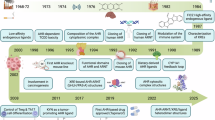
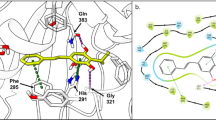
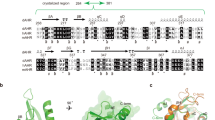
Abstract
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor originally identified as the target mediating the toxic effects of environmental pollutants including polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and dioxins. For years, AHR activation was actively avoided during drug development. However, the AHR was later identified as an important physiological regulator of the immune response. These findings triggered a paradigm shift that resulted in identification of the AHR as a regulator of both innate and adaptive immunity and outlined a pathway for its modulation by the diet, commensal flora and metabolism in the context of autoimmunity, cancer and infection. Moreover, the AHR was revealed as a candidate target for the therapeutic modulation of the immune response. Indeed, the first AHR-activating drug (tapinarof) was recently approved for the treatment of psoriasis. Clinical trials are underway to evaluate the effects of tapinarof and other AHR-targeting therapeutics in inflammatory diseases, cancer and infections. This Review outlines the molecular mechanism of AHR action, and describes how it regulates the immune response. We also discuss links to disease and AHR-targeting therapeutics that have been tested in past and ongoing clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Poland, A., Glover, E. & Kende, A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 251, 4936–4946 (1976). This study is the first to identify TCDD as an AHR ligand.
Poland, A. & Knutson, J. C. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 22, 517–554 (1982).
Kewley, R. J., Whitelaw, M. L. & Chapman-Smith, A. The mammalian basic helix–loop–helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 36, 189–204 (2004).
Gu, Y. Z., Hogenesch, J. B. & Bradfield, C. A. The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 40, 519–561 (2000).
Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 35, 307–340 (1995).
Swanson, H. I. & Bradfield, C. A. The AH-receptor: genetics, structure and function. Pharmacogenetics 3, 213–230 (1993).
Elferink, C. J., Ge, N. L. & Levine, A. Maximal aryl hydrocarbon receptor activity depends on an interaction with the retinoblastoma protein. Mol. Pharmacol. 59, 664–673 (2001).
Shivanna, S. et al. The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J. Am. Soc. Nephrol. 27, 189–201 (2016).
Furman, D. P., Oshchepkova, E. A., Oshchepkov, D. Y., Shamanina, M. Y. & Mordvinov, V. A. Promoters of the genes encoding the transcription factors regulating the cytokine gene expression in macrophages contain putative binding sites for aryl hydrocarbon receptor. Comput. Biol. Chem. 33, 465–468 (2009).
Durrin, L. K., Jones, P. B., Fisher, J. M., Galeazzi, D. R. & Whitlock, J. P. Jr 2,3,7,8-Tetrachlorodibenzo-p-dioxin receptors regulate transcription of the cytochrome P1-450 gene. J. Cell Biochem. 35, 153–160 (1987).
Mimura, J. et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes. Cell 2, 645–654 (1997).
Vorderstrasse, B. A., Fenton, S. E., Bohn, A. A., Cundiff, J. A. & Lawrence, B. P. A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol. Sci. 78, 248–257 (2004).
Moennikes, O. et al. A constitutively active dioxin/aryl hydrocarbon receptor promotes hepatocarcinogenesis in mice. Cancer Res. 64, 4707–4710 (2004).
Andersson, P. et al. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc. Natl Acad. Sci. USA 99, 9990–9995 (2002).
Mulero-Navarro, S. et al. Immortalized mouse mammary fibroblasts lacking dioxin receptor have impaired tumorigenicity in a subcutaneous mouse xenograft model. J. Biol. Chem. 280, 28731–28741 (2005).
Zudaire, E. et al. The aryl hydrocarbon receptor repressor is a putative tumor suppressor gene in multiple human cancers. J. Clin. Invest. 118, 640–650 (2008).
Barroso, A., Mahler, J. V., Fonseca-Castro, P. H. & Quintana, F. J. The aryl hydrocarbon receptor and the gut–brain axis. Cell. Mol. Immunol. 18, 259–268 (2021).
Rothhammer, V. et al. Detection of aryl hydrocarbon receptor agonists in human samples. Sci. Rep. 8, 1–8 (2018).
Denison, M. S. & Faber, S. C. And now for something completely different: diversity in ligand-dependent activation of Ah receptor responses. Curr. Opin. Toxicol. 2, 124–131 (2017).
Stejskalova, L., Dvorak, Z. & Pavek, P. Endogenous and exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr. Drug Metab. 12, 198–212 (2011).
Lamas, B., Natividad, J. M. & Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 11, 1024–1038 (2018).
Denison, M. S. & Nagy, S. R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43, 309–334 (2003).
Bissonnette, R., Gold, L. S., Rubenstein, D. S., Tallman, A. M. & Armstrong, A. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor–modulating agent. J. Am. Acad. Dermatol. 84, 1059–1067 (2021).
Larigot, L., Juricek, L., Dairou, J. & Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 7, 1–9 (2018).
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008). Together with Quintana et al. (2008), this work shows that different AHR ligands trigger varying immune responses, shaping immune regulation.
Dermavant Sciences. VTAMA (tapinarof) cream 1%: US prescribing information. VTAMA https://www.vtama.com/PI/ (2022).
Pandini, A., Denison, M. S., Song, Y., Soshilov, A. A. & Bonati, L. Structural and functional characterization of the aryl hydrocarbon receptor ligand binding domain by homology modeling and mutational analysis. Biochemistry 46, 696–708 (2007).
Fukunaga, B. N., Probst, M. R., Reisz-Porszasz, S. & Hankinson, O. Identification of functional domains of the aryl hydrocarbon receptor. J. Biol. Chem. 270, 29270–29278 (1995).
Pongratz, I., Antonsson, C., Whitelaw, M. L. & Poellinger, L. Role of the PAS domain in regulation of dimerization and DNA binding specificity of the dioxin receptor. Mol. Cell Biol. 18, 4079–4088 (1998).
Reyes, H., Reisz-Porszasz, S. & Hankinson, O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science 256, 1193–1195 (1992).
Wu, D., Potluri, N., Kim, Y. & Rastinejad, F. Structure and dimerization properties of the aryl hydrocarbon receptor PAS-A domain. Mol. Cell Biol. 33, 4346–4356 (2013).
Soshilov, A. & Denison, M. S. Role of the Per/Arnt/Sim domains in ligand-dependent transformation of the aryl hydrocarbon receptor. J. Biol. Chem. 283, 32995–33005 (2008).
Schulte, K. W., Green, E., Wilz, A., Platten, M. & Daumke, O. Structural basis for aryl hydrocarbon receptor-mediated gene activation. Structure 25, 1025–1033.e3 (2017).
