Abstract
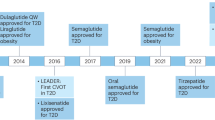
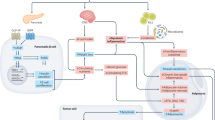
Glucagon-like peptide 1 (GLP-1)-based therapies, such as semaglutide and tirzepatide, represent highly effective treatment options for people with type 2 diabetes and obesity, enabling effective control of glucose and weight loss, while reducing cardiovascular and renal morbidity and mortality. The success of these medicines has spurred development of next-generation GLP-1-based drugs, promising greater weight loss, improved tolerability and additional options for the route and frequency of dosing. This Review profiles established and emerging GLP-1-based medicines, discussing optimization of pharmacokinetics and tolerability, engagement of new therapeutically useful pathways and safety aspects. Structurally unique GLP-1-based medicines that achieve substantially greater and rapid weight loss may impact musculoskeletal health, providing a rationale for therapeutics that more selectively target adipose tissue loss while preserving muscle mass and strength. Ongoing clinical trials in peripheral vascular disease, neuropsychiatric and substance use disorders, metabolic liver disease, arthritis, hypertension and neurodegenerative disorders may broaden indications for GLP-1-based therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
28 May 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41573-025-01231-3
References
Drucker, D. J., Philippe, J., Mojsov, S., Chick, W. L. & Habener, J. F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl Acad. Sci. USA 84, 3434–3438 (1987).
Mojsov, S., Weir, G. C. & Habener, J. F. Insulinotropin: glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J. Clin. Invest. 79, 616–619 (1987).
Holst, J. J., Orskov, C., Nielsen, O. V. & Schwartz, T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 211, 169–174 (1987).
Drucker, D. J., Habener, J. F. & Holst, J. J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 127, 4217–4227 (2017).
Turton, M. D. et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69–72 (1996).
Tang-Christensen, M. et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am. J. Physiol. 271, R848–R856 (1996).
Scrocchi, L. A. et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 2, 1254–1258 (1996).
Zander, M., Madsbad, S., Madsen, J. L. & Holst, J. J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: a parallel-group study. Lancet 359, 824–830 (2002).
Hammoud, R. & Drucker, D. J. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat. Rev. Endocrinol. 19, 201–216 (2023).
Drucker, D. J. et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 372, 1240–1250 (2008).
Fineman, M. S. et al. Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes. Metab. 14, 546–554 (2012).
Knudsen, L. B. Inventing liraglutide, a glucagon-like peptide-1 analogue, for the treatment of diabetes and obesity. ACS Pharmacol. Transl. Sci. 2, 468–484 (2019).
Drucker, D. J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 57, 101351 (2022).
Drucker, D. J. Efficacy and safety of GLP-1 medicines for type 2 diabetes and obesity. Diabetes Care 47, 1873–1888 (2024).
Drucker, D. J. The GLP-1 journey: from discovery science to therapeutic impact. J. Clin. Invest. 134, e175634 (2024).
Drucker, D. J. The biology of incretin hormones. Cell Metab. 3, 153–165 (2006).
Jalleh, R. J., Jones, K. L., Nauck, M. & Horowitz, M. Accurate measurements of gastric emptying and gastrointestinal symptoms in the evaluation of glucagon-like peptide-1 receptor agonists. Ann. Intern. Med. 176, 1542–1543 (2023).
Patel, H. et al. Gastrointestinal adverse events and weight reduction in people with type 2 diabetes treated with tirzepatide in the SURPASS clinical trials. Diabetes Obes. Metab. 26, 473–481 (2024).
Wharton, S. et al. Gastrointestinal tolerability of once-weekly semaglutide 2.4 mg in adults with overweight or obesity, and the relationship between gastrointestinal adverse events and weight loss. Diabetes Obes. Metab. 24, 94–105 (2022).
Blundell, J. et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 19, 1242–1251 (2017).
Drucker, D. J. & Nauck, M. A. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368, 1696–1705 (2006).
Rosenstock, J. et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X). Diabetes Care 36, 2945–2951 (2013).
