.L.B., K.R.W., M.S. and P.S. researched data for the article. All authors contributed substantially to discussion of the content. J.L.C., C.E.T., B.K.F., I.L.B., K.R.W., M.S. and P.S. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.L.C. has provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, ALZpath, Aprinoia, AriBio, Artery, Biogen, Biohaven, BioVie, BioXcel, Bristol-Myers Squib, Cassava, Cerecin, Diadem, Eisai, GAP Foundation, GemVax, Janssen, Jocasta, Karuna, Lighthouse, Lilly, Lundbeck, LSP/eqt, Merck, NervGen, New Amsterdam, Novo Nordisk, Oligomerix, Optoceutics, Ono, Otsuka, Oxford Brain Diagnostics, Prothena, ReMYND, Roche, Sage Therapeutics, Signant Health, Simcere, Sinaptica, Suven, TrueBinding, Vaxxinity, and Wren pharmaceutical, assessment, and investment companies. B.K.F. is a full-time employee of The Michael J. Fox Foundation for Parkinson’s Research and has no conflicts of interest to disclose. I.L.B. has served on a Medical Advisory Board and has a consultancy agreement with Alector and Prevail Therapeutics/Lilly. C.E.T. performed contract research for Acumen, ADx Neurosciences, AC Immune, Alamar, Aribio, Axon Neurosciences, Beckman–Coulter, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Eli Lilly, Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, Olink, PeopleBio, Quanterix, Roche, Toyama, Vivoryon. C.E.T. is editor in chief of Alzheimer’s Research & Therapy, and serves on editorial boards of Medidact Neurologie/Springer, and Neurology: Neuroimmunology & Neuroinflammation, Alzheimer’s & Dementia and Molecular Neurodegeneration. K.R.W. is a full-time employee of Eisai. M.S. has served or serves on advisory boards for Arvakor, Eli Lilly, Novo Nordisk and Roche, has received speaker honoraria from Bioarctic, Genentech, Novo Nordisk, Eli Lilly, Roche and Triolabs and receives research support (paid to institution) from Alzpath, Beckman–Coulter, Bioarctic, Gates Ventures, Novo Nordisk and Roche. He is a co-founder and stakeholder of Centile Bioscience and serves as Associate Editor of Alzheimer’s Research & Therapy. B.D. has provided consultation to Prothena Biosciences. P.S. is a full-time employee of EQT Life Sciences (formerly LSP). He reports having received consultancy fees (paid to Vrije Universiteit Amsterdam) from AC Immune, Alzheon, BrainStorm Cell, FUJIFILM/Toyama, Green Valley and Novo Nordisk.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Naazneen Khan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
FDA Biomarker Qualification Program: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/biomarker-qualification-program
Supplementary information
Glossary
- Amyloid plaques
-
Extracellular aggregates of Aβ protein in the brain.
- Amyloid-related imaging abnormalities
-
Brain effusions or microhaemorrhages that occur in some patients receiving treatment with anti-amyloid monoclonal antibodies.
- Dipeptide protein repeats
-
Toxic proteins related to the GGGCC expansion of the C9orf72 gene that occurs in some genetic forms of frontotemporal dementia and amyotrophic lateral sclerosis.
- Levodopa-induced dyskinesias
-
Choreiform movements that may emerge after long-term treatment with levodopa or other dopaminergic agents.
- Neurofibrillary tangles
-
Intracellular aggregates of paired helical filaments composed of hyperphosphorylated tau protein.
- Oligomers
-
Molecules that consist of a few or many repeating subunits (for example, Aβ subunits in Alzheimer disease).
- Polygenic risk score
-
The relative estimate of an individual’s genetic liability to develop a disease.
- Positron emission tomography
-
An imaging technology that uses ligands labelled with positron-emitting isotopes to allow detection of abnormalities (for example, amyloid or tau protein deposits in Alzheimer disease) labelled by the administered ligand.
- Target engagement
-
Describes how well a drug binds to its intended target in a living system.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cummings, J.L., Teunissen, C.E., Fiske, B.K. et al. Biomarker-guided decision making in clinical drug development for neurodegenerative disorders. Nat Rev Drug Discov 24, 589–609 (2025). https://doi.org/10.1038/s41573-025-01165-w
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41573-025-01165-w
This article is cited by
-
Biochemical landscape of breast cancer: integrating serum markers with clinical prognosis and staging
Clinical and Translational Oncology (2025)