Reisz-Porszasz, S., Probst, M. R., Fukunaga, B. N. & Hankinson, O. Identification of functional domains of the aryl hydrocarbon receptor nuclear translocator protein (ARNT). Mol. Cell Biol. 14, 6075–6086 (1994).
Jain, S., Dolwick, K. M., Schmidt, J. V. & Bradfield, C. A. Potent transactivation domains of the Ah receptor and the Ah receptor nuclear translocator map to their carboxyl termini. J. Biol. Chem. 269, 31518–31524 (1994).
Seok, S. H. et al. Structural hierarchy controlling dimerization and target DNA recognition in the AHR transcriptional complex. Proc. Natl Acad. Sci. USA 114, 5431–5436 (2017).
Dai, S. Q. et al. Structural insight into the ligand binding mechanism of aryl hydrocarbon receptor. Nat. Commun. 13, 6234 (2022).
Wen, Z. et al. Cryo-EM structure of the cytosolic AhR complex. Structure 31, 295–308.e4 (2023).
Gruszczyk, J. et al. Cryo-EM structure of the agonist-bound Hsp90–XAP2–AHR cytosolic complex. Nat. Commun. 13, 7010 (2022). Together with Dai et al. (2022) and Wen et al. (2023), this work defines structural mechanisms that regulate AHR activation.
Perdew, G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J. Biol. Chem. 263, 13802–13805 (1988).
Denis, M., Cuthill, S., Wikstrom, A. C., Poellinger, L. & Gustafsson, J. A. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem. Biophys. Res. Commun. 155, 801–807 (1988).
Grenert, J. P. et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 272, 23843–23850 (1997).
Nair, S. C. et al. A pathway of multi-chaperone interactions common to diverse regulatory proteins: estrogen receptor, Fes tyrosine kinase, heat shock transcription factor Hsf1, and the aryl hydrocarbon receptor. Cell Stress. Chaperones 1, 237–250 (1996).
Meyer, B. K. & Perdew, G. H. Characterization of the AhR–hsp90–XAP2 core complex and the role of the immunophilin-related protein XAP2 in AhR stabilization. Biochemistry 38, 8907–8917 (1999).
Carver, L. A. & Bradfield, C. A. Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J. Biol. Chem. 272, 11452–11456 (1997).
Dong, B. et al. FRET analysis of protein tyrosine kinase c-Src activation mediated via aryl hydrocarbon receptor. Biochim. Biophys. Acta 1810, 427–431 (2011).
Antonsson, C., Whitelaw, M. L., McGuire, J., Gustafsson, J. A. & Poellinger, L. Distinct roles of the molecular chaperone hsp90 in modulating dioxin receptor function via the basic helix–loop–helix and PAS domains. Mol. Cell Biol. 15, 756–765 (1995).
Kudo, I. et al. The regulation mechanisms of AhR by molecular chaperone complex. J. Biochem. 163, 223–232 (2018).
Meyer, B. K., Pray-Grant, M. G., Vanden Heuvel, J. P. & Perdew, G. H. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol. Cell Biol. 18, 978–988 (1998).
Ramadoss, P., Petrulis, J. R., Hollingshead, B. D., Kusnadi, A. & Perdew, G. H. Divergent roles of hepatitis B virus X-associated protein 2 (XAP2) in human versus mouse Ah receptor complexes. Biochemistry 43, 700–709 (2004).
Petrulis, J. R. & Perdew, G. H. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem. Biol. Interact. 141, 25–40 (2002).
Petrulis, J. R., Kusnadi, A., Ramadoss, P., Hollingshead, B. & Perdew, G. H. The hsp90 co-chaperone XAP2 alters importin beta recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J. Biol. Chem. 278, 2677–2685 (2003).
Kazlauskas, A., Poellinger, L. & Pongratz, I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (aryl hydrocarbon) receptor. J. Biol. Chem. 274, 13519–13524 (1999).
Pappas, B. et al. p23 protects the human aryl hydrocarbon receptor from degradation via a heat shock protein 90-independent mechanism. Biochem. Pharmacol. 152, 34–44 (2018).
Enan, E. & Matsumura, F. Identification of c-Src as the integral component of the cytosolic Ah receptor complex, transducing the signal of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) through the protein phosphorylation pathway. Biochem. Pharmacol. 52, 1599–1612 (1996).
Backlund, M. & Ingelman-Sundberg, M. Regulation of aryl hydrocarbon receptor signal transduction by protein tyrosine kinases. Cell Signal. 17, 39–48 (2005).
Soshilov, A. A. & Denison, M. S. Ligand promiscuity of aryl hydrocarbon receptor agonists and antagonists revealed by site-directed mutagenesis. Mol. Cell Biol. 34, 1707–1719 (2014).
Guyot, E., Chevallier, A., Barouki, R. & Coumoul, X. The AhR twist: ligand-dependent AhR signaling and pharmaco-toxicological implications. Drug Discov. Today 18, 479–486 (2013).
Safe, S., Jin, U. H., Park, H., Chapkin, R. S. & Jayaraman, A. Aryl hydrocarbon receptor (AHR) ligands as selective AHR modulators (SAhRMs). Int. J. Mol. Sci. https://doi.org/10.3390/ijms21186654 (2020).
Giani Tagliabue, S., Faber, S. C., Motta, S., Denison, M. S. & Bonati, L. Modeling the binding of diverse ligands within the Ah receptor ligand binding domain. Sci. Rep. 9, 10693 (2019).
Bisson, W. H. et al. Modeling of the aryl hydrocarbon receptor (AhR) ligand binding domain and its utility in virtual ligand screening to predict new AhR ligands. J. Med. Chem. 52, 5635–5641 (2009).
Avilla, M. N., Malecki, K. M. C., Hahn, M. E., Wilson, R. H. & Bradfield, C. A. The Ah receptor: adaptive metabolism, ligand diversity, and the xenokine model. Chem. Res. Toxicol. 33, 860–879 (2020).
Bradfield, C. A. & Bjeldanes, L. F. Structure–activity relationships of dietary indoles: a proposed mechanism of action as modifiers of xenobiotic metabolism. J. Toxicol. Environ. Health 21, 311–323 (1987).
Reyes-Hernandez, O. D. et al. 3,3′-Diindolylmethane and indole-3-carbinol: potential therapeutic molecules for cancer chemoprevention and treatment via regulating cellular signaling pathways. Cancer Cell Int. 23, 180 (2023).
De Kruif, C. A. et al. Structure elucidation of acid reaction products of indole-3-carbinol: detection in vivo and enzyme induction in vitro. Chem. Biol. Interact. 80, 303–315 (1991).
Bjeldanes, L. F., Kim, J. Y., Grose, K. R., Bartholomew, J. C. & Bradfield, C. A. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl Acad. Sci. USA 88, 9543–9547 (1991). This work describes the pro-ligand concept for AHR activation.