Buse, J. B. et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 381, 117–124 (2013).
Sanford, M. Dulaglutide: first global approval. Drugs 74, 2097–2103 (2014).
Dungan, K. M. et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 384, 1349–1357 (2014).
Buse, J. B. et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care 33, 1255–1261 (2010).
Willard, F. S. et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI insight 5, e140532 (2020).
Pratley, R. E. et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 6, 275–286 (2018).
Frias, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515 (2021).
Aroda, V. R. et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 5, 355–366 (2017).
Frias, J. P. et al. Efficacy and safety of once-weekly semaglutide 2.0 mg versus 1.0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol. 9, 563–574 (2021).
Rubino, D. M. et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA 327, 138–150 (2022).
Coskun, T. et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol. Metab. 18, 3–14 (2018).
El, K. et al. The incretin co-agonist tirzepatide requires GIPR for hormone secretion from human islets. Nat. Metab. 5, 945–954 (2023).
Finan, B. et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 5, 209ra151 (2013).
Frias, J. P. et al. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 26, 343–352.e2 (2017).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Garvey, W. T. et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 402, 613–626 (2023).
Aronne, L. J. et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA 331, 38–48 (2024).
Wadden, T. A. et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat. Med. 29, 2909–2918 (2023).
Wilding, J. P. H. et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes. Metab. 24, 1553–1564 (2022).
German, J. et al. Association between plausible genetic factors and weight loss from GLP1-RA and bariatric surgery. Nat. Med. https://doi.org/10.1038/s41591-025-03645-3 (2025).
Kinzig, K. P., D’Alessio, D. A. & Seeley, R. J. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J. Neurosci. 22, 10470–10476 (2002).
Huang, K. P. et al. Dissociable hindbrain GLP1R circuits for satiety and aversion. Nature 632, 585–593 (2024).
Borner, T. et al. GIP receptor agonism attenuates GLP-1 receptor agonist-induced nausea and emesis in preclinical models. Diabetes 70, 2545–2553 (2021).
Knop, F. K. et al. A long-acting glucose-dependent insulinotropic polypeptide receptor agonist improves the gastrointestinal tolerability of glucagon-like peptide-1 receptor agonist therapy. Diabetes Obes. Metab. 26, 5474–5478 (2024).
Overgaard, R. V., Hertz, C. L., Ingwersen, S. H., Navarria, A. & Drucker, D. J. Levels of circulating semaglutide determine reductions in HbA1c and body weight in people with type 2 diabetes. Cell Rep. Med. 2, 100387 (2021).
He, L. et al. Association of glucagon-like peptide-1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 182, 513–519 (2022).
Thompson, C. A. & Sturmer, T. Putting GLP-1 RAs and thyroid cancer in context: additional evidence and remaining doubts. Diabetes Care 46, 249–251 (2023).
Drucker, D. J. Advances in oral peptide therapeutics. Nat. Rev. Drug. Discov. 19, 277–289 (2020).
Haggag, A. Z. et al. Non-clinical and first-in-human characterization of ECC5004/AZD5004, a novel once-daily, oral small-molecule GLP-1 receptor agonist. Diabetes, Obes. Metab. https://doi.org/10.1111/dom.16047 (2024).
Griffith, D. A. et al. A small-molecule oral agonist of the human glucagon-like peptide-1 receptor. J. Med. Chem. 65, 8208–8226 (2022).
Sloop, K. W. et al. The pharmacological basis for nonpeptide agonism of the GLP-1 receptor by orforglipron. Sci. Transl. Med. 16, eadp5765 (2024).
Saxena, A. R. et al. Efficacy and safety of oral small molecule glucagon-like peptide 1 receptor agonist danuglipron for glycemic control among patients with type 2 diabetes: a randomized clinical trial. JAMA Netw. Open. 6, e2314493 (2023).
Saxena, A. R. et al. Tolerability, safety and pharmacodynamics of oral, small-molecule glucagon-like peptide-1 receptor agonist danuglipron for type 2 diabetes: a 12-week, randomized, placebo-controlled, phase 2 study comparing different dose-escalation schemes. Diabetes Obes. Metab. 25, 2805–2814 (2023).
Fediuk, D. J. et al. Effect of renal impairment on the pharmacokinetics of a single oral dose of danuglipron in participants with type 2 diabetes. J. Clin. Pharmacol. 64, 449–460 (2024).
Kawai, T. et al. Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist. Proc. Natl Acad. Sci. USA 117, 29959–29967 (2020).
Frias, J. P. et al. Efficacy and safety of oral orforglipron in patients with type 2 diabetes: a multicentre, randomised, dose-response, phase 2 study. Lancet 402, 472–483 (2023).
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2302392 (2023).
Ma, X. et al. Effect of food consumption on the pharmacokinetics, safety, and tolerability of once-daily orally administered orforglipron (LY3502970), a non-peptide GLP-1 receptor agonist. Diabetes Ther. 15, 819–832 (2024).
Guo, W. et al. Discovery of ecnoglutide—a novel, long-acting, cAMP-biased glucagon-like peptide-1 (GLP-1) analog. Mol. Metab. 75, 101762 (2023).
Zhu, D. et al. Efficacy and safety of GLP-1 analog ecnoglutide in adults with type 2 diabetes: a randomized, double-blind, placebo-controlled phase 2 trial. Nat. Commun. 15, 8408 (2024).
Zhang, M. et al. GZR18, a novel long-acting GLP-1 analog, demonstrated positive in vitro and in vivo pharmacokinetic and pharmacodynamic characteristics in animal models. Eur. J. Pharmacol. 928, 175107 (2022).
Finan, B. et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 21, 27–36 (2015).
Zhang, Q. et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 33, 833–844.e5 (2021).
Killion, E. A. et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci. Transl. Med. 10, eaat3392 (2018).
Gastaldelli, A. et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 10, 393–406 (2022).
Sattar, N. et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat. Med. 28, 591–598 (2022).
Davies, M. et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 397, 971–984 (2021).
Garvey, W. T. et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat. Med. 28, 2083–2091 (2022).
Gewitz, A. et al. Pharmacokinetics and pharmacodynamics of mibavademab (a leptin receptor agonist): results from a first-in-human phase I study. Clin. Transl. Sci. 17, e13762 (2024).
Styrkarsdottir, U. et al. Obesity variants in the GIPR gene are not associated with risk of fracture or bone mineral density. J. Clin. Endocrinol. Metab. 109, e1608–e1615 (2024).
Yuan, J. et al. Molecular dynamics-guided optimization of BGM0504 enhances dual-target agonism for combating diabetes and obesity. Sci. Rep. 14, 16680 (2024).
Fan, Y. et al. The safety, tolerability, pharmacokinetics and pharmacodynamics of an optimized dual GLP-1/GIP receptor agonist (BGM0504) in healthy volunteers: a dose-escalation phase I study. Diabetes Obes. Metab. 27, 2110–2119 (2025).
Baggio, L. L. & Drucker, D. J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 46, 101090 (2021).
Campbell, J. E. et al. GIPR/GLP-1R dual agonist therapies for diabetes and weight loss-chemistry, physiology, and clinical applications. Cell Metab. 35, 1519–1529 (2023).
Kizilkaya, H. S. et al. Characterization of genetic variants of GIPR reveals a contribution of β-arrestin to metabolic phenotypes. Nat. Metab. 6, 1268–1281 (2024).
Killion, E. A. et al. Chronic glucose-dependent insulinotropic polypeptide receptor (GIPR) agonism desensitizes adipocyte GIPR activity mimicking functional GIPR antagonism. Nat. Commun. 11, 4981 (2020).
Veniant, M. M. et al. A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Nat. Metab. 6, 290–303 (2024).
Jensen, M. H. et al. AT-7687, a novel GIPR peptide antagonist, combined with a GLP-1 agonist, leads to enhanced weight loss and metabolic improvements in cynomolgus monkeys. Mol. Metab. 88, 102006 (2024).