Seok, S. H. et al. Trace derivatives of kynurenine potently activate the aryl hydrocarbon receptor (AHR). J. Biol. Chem. 293, 1994–2005 (2018).
Bittinger, M. A., Nguyen, L. P. & Bradfield, C. A. Aspartate aminotransferase generates proagonists of the aryl hydrocarbon receptor. Mol. Pharmacol. 64, 550–556 (2003).
Nguyen, L. P. et al. d-Amino acid oxidase generates agonists of the aryl hydrocarbon receptor from d-tryptophan. Chem. Res. Toxicol. 22, 1897–1904 (2009).
Wei, G. Z. et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2021091118 (2021).
Nguyen, L. P. & Bradfield, C. A. The search for endogenous activators of the aryl hydrocarbon receptor. Chem. Res. Toxicol. 21, 102–116 (2008).
Schmidt, J. V. & Bradfield, C. A. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 12, 55–89 (1996).
Ikuta, T., Eguchi, H., Tachibana, T., Yoneda, Y. & Kawajiri, K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J. Biol. Chem. 273, 2895–2904 (1998).
Ikuta, T. et al. Nucleocytoplasmic shuttling of the aryl hydrocarbon receptor. J. Biochem. 127, 503–509 (2000).
Ikuta, T., Kobayashi, Y. & Kawajiri, K. Phosphorylation of nuclear localization signal inhibits the ligand-dependent nuclear import of aryl hydrocarbon receptor. Biochem. Biophys. Res. Commun. 317, 545–550 (2004).
Tsuji, N. et al. The activation mechanism of the aryl hydrocarbon receptor (AhR) by molecular chaperone HSP90. FEBS Open. Bio 4, 796–803 (2014).
Eguchi, H., Ikuta, T., Tachibana, T., Yoneda, Y. & Kawajiri, K. A nuclear localization signal of human aryl hydrocarbon receptor nuclear translocator/hypoxia-inducible factor 1β is a novel bipartite type recognized by the two components of nuclear pore-targeting complex. J. Biol. Chem. 272, 17640–17647 (1997).
Soshilov, A. A., Motta, S., Bonati, L. & Denison, M. S. Transitional states in ligand-dependent transformation of the aryl hydrocarbon receptor into its DNA-binding form. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21072474 (2020).
Wright, E. J., De Castro, K. P., Joshi, A. D. & Elferink, C. J. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr. Opin. Toxicol. 2, 87–92 (2017).
Swanson, H. I., Tullis, K. & Denison, M. S. Binding of transformed Ah receptor complex to a dioxin responsive transcriptional enhancer: evidence for two distinct heteromeric DNA-binding forms. Biochemistry 32, 12841–12849 (1993).
Yao, E. F. & Denison, M. S. DNA sequence determinants for binding of transformed Ah receptor to a dioxin-responsive enhancer. Biochemistry 31, 5060–5067 (1992).
Sun, Y. V., Boverhof, D. R., Burgoon, L. D., Fielden, M. R. & Zacharewski, T. R. Comparative analysis of dioxin response elements in human, mouse and rat genomic sequences. Nucleic Acids Res. 32, 4512–4523 (2004).
Apetoh, L. et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 (2010).
Gandhi, R. et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3+ regulatory T cells. Nat. Immunol. 11, 846–853 (2010).
Yeste, A. et al. IL-21 induces IL-22 production in CD4 + T cells. Nat. Commun. 5, 3753 (2014).
Mascanfroni, I. D. et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat. Med. 21, 638–646 (2015). Together with Gandhi et al. (2010) and Yeste et al. (2014), this work uncovers the role of the AHR in T cell regulation.
Nebert, D. W. & Karp, C. L. Endogenous functions of the aryl hydrocarbon receptor (AHR): intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR biology. J. Biol. Chem. 283, 36061–36065 (2008).
Mimura, J., Ema, M., Sogawa, K. & Fujii-Kuriyama, Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes. Dev. 13, 20–25 (1999).
Sakurai, S., Shimizu, T. & Ohto, U. The crystal structure of the AhRR–ARNT heterodimer reveals the structural basis of the repression of AhR-mediated transcription. J. Biol. Chem. 292, 17609–17616 (2017).
Ma, Q. Induction and superinduction of 2,3,7,8-tetrachlorodibenzo-rho-dioxin-inducible poly(ADP-ribose) polymerase: role of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator transcription activation domains and a labile transcription repressor. Arch. Biochem. Biophys. 404, 309–316 (2002).
MacPherson, L. et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin poly(ADP-ribose) polymerase (TiPARP, ARTD14) is a mono-ADP-ribosyltransferase and repressor of aryl hydrocarbon receptor transactivation. Nucleic Acids Res. 41, 1604–1621 (2013).
Ma, Q. & Baldwin, K. T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced degradation of aryl hydrocarbon receptor (AhR) by the ubiquitin-proteasome pathway. Role of the transcription activaton and DNA binding of AhR. J. Biol. Chem. 275, 8432–8438 (2000).
Davarinos, N. A. & Pollenz, R. S. Aryl hydrocarbon receptor imported into the nucleus following ligand binding is rapidly degraded via the cytosplasmic proteasome following nuclear export. J. Biol. Chem. 274, 28708–28715 (1999).
Schiering, C. et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 542, 242–245 (2017).
Takenaka, M. C. et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 22, 729–740 (2019).
Chinen, I. et al. The aryl hydrocarbon receptor/microRNA-212/132 axis in T cells regulates IL-10 production to maintain intestinal homeostasis. Int. Immunol. 27, 405–415 (2015).
Garcia, G. R. et al. In vivo characterization of an AHR-dependent long noncoding RNA required for proper Sox9b expression. Mol. Pharmacol. 91, 609–619 (2017).
Hecht, E. et al. Aryl hydrocarbon receptor-dependent regulation of miR-196a expression controls lung fibroblast apoptosis but not proliferation. Toxicol. Appl. Pharmacol. 280, 511–525 (2014).
Nakahama, T. et al. Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc. Natl Acad. Sci. USA 110, 11964–11969 (2013).
Zhang, D. D. et al. Long noncoding RNA LINC00305 promotes inflammation by activating the AHRR-NF-κB pathway in human monocytes. Sci. Rep. 7, 46204 (2017).
Vogel, C. F. et al. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol. Endocrinol. 21, 2941–2955 (2007).
Ishihara, Y., Kado, S. Y., Hoeper, C., Harel, S. & Vogel, C. F. A. Role of NF-κB RelB in aryl hydrocarbon receptor-mediated ligand specific effects. Int. J. Mol. Sci. https://doi.org/10.3390/ijms20112652 (2019).