Riddle, M. C. & Drucker, D. J. Emerging therapies mimicking the effects of amylin and glucagon-like peptide 1. Diabetes care 29, 435–449 (2006).
Ravussin, E. et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity 17, 1736–1743 (2009).
Kruse, T. et al. Development of cagrilintide, a long-acting amylin analogue. J. Med. Chem. 64, 11183–11194 (2021).
Cao, J. et al. A structural basis for amylin receptor phenotype. Science 375, eabm9609 (2022).
Lau, D. C. W. et al. Once-weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet 398, 2160–2172 (2021).
Frias, J. P. et al. Efficacy and safety of co-administered once-weekly cagrilintide 2.4 mg with once-weekly semaglutide 2.4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 402, 720–730 (2023).
Boland, M. L. et al. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat. Metab. 2, 413–431 (2020).
Romero-Gomez, M. et al. A phase IIa active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non-alcoholic fatty liver disease. J. Hepatol. 79, 888–897 (2023).
le Roux, C. W. et al. Glucagon and GLP-1 receptor dual agonist survodutide for obesity: a randomised, double-blind, placebo-controlled, dose-finding phase 2 trial. Lancet Diabetes Endocrinol. 12, 162–173 (2024).
Jastreboff, A. M. et al. Triple-hormone-receptor agonist retatrutide for obesity—a phase 2 trial. N. Engl. J. Med. 389, 514–526 (2023).
Rosenstock, J. et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 402, 529–544 (2023).
Friedrichsen, M. H. et al. Results from three phase 1 trials of NNC9204-1177, a glucagon/GLP-1 receptor co-agonist: effects on weight loss and safety in adults with overweight or obesity. Mol. Metab. 78, 101801 (2023).
Nestor, J. J., Parkes, D., Feigh, M., Suschak, J. J. & Harris, M. S. Effects of ALT-801, a GLP-1 and glucagon receptor dual agonist, in a translational mouse model of non-alcoholic steatohepatitis. Sci. Rep. 12, 6666 (2022).
Jiang, H. et al. A phase 1b randomised controlled trial of a glucagon-like peptide-1 and glucagon receptor dual agonist IBI362 (LY3305677) in Chinese patients with type 2 diabetes. Nat. Commun. 13, 3613 (2022).
Ji, L. et al. A phase 2 randomised controlled trial of mazdutide in Chinese overweight adults or adults with obesity. Nat. Commun. 14, 8289 (2023).
Zimmermann, T. et al. BI 456906: discovery and preclinical pharmacology of a novel GCGR/GLP-1R dual agonist with robust anti-obesity efficacy. Mol. Metab. 66, 101633 (2022).
Coskun, T. et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: from discovery to clinical proof of concept. Cell Metab. 34, 1234–1247.e9 (2022).
Finan, B. et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 18, 1847–1856 (2012).
Quarta, C. et al. Molecular integration of incretin and glucocorticoid action reverses immunometabolic dysfunction and obesity. Cell Metab. 26, 620–632.e6 (2017).
Petersen, J. et al. GLP-1-directed NMDA receptor antagonism for obesity treatment. Nature 629, 1133–1141 (2024).
Drucker, D. J. Prevention of cardiorenal complications in people with type 2 diabetes and obesity. Cell Metab. 36, 338–353 (2024).
Drucker, D. J. The benefits of GLP-1 drugs beyond obesity. Science 385, 258–260 (2024).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Ussher, J. R. & Drucker, D. J. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 20, 463–474 (2023).
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
McGuire, D. K. et al. Oral semaglutide and cardiovascular outcomes in high-risk type 2 diabetes. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2501006 (2025).
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 389, 2221–2232 (2023).
Ryan, D. H. et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat. Med. 30, 2049–2057 (2024).
Verma, S. et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT): outcomes by sex. J. Am. Coll. Cardiol. 84, 1678–1682 (2024).
Kosiborod, M. N. et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 389, 1069–1084 (2023).
Kosiborod, M. N. et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N. Engl. J. Med. 390, 1394–1407 (2024).