Kim, D. W. et al. The RelA NF-κB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 19, 5498–5506 (2000).
Tian, Y., Ke, S., Denison, M. S., Rabson, A. B. & Gallo, M. A. Ah receptor and NF-κB interactions, a potential mechanism for dioxin toxicity. J. Biol. Chem. 274, 510–515 (1999).
McBerry, C., Gonzalez, R. M., Shryock, N., Dias, A. & Aliberti, J. SOCS2-induced proteasome-dependent TRAF6 degradation: a common anti-inflammatory pathway for control of innate immune responses. PLoS ONE 7, e38384 (2012).
Ovrevik, J. et al. AhR and Arnt differentially regulate NF-κB signaling and chemokine responses in human bronchial epithelial cells. Cell Commun. Signal. 12, 48 (2014).
Salisbury, R. L. & Sulentic, C. E. The AhR and NF-κB/Rel proteins mediate the inhibitory effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the 3′ immunoglobulin heavy chain regulatory region. Toxicol. Sci. 148, 443–459 (2015).
Sanmarco, L. M. et al. Identification of environmental factors that promote intestinal inflammation. Nature 611, 801–809 (2022).
Wilson, S. R., Joshi, A. D. & Elferink, C. J. The tumor suppressor Krüppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. J. Pharmacol. Exp. Ther. 345, 419–429 (2013).
Kimura, A., Naka, T., Nohara, K., Fujii-Kuriyama, Y. & Kishimoto, T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of TH17 cells. Proc. Natl Acad. Sci. USA 105, 9721–9726 (2008).
Esteban, J. et al. Role of aryl hydrocarbon receptor (AHR) in overall retinoid metabolism: response comparisons to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure between wild-type and AHR knockout mice. Reprod. Toxicol. 101, 33–49 (2021).
Widerak, M. et al. The aryl hydrocarbon receptor activates the retinoic acid receptoralpha through SMRT antagonism. Biochimie 88, 387–397 (2006).
Ge, N. L. & Elferink, C. J. A direct interaction between the aryl hydrocarbon receptor and retinoblastoma protein. Linking dioxin signaling to the cell cycle. J. Biol. Chem. 273, 22708–22713 (1998).
Puga, A. et al. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J. Biol. Chem. 275, 2943–2950 (2000).
Giovannoni, F. et al. AHR is a Zika virus host factor and a candidate target for antiviral therapy. Nat. Neurosci. 23, 939–951 (2020).
Ohtake, F. et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature 423, 545–550 (2003).
Lee, H. G. et al. Disease-associated astrocyte epigenetic memory promotes CNS pathology. Nature https://doi.org/10.1038/s41586-024-07187-5 (2024).
Ohtake, F. et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature 446, 562–566 (2007).
Luecke-Johansson, S. et al. A molecular mechanism to switch the aryl hydrocarbon receptor from a transcription factor to an E3 ubiquitin ligase. Mol. Cell Biol. https://doi.org/10.1128/MCB.00630-16 (2017).
Bessede, A. et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511, 184–190 (2014).
Han, Z. et al. Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells. Cardiovasc. Res. 77, 809–818 (2008).
Xiao, W., Son, J., Vorrink, S. U., Domann, F. E. & Goswami, P. C. Ligand-independent activation of aryl hydrocarbon receptor signaling in PCB3-quinone treated HaCaT human keratinocytes. Toxicol. Lett. 233, 258–266 (2015).
Hahn, M. E., Karchner, S. I. & Merson, R. R. Diversity as opportunity: insights from 600 million years of AHR evolution. Curr. Opin. Toxicol. 2, 58–71 (2017).
Stockinger, B., Di Meglio, P., Gialitakis, M. & Duarte, J. H. The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 32, 403–432 (2014).
Gutierrez-Vazquez, C. & Quintana, F. J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48, 19–33 (2018).
Esser, C., Rannug, A. & Stockinger, B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 30, 447–454 (2009).
Cabeza-Cabrerizo, M., Cardoso, A., Minutti, C. M., Pereira da Costa, M. & Reis e Sousa, C. Dendritic cells revisited. Annu. Rev. Immunol. 39, 131–166 (2021).
Quintana, F. J., Yeste, A. & Mascanfroni, I. D. Role and therapeutic value of dendritic cells in central nervous system autoimmunity. BMC Neurol. 22, 215–224 (2015).
Thordardottir, S. et al. The aryl hydrocarbon receptor antagonist StemRegenin 1 promotes human plasmacytoid and myeloid dendritic cell development from CD34+ hematopoietic progenitor cells. Stem Cell Dev. 23, 955–967 (2014).
Goudot, C. et al. Aryl hydrocarbon receptor controls monocyte differentiation into dendritic cells versus macrophages. Immunity 47, 582–596.e6 (2017).
Vogel, C. F. et al. Aryl hydrocarbon receptor signaling regulates NF-κB RelB activation during dendritic-cell differentiation. Immunol. Cell Biol. 91, 568–575 (2013).
Platzer, B. et al. Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J. Immunol. 183, 66–74 (2009).
Hwang, W. B., Kim, D. J., Oh, G. S. & Park, J. H. Aryl hydrocarbon receptor ligands indoxyl 3-sulfate and indole-3-carbinol inhibit FMS-like tyrosine kinase 3 ligand-induced bone marrow-derived plasmacytoid dendritic cell differentiation. Immune Netw. 18, e35 (2018).
Ettmayer, P. et al. A novel low molecular weight inhibitor of dendritic cells and B cells blocks allergic inflammation. Am. J. Respir. Crit. Care Med. 173, 599–606 (2006).
Quintana, F. J. et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 107, 20768–20773 (2010).
Mascanfroni, I. D. et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 14, 1054–1063 (2013).
Nguyen, N. T. et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl Acad. Sci. USA 107, 19961–19966 (2010). Together with Quintana et al. (2010) and Mascanfroni et al. (2013), this work shows that AHR activation in DCs affects T cell responses.
Mezrich, J. D. et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185, 3190–3198 (2010).
Chng, S. H. et al. Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity. Sci. Rep. 6, 23820 (2016).
Cros, A. et al. Homeostatic activation of aryl hydrocarbon receptor by dietary ligands dampens cutaneous allergic responses by controlling Langerhans cells migration. eLife https://doi.org/10.7554/eLife.86413 (2023).
Cui, X. et al. Aryl hydrocarbon receptor activation ameliorates experimental colitis by modulating the tolerogenic dendritic and regulatory T cell formation. Cell Biosci. 12, 46 (2022).
Bankoti, J., Rase, B., Simones, T. & Shepherd, D. M. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol. Appl. Pharmacol. 246, 18–28 (2010).
Bankoti, J. et al. Effects of TCDD on the fate of naive dendritic cells. Toxicol. Sci. 115, 422–434 (2010).