Kosiborod, M. N. et al. Semaglutide versus placebo in patients with heart failure and mildly reduced or preserved ejection fraction: a pooled analysis of the SELECT, FLOW, STEP-HFpEF, and STEP-HFpEF DM randomised trials. Lancet 404, 949–961 (2024).
Nicholls, S. J. et al. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am. Heart J. 267, 1–11 (2024).
Bonaca, M. P. et al. Design and baseline characteristics of the STRIDE trial: evaluating semaglutide in people with symptomatic peripheral artery disease and type 2 diabetes. Eur. Heart J. Cardiovasc. Pharmacother. 10, 728–737 (2025).
Bonaca, M. P. et al. Semaglutide and walking capacity in people with symptomatic peripheral artery disease and type 2 diabetes (STRIDE): a phase 3b, double-blind, randomised, placebo-controlled trial. Lancet https://doi.org/10.1016/S0140-6736(25)00509-4 (2025).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Gragnano, F., De Sio, V. & Calabro, P. FLOW trial stopped early due to evidence of renal protection with semaglutide. Eur. Heart J. Cardiovasc. Pharmacother. 10, 7–9 (2024).
Mann, J. F. E. et al. Effects of semaglutide with and without concomitant SGLT2 inhibitor use in participants with type 2 diabetes and chronic kidney disease in the FLOW trial. Nat. Med. 30, 2849–2856 (2024).
Colhoun, H. M. et al. Long-term kidney outcomes of semaglutide in obesity and cardiovascular disease in the SELECT trial. Nat. Med. 30, 2058–2066 (2024).
Bliddal, H. et al. Once-weekly semaglutide in persons with obesity and knee osteoarthritis. N. Engl. J. Med. 391, 1573–1583 (2024).
Malhotra, A. et al. Tirzepatide for the treatment of obstructive sleep apnea and obesity. N. Engl. J. Med. 391, 1193–1205 (2024).
Harrison, S. A. et al. Effect of pemvidutide, a GLP-1/glucagon dual receptor agonist, on MASLD: a randomized, double-blind, placebo-controlled study. J. Hepatol. 82, 7–17 (2025).
Loomba, R. et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N. Engl. J. Med. 391, 299–310 (2024).
Sanyal, A. J. et al. A phase 2 randomized trial of survodutide in MASH and fibrosis. N. Engl. J. Med. 391, 311–319 (2024).
Newsome, P. N. et al. Semaglutide 2.4 mg in participants with metabolic dysfunction-associated steatohepatitis: baseline characteristics and design of the phase 3 ESSENCE trial. Aliment. Pharmacol. Ther. 60, 1525–1533 (2024).
Sanyal, A. J. et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction-associated steatotic liver disease: a randomized phase 2a trial. Nat. Med. 30, 2037–2048 (2024).
Bruns Vi, N., Tressler, E. H., Vendruscolo, L. F., Leggio, L. & Farokhnia, M. IUPHAR review—glucagon-like peptide-1 (GLP-1) and substance use disorders: an emerging pharmacotherapeutic target. Pharmacol. Res. 207, 107312 (2024).
Luthi, H. et al. Effect of dulaglutide in promoting abstinence during smoking cessation: 12-month follow-up of a single-centre, randomised, double-blind, placebo-controlled, parallel group trial. EClinicalMedicine 68, 102429 (2024).
Klausen, M. K. et al. Exenatide once weekly for alcohol use disorder investigated in a randomized, placebo-controlled clinical trial. JCI Insight 7, e159863 (2022).
Hendershot, C. S. et al. Once-weekly semaglutide in adults with alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 82, 395–405 (2025).
Lahteenvuo, M. et al. Repurposing semaglutide and liraglutide for alcohol use disorder. JAMA Psychiatry 82, 94–98 (2025).
Xie, Y., Choi, T. & Al-Aly, Z. Mapping the effectiveness and risks of GLP-1 receptor agonists. Nat. Med. https://doi.org/10.1038/s41591-024-03412-w (2025).
Gerstein, H. C. et al. The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. 8, 106–114 (2020).