Hong, C. H., Lin, S. H., Clausen, B. E. & Lee, C. H. Selective AhR knockout in langerin-expressing cells abates Langerhans cells and polarizes TH2/Tr1 in epicutaneous protein sensitization. Proc. Natl Acad. Sci. USA 117, 12980–12990 (2020).
Sadeghi Shermeh, A. et al. Differential modulation of dendritic cell biology by endogenous and exogenous aryl hydrocarbon receptor ligands. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24097801 (2023).
Vivier, E. The discovery of innate lymphoid cells. Nat. Rev. Immunol. 21, 616 (2021).
Robinette, M. L. et al. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat. Immunol. 16, 306–317 (2015).
Zhang, L. H., Shin, J. H., Haggadone, M. D. & Sunwoo, J. B. The aryl hydrocarbon receptor is required for the maintenance of liver-resident natural killer cells. J. Exp. Med. 213, 2249–2257 (2016).
Curio, S. & Belz, G. T. The unique role of innate lymphoid cells in cancer and the hepatic microenvironment. Cell Mol. Immunol. 19, 1012–1029 (2022).
Mjosberg, J. M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 12, 1055–1062 (2011).
Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2012).
Lee, J. S. et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13, 144–151 (2011).
Song, C. et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J. Exp. Med. 212, 1869–1882 (2015).
Qiu, J. et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity 39, 386–399 (2013).
Kiss, E. A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011).
Li, S., Bostick, J. W. & Zhou, L. Regulation of innate lymphoid cells by aryl hydrocarbon receptor. Front. Immunol. 8, 1909 (2017).
Schnell, A., Littman, D. R. & Kuchroo, V. K. TH17 cell heterogeneity and its role in tissue inflammation. Nat. Immunol. 24, 19–29 (2023).
Nurieva, R. et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 448, 480–483 (2007).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
McGeachy, M. J. GM-CSF: the secret weapon in the TH17 arsenal. Nat. Immunol. 12, 521–522 (2011).
Quintana, F. J. et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat. Immunol. 13, 770–777 (2012).
Yang, X. P. et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 12, 247–254 (2011).
Veldhoen, M., Hirota, K., Christensen, J., O’Garra, A. & Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of TH17 T cells. J. Exp. Med. 206, 43–49 (2009).
Basu, R. et al. TH22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37, 1061–1075 (2012).
Ramirez, J. M. et al. Activation of the aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T helper cells. Eur. J. Immunol. 40, 2450–2459 (2010).
Trifari, S., Kaplan, C. D., Tran, E. H., Crellin, N. K. & Spits, H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. 10, 864–871 (2009).
Duhen, T., Geiger, R., Jarrossay, D., Lanzavecchia, A. & Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 10, 857–863 (2009).
Groux, H. et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 (1997).
Fitzgerald, D. C. et al. Suppressive effect of IL-27 on encephalitogenic TH17 cells and the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 179, 3268–3275 (2007).
Stumhofer, J. S. et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7, 937–945 (2006).
Spolski, R., Kim, H. P., Zhu, W., Levy, D. E. & Leonard, W. J. IL-21 mediates suppressive effects via its induction of IL-10. J. Immunol. 182, 2859–2867 (2009).
Takenaka, M. C., Robson, S. & Quintana, F. J. Regulation of the T cell response by CD39. Trends Immunol. 37, 427–439 (2016).
Gagliani, N. et al. TH17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015).
Dean, J. W. et al. The aryl hydrocarbon receptor cell intrinsically promotes resident memory CD8+ T cell differentiation and function. Cell Rep. 42, 111963 (2023).
Zaid, A. et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl Acad. Sci. USA 111, 5307–5312 (2014).
Liu, Y. et al. IL-2 regulates tumor-reactive CD8+ T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 22, 358–369 (2021).
Lawrence, B. P., Roberts, A. D., Neumiller, J. J., Cundiff, J. A. & Woodland, D. L. Aryl hydrocarbon receptor activation impairs the priming but not the recall of influenza virus-specific CD8+ T cells in the lung. J. Immunol. 177, 5819–5828 (2006).
Winans, B. et al. Linking the aryl hydrocarbon receptor with altered DNA methylation patterns and developmentally induced aberrant antiviral CD8+ T cell responses. J. Immunol. 194, 4446–4457 (2015).
Stavnezer, J., Guikema, J. E. & Schrader, C. E. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 26, 261–292 (2008).
Vaidyanathan, B. et al. The aryl hydrocarbon receptor controls cell-fate decisions in B cells. J. Exp. Med. 214, 197–208 (2017).
Piper, C. J. M. et al. Aryl hydrocarbon receptor contributes to the transcriptional program of IL-10-producing regulatory B cells. Cell Rep. 29, 1878–1892.e7 (2019).
McGovern, K. et al. Discovery and characterization of a novel aryl hydrocarbon receptor inhibitor, IK-175, and its inhibitory activity on tumor immune suppression. Mol. Cancer Ther. 21, 1261–1272 (2022).
Perdew, G. H. et al. The Ah receptor from toxicity to therapeutics: report from the 5th AHR Meeting at Penn State University, USA, June 2022. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24065550 (2023).
Di Meglio, P. et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity 40, 989–1001 (2014). This work highlights the crucial role of the AHR in inflammatory skin diseases.
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Tsuji, G. et al. Difamilast, a topical phosphodiesterase 4 inhibitor, produces soluble ST2 via the AHR–NRF2 axis in human keratinocytes. Int. J. Mol. Sci. https://doi.org/10.3390/ijms25147910 (2024).
Smith, S. H. et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Investig. Dermatol. 137, 2110–2119 (2017).
Bissonnette, R., Saint-Cyr Proulx, E., Jack, C. & Maari, C. Tapinarof for psoriasis and atopic dermatitis: 15 years of clinical research. J. Eur. Acad. Dermatol. Venereol. 37, 1168–1174 (2023).
Bissonnette, R. et al. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: results from a randomized double-blind placebo-controlled, phase II trial. J. Eur. Acad. Dermatol. Venereol. 26, 1516–1521 (2012).
Bissonnette, R. et al. Efficacy and safety of topical WBI-1001 in the treatment of atopic dermatitis: results from a phase 2A, randomized, placebo-controlled clinical trial. Arch. Dermatol. 146, 446–449 (2010).
Bissonnette, R. et al. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: results from a 12-week, multicentre, randomized, placebo-controlled double-blind trial. Br. J. Dermatol. 166, 853–860 (2012).
Peppers, J. et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 80, 89–98.e3 (2019).
Robbins, K. et al. Phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. J. Am. Acad. Dermatol. 80, 714–721 (2019).
Sutter, C. H. et al. Ligand activation of the aryl hydrocarbon receptor upregulates epidermal uridine diphosphate glucose ceramide glucosyltransferase and glucosylceramides. J. Invest. Dermatol. 143, 1964–1972.e4 (2023).
Koch, M. et al. Quantitative proteomics identifies reduced NRF2 activity and mitochondrial dysfunction in atopic dermatitis. J. Invest. Dermatol. 143, 220–231.e7 (2023).
Lebwohl, M. G. et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N. Engl. J. Med. 385, 2219–2229 (2021).
Strober, B. et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J. Am. Acad. Dermatol. 87, 800–806 (2022).
Mooney, N. et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memeory T-cells in vitro. J. Investig. Dermatol. 142, B26 (2022).
Jett, J. E. et al. Tapinarof cream 1% for extensive plaque psoriasis: a maximal use trial on safety, tolerability, and pharmacokinetics. Am. J. Clin. Dermatol. 23, 83–91 (2022).
Igarashi, A., Tsuji, G., Fukasawa, S., Murata, R. & Yamane, S. Tapinarof cream for the treatment of plaque psoriasis: efficacy and safety results from 2 Japanese phase 3 trials. J. Dermatol. https://doi.org/10.1111/1346-8138.17423 (2024).
Silverberg, J. I. et al. in 32nd European Academy of Dermatology and Venereology Congress (Medicom, 2023).
Silverberg, J. I., Boguniewicz, M., Rubenstein, D., Tallman, A. & Brown, P. Tapinarof cream improved itch on two phase 3 trials of moderate to severe artopic dermatitis. Ann. Allergy Asthma Immunol. 131, P282 (2023).
Rodríguez Murúa, S., Farez, M. F. & Quintana, F. J. The immune response in multiple sclerosis. Annu. Rev. Pathol. Mech. Dis. 17, 121–139 (2022).
Rothhammer, V. et al. Dynamic regulation of serum aryl hydrocarbon receptor agonists in MS. Neurol. Neuroimmunol. Neuroinflamm. 4, e359 (2017).
Rouse, M., Singh, N. P., Nagarkatti, P. S. & Nagarkatti, M. Indoles mitigate the development of experimental autoimmune encephalomyelitis by induction of reciprocal differentiation of regulatory T cells and TH17 cells. Br. J. Pharmacol. 169, 1305–1321 (2013).
Charabati, M., Wheeler, M. A., Weiner, H. L. & Quintana, F. J. Multiple sclerosis: neuroimmune crosstalk and therapeutic targeting. Cell 186, 1309–1327 (2023).
Dossi, E., Vasile, F. & Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 136, 139–156 (2018).
Wheeler, M. A. & Quintana, F. J. Regulation of astrocyte functions in multiple sclerosis. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a029009 (2019).
Colonna, M. & Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35, 441–468 (2017).
Buttgereit, A. et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat. Immunol. 17, 1397–1406 (2016).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Rothhammer, V. et al. Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728 (2018). Together with Rothhammer et al. (2016), this work identifies the AHR in astrocytes and microglia as a regulator of CNS inflammation.
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Law, C. et al. Interferon subverts an AHR–JUN axis to promote CXCL13+ T cells in lupus. Nature 631, 857–866 (2024). This work shows that the AHR is a negative regulator of the pathogenic immune response in SLE.
Shinde, R. et al. Apoptotic cell-induced AhR activity is required for immunological tolerance and suppression of systemic lupus erythematosus in mice and humans. Nat. Immunol. 19, 571–582 (2018).
Mohammadi, S., Memarian, A., Sedighi, S., Behnampour, N. & Yazdani, Y. Immunoregulatory effects of indole-3-carbinol on monocyte-derived macrophages in systemic lupus erythematosus: a crucial role for aryl hydrocarbon receptor. Autoimmunity 51, 199–209 (2018).
Yu, H. et al. Association between the ratio of aryl hydrocarbon receptor (AhR) in TH17 cells to AhR in Treg cells and SLE skin lesions. Int. Immunopharmacol. 69, 257–262 (2019).
Pernomian, L., Duarte-Silva, M. & de Barros Cardoso, C. R. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 59, 382–390 (2020).
Schiering, C., Vonk, A., Das, S., Stockinger, B. & Wincent, E. Cytochrome P4501-inhibiting chemicals amplify aryl hydrocarbon receptor activation and IL-22 production in T helper 17 cells. Biochem. Pharmacol. 151, 47–58 (2018).
Keir, M., Yi, Y., Lu, T. & Ghilardi, N. The role of IL-22 in intestinal health and disease. J. Exp. Med. 217, e20192195 (2020).
Gronke, K. et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253 (2019).
Furumatsu, K. et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig. Dis. Sci. 56, 2532–2544 (2011).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016). This study describes how IBD-linked mutations in CARD9 diminish the abundance of AHR agonist-producing members of the intestinal microbiome.
Monteleone, I. et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 141, 237–248, 248.e1 (2011).
Song, J. et al. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc. Natl Acad. Sci. USA 99, 14694–14699 (2002).
Goettel, J. A. et al. AHR activation is protective against colitis driven by T cells in humanized mice. Cell Rep. 17, 1318–1329 (2016).
Uchiyama, K. et al. Efficacy and safety of short-term therapy with indigo naturalis for ulcerative colitis: an investigator-initiated multicenter double-blind clinical trial. PLoS ONE 15, e0241337 (2020).
Casaro, M., Souza, V. R., Oliveira, F. A. & Ferreira, C. M. OVA-induced allergic airway inflammation mouse model. Methods Mol. Biol. 1916, 297–301 (2019).
Traboulsi, H. et al. Differential regulation of the asthmatic phenotype by the aryl hydrocarbon receptor. Front. Physiol. 12, 720196 (2021).
Xu, T. et al. Aryl hydrocarbon receptor protects lungs from cockroach allergen-induced inflammation by modulating mesenchymal stem cells. J. Immunol. 195, 5539–5550 (2015).
Caminati, M., Vaia, R., Furci, F., Guarnieri, G. & Senna, G. Uncontrolled asthma: unmet needs in the management of patients. J. Asthma Allergy 14, 457–466 (2021).
Fadadu, R. P. et al. Association of wildfire air pollution with clinic visits for psoriasis. JAMA Netw. Open 6, e2251553 (2023).
Fadadu, R. P. et al. Association of exposure to wildfire air pollution with exacerbations of atopic dermatitis and itch among older adults. JAMA Netw. Open. 5, e2238594 (2022).
Fadadu, R. P. et al. Association of wildfire air pollution and health care use for atopic dermatitis and itch. JAMA Dermatol. 157, 658–666 (2021).
Kim, B. E. et al. Particulate matter causes skin barrier dysfunction. JCI Insight https://doi.org/10.1172/jci.insight.145185 (2021).