Strain, W. D. et al. Effects of semaglutide on stroke subtypes in type 2 diabetes: post hoc analysis of the randomized SUSTAIN 6 and PIONEER 6. Stroke 53, 2749–2757 (2022).
Aviles-Olmos, I. et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Invest. 123, 2730–2736 (2013).
Athauda, D. et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390, 1664–1675 (2017).
Meissner, W. G. et al. Trial of lixisenatide in early Parkinson’s disease. N. Engl. J. Med. 390, 1176–1185 (2024).
McGarry, A. et al. Safety, tolerability, and efficacy of NLY01 in early untreated Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 23, 37–45 (2024).
Vijiaratnam, N. et al. Exenatide once a week versus placebo as a potential disease-modifying treatment for people with Parkinson’s disease in the UK: a phase 3, multicentre, double-blind, parallel-group, randomised, placebo-controlled trial. Lancet 405, 627–636 (2025).
Cukierman-Yaffe, T. et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 19, 582–590 (2020).
Norgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement. (N. Y.) 8, e12268 (2022).
Wang, W. et al. Associations of semaglutide with first-time diagnosis of Alzheimer’s disease in patients with type 2 diabetes: target trial emulation using nationwide real-world data in the US. Alzheimers Dement. 20, 8661–8672 (2024).
Cummings, J. L. et al. EVOKE and EVOKE+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease. Alzheimers Res. Ther. 17, 14 (2025).
Lachin, J. M., Nathan, D. M. & Group, D. E. R. Understanding metabolic memory: the prolonged influence of glycemia during the Diabetes Control and Complications Trial (DCCT) on future risks of complications during the study of the epidemiology of diabetes interventions and complications (EDIC). Diabetes care 44, 2216–2224 (2021).
Lund, P. K., Goodman, R. H., Dee, P. C. & Habener, J. F. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc. Natl Acad. Sci. USA 79, 345–349 (1982).
Bell, G. I., Sanchez-Pescador, R., Laybourn, P. J. & Najarian, R. C. Exon duplication and divergence in the human preproglucagon gene. Nature 304, 368–371 (1983).
Bell, G. I., Santerre, R. F. & Mullenbach, G. T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 302, 716–718 (1983).
Eng, J., Kleinman, W. A., Singh, L., Singh, G. & Raufman, J. P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J. Biol. Chem. 267, 7402–7405 (1992).
Davidson, M. B., Bate, G. & Kirkpatrick, P. Exenatide. Nat. Rev. Drug Discov. 4, 713–714 (2005).
Drucker, D., Easley, C. & Kirkpatrick, P. Sitagliptin. Nat. Rev. Drug Discov. 6, 109–110 (2007).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Valentine, V., Goldman, J. & Shubrook, J. H. Rationale for, initiation and titration of the basal insulin/GLP-1RA fixed-ratio combination products, IDegLira and IGlarLixi, for the management of type 2 diabetes. Diabetes Ther. 8, 739–752 (2017).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Syed, Y. Y. Tirzepatide: first approval. Drugs 82, 1213–1220 (2022).
Abbasi, J. FDA green-lights tirzepatide, marketed as Zepbound, for chronic weight management. JAMA 330, 2143–2144 (2023).
Packer, M. et al. Tirzepatide for heart failure with preserved ejection fraction and obesity. N. Engl. J. Med. 392, 427–437 (2025).
McLean, B. A. et al. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr. Rev. 42, 101–132 (2021).
Lv, D. et al. Neuroprotective effects of GLP-1 class drugs in Parkinson’s disease. Front. Neurol. 15, 1462240 (2024).
Park, B. et al. GLP-1 receptor agonists and atherosclerosis protection: the vascular endothelium takes center stage. Am. J. Physiol. Heart Circ. Physiol. 326, H1159–H1176 (2024).
Wong, C. K. et al. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 34, 1514–1531.e7 (2022).
Wong, C. K. et al. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metab. 36, 130–143.e5 (2024).
Benz, E. et al. Sarcopenia and sarcopenic obesity and mortality among older people. JAMA