Wong, T. H. et al. A prominent air pollutant, indeno[1,2,3-cd]pyrene, enhances allergic lung inflammation via aryl hydrocarbon receptor. Sci. Rep. 8, 5198 (2018).
Chiba, T., Chihara, J. & Furue, M. Role of the arylhydrocarbon receptor (AhR) in the pathology of asthma and COPD. J. Allergy 2012, 372384 (2012).
Jasper, A. E., McIver, W. J., Sapey, E. & Walton, G. M. Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Res https://doi.org/10.12688/f1000research.18411.1 (2019).
Loughman, J. A., Yarbrough, M. L., Tiemann, K. M. & Hunstad, D. A. Local generation of kynurenines mediates inhibition of neutrophil chemotaxis by uropathogenic Escherichia coli. Infect. Immun. 84, 1176–1183 (2016).
Nganou-Makamdop, K. et al. Type I IFN signaling blockade by a PASylated antagonist during chronic SIV infection suppresses specific inflammatory pathways but does not alter T cell activation or virus replication. PLoS Pathog. 14, e1007246 (2018).
Yamada, T. et al. Constitutive aryl hydrocarbon receptor signaling constrains type I interferon-mediated antiviral innate defense. Nat. Immunol. 17, 687–694 (2016). This work identifies the AHR as a negative regulator of type I interferon responses against viruses.
Giovannoni, F. et al. AHR signaling is induced by infection with coronaviruses. Nat. Commun. 12, 5148 (2021).
Silginer, M. et al. The aryl hydrocarbon receptor links integrin signaling to the TGF-β pathway. Oncogene 35, 3260–3271 (2016).
Yang, X. et al. Constitutive regulation of CYP1B1 by the aryl hydrocarbon receptor (AhR) in pre-malignant and malignant mammary tissue. J. Cell Biochem. 104, 402–417 (2008).
Stanford, E. A. et al. Role for the aryl hydrocarbon receptor and diverse ligands in oral squamous cell carcinoma migration and tumorigenesis. Mol. Cancer Res. 14, 696–706 (2016).
Stanford, E. A. et al. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 14, 20 (2016).
Jin, U. H., Kim, S. B. & Safe, S. Omeprazole inhibits pancreatic cancer cell invasion through a nongenomic aryl hydrocarbon receptor pathway. Chem. Res. Toxicol. 28, 907–918 (2015).
Wang, K. et al. An endogenous aryl hydrocarbon receptor ligand inhibits proliferation and migration of human ovarian cancer cells. Cancer Lett. 340, 63–71 (2013).
Chen, J. Y. et al. Cancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progression. Breast Cancer Res. 16, 410 (2014).
D’Amato, N. C. et al. A TDO2–AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 75, 4651–4664 (2015).
Opitz, C. A. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 (2011). This work shows that cancer cells produce AHR agonists to boost tumour growth.
Liu, Y. et al. Blockade of IDO–kynurenine–AhR metabolic circuitry abrogates IFN-γ-induced immunologic dormancy of tumor-repopulating cells. Nat. Commun. 8, 15207 (2017).
Kenison, J. E. et al. The aryl hydrocarbon receptor suppresses immunity to oral squamous cell carcinoma through immune checkpoint regulation. Proc. Natl. Acad. Sci. USA https://doi.org/10.1073/pnas.2012692118 (2021).
Sadik, A. et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 182, 1252–1270.e34 (2020).
Balachandran, V. P. et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat. Med. 17, 1094–1100 (2011).
Muller, A. J., DuHadaway, J. B., Donover, P. S., Sutanto-Ward, E. & Prendergast, G. C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 11, 312–319 (2005).
Pilotte, L. et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc. Natl Acad. Sci. USA 109, 2497–2502 (2012).
Hezaveh, K. et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55, 324–340.e8 (2022).
Fong, W. et al. Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis. Gut 72, 2272–2285 (2023).
St Paul, M. et al. IL6 induces an IL22+CD8+ T-cell subset with potent antitumor function. Cancer Immunol. Res. 8, 321–333 (2020).
Bender, M. J. et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186, 1846–1862.e26 (2023).
Sugatani, J. et al. Effects of dietary inulin, statin, and their co-treatment on hyperlipidemia, hepatic steatosis and changes in drug-metabolizing enzymes in rats fed a high-fat and high-sucrose diet. Nutr. Metab. 9, 23 (2012).
Adachi, J. et al. Indirubin and indigo are potent aryl hydrocarbon receptor ligands present in human urine. J. Biol. Chem. 276, 31475–31478 (2001).
Chauvigne-Hines, L. M., Clarke, C. A., Gernert, D. L., Green, S. J. & Watson, B. M. AHR agonists. US Patent 2023/0159493 (2023).
Carson, C. A., Clarke, C. A., Gernert, D. L., Holloway, W. G. & Barrett, D. G. AHR agonists. US Patent 2023/0127797 (2023).
Kaye, J. et al. Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc. Natl Acad. Sci. USA 113, E6145–E6152 (2016).
Comi, G. et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N. Engl. J. Med. 366, 1000–1009 (2012).
Vollmer, T. L. et al. A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. J. Neurol. 261, 773–783 (2014).
Garhöfer, G. et al. in International Ocular Inflammation Society (IOIS) Meeting (ed. van de Kerkhof, P.) (Medicom, 2023).
Rothhammer, V. et al. Aryl hydrocarbon receptor activation in astrocytes by laquinimod ameliorates autoimmune inflammation in the CNS. Neurol. Neuroimmunol. Neuroinflamm. https://doi.org/10.1212/NXI.0000000000000946 (2021).
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R. & Rastall, R. A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 605–616 (2019).
Khalesi, S. et al. A review of probiotic supplementation in healthy adults: helpful or hype? Eur. J. Clin. Nutr. 73, 24–37 (2019).
Suez, J., Zmora, N., Segal, E. & Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 25, 716–729 (2019).
Canale, F. P. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021).
Sanmarco, L. M. et al. Lactate limits CNS autoimmunity by stabilizing HIF-1ɑ in dendritic cells. Nature 620, 881–889 (2023).
Scott, B. M. et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat. Rev. Genet. 27, 1212–1222 (2021).
Takamura, T. et al. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol. Cell Biol. 89, 817–822 (2011).
Fukumoto, S. et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol. 92, 460–465 (2014).
Xia, Y. et al. Lactobacillus-derived indole-3-lactic acid ameliorates colitis in cesarean-born offspring via activation of aryl hydrocarbon receptor. iScience 26, 108279 (2023).
Gu, Z. et al. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 12, 10184–10195 (2021).
Cui, Q. Y. et al. Bifidobacterium bifidum relieved DSS-induced colitis in mice potentially by activating the aryl hydrocarbon receptor. Food Funct. 13, 5115–5123 (2022).
Cui, Q. et al. Bifidobacterium bifidum ameliorates DSS-induced colitis in mice by regulating AHR/NRF2/NLRP3 inflammasome pathways through indole-3-lactic acid production. J. Agric. Food Chem. 71, 1970–1981 (2023).
Fei, Y. et al. The role of dihydroresveratrol in enhancing the synergistic effect of Ligilactobacillus salivarius Li01 and resveratrol in ameliorating colitis in mice. Research 2022, 9863845 (2022).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Hou, K. et al. Microbiota in health and diseases. Signal. Transduct. Target. Ther. 7, 135 (2022).
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Rankin, L. C. et al. Dietary tryptophan deficiency promotes gut RORγt+ Treg cells at the expense of Gata3+ Treg cells and alters commensal microbiota metabolism. Cell Rep. 42, 112135 (2023).
Hou, Q. et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 25, 1657–1670 (2018).
Cervantes-Barragan, L. et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8ɑ ɑ+ T cells. Science 357, 806–810 (2017).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Petros, R. A. & DeSimone, J. M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 9, 615–627 (2010).
Chen, Y. et al. Colon-targeted delivery of indole acetic acid helps regulate gut motility by activating the AHR signaling pathway. Nutrients https://doi.org/10.3390/nu15194282 (2023).
Zhu, M. Z. et al. Edible exosome-like nanoparticles from Portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+ T cells expansion. J. Nanobiotechnol. 21, 309 (2023).
Yeste, A., Nadeau, M., Burns, E. J., Weiner, H. L. & Quintana, F. J. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 109, 11270–11275 (2012).
Yeste, A. et al. Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci. Signal. 9, ra61 (2016).
Kenison, J. E. et al. Tolerogenic nanoparticles suppress central nervous system inflammation. Proc. Natl Acad. Sci. USA 117, 32017–32028 (2020).
Zdioruk, M. et al. PPRX-1701, a nanoparticle formulation of 6′-bromoindirubin acetoxime, improves delivery and shows efficacy in preclinical GBM models. Cell Rep. Med. 4, 101019 (2023).
Quintana, F. J. & Sherr, D. H. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 65, 1148–1161 (2013).
Dolciami, D. et al. Targeting aryl hydrocarbon receptor for next-generation immunotherapies: selective modulators (SAhRMs) versus rapidly metabolized ligands (RMAhRLs). Eur. J. Med. Chem. 185, 111842 (2020).
Hitch, T. C. A. et al. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol. 15, 1095–1113 (2022).
Paris, A. et al. The AhR–SRC axis as a therapeutic vulnerability in BRAFi-resistant melanoma. EMBO Mol. Med. 14, e15677 (2022).
DiNatale, B. C., Schroeder, J. C. & Perdew, G. H. Ah receptor antagonism inhibits constitutive and cytokine inducible IL6 production in head and neck tumor cell lines. Mol. Carcinog. 50, 173–183 (2011).
Litzenburger, U. M. et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget 5, 1038–1051 (2014).
Novikov, O. et al. An aryl hydrocarbon receptor-mediated amplification loop that enforces cell migration in ER-/PR-/Her2- human breast cancer cells. Mol. Pharmacol. 90, 674–688 (2016).
Esser, C. et al. Old receptor, new tricks-the ever-expanding universe of aryl hydrocarbon receptor functions. Report from the 4th AHR Meeting, 29–31 August 2018 in Paris, France. Int. J. Mol. Sci. https://doi.org/10.3390/ijms19113603 (2018).
Riemschneider, S. et al. Aryl hydrocarbon receptor activation by benzo(a)pyrene inhibits proliferation of myeloid precursor cells and alters the differentiation state as well as the functional phenotype of murine bone marrow-derived macrophages. Toxicol. Lett. 296, 106–113 (2018).
Hwang, J. A., Lee, J. A., Cheong, S. W., Youn, H. J. & Park, J. H. Benzo(a)pyrene inhibits growth and functional differentiation of mouse bone marrow-derived dendritic cells. Downregulation of RelB and eIF3 p170 by benzo(a)pyrene. Toxicol. Lett. 169, 82–90 (2007).
Lee, J. A. et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modulates functional differentiation of mouse bone marrow-derived dendritic cells. Downregulation of RelB by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Lett. 173, 31–40 (2007).
Crunkhorn, S. Autoimmune disease: aryl hydrocarbon receptor suppresses inflammation. Nat. Rev. Drug Discov. 17, 470 (2018).
Sameir, M. et al. The increased frequency of type 1 regulatory T (Tr1) cells and the altered expression of aryl hydrocarbon receptor (AHR) and interferon regulatory factor-4 (IRF4) genes in type 1 diabetes: a case–control study. Cureus 16, e65749 (2024).
Acknowledgements
The authors thank all other Quintana laboratory members for helpful discussion related to this study. This work was supported by grants NS102807, ES02530, ES029136, AI126880 from the National Institutes of Health (NIH); RG4111A1 and JF2161-A-5 from the National Multiple Sclerosis Society (NMSS); RSG-14-198-01-LIB from the American Cancer Society; and PA-1604-08459 from the International Progressive MS Alliance. C.M.P. is supported by NMSS (FG-2307-42209) and Mayer Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.A.M. and D.R. are employees of Dermavant Sciences, for which they receive financial compensation in the form of salary and stock options. D.H.S. holds equity in and is a co-founder of Hercules Pharmaceuticals. F.J.Q is the Scientific Founder of AnTolRx and Violet Therapeutics, companies developing novel therapies for inflammatory and neurologic disorders; and is a consultant for Dermavant Sciences. C.M.P. declares no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Christopher Bradfield, who co-reviewed with Alex Veith; Marco Colonna; and Ari Waisman for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Polonio, C.M., McHale, K.A., Sherr, D.H. et al. The aryl hydrocarbon receptor: a rehabilitated target for therapeutic immune modulation. Nat Rev Drug Discov 24, 610–630 (2025). https://doi.org/10.1038/s41573-025-01172-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-025-01172-x
This article is cited by
-
Integrating genomic and exposomic data identifies endocrine disruptors potentially associated with chronic obstructive pulmonary disease
Human Genomics (2026)
-
Hepatic tryptophan metabolism links chronic stress to liver cancer
Nature Metabolism (2026)
-
The aryl hydrocarbon receptor: structure, signaling, physiology and pathology
Signal Transduction and Targeted Therapy (2026)
-
Interactions between gut microbiota and parkinson’s disease: the role of tryptophan metabolism
Cell Communication and Signaling (2025)
-
Prognostic value of neutrophil-to-lymphocyte ratio for the clinical outcomes of chronic kidney diseases: an update systematic review and meta-analysis
BMC Nephrology (2025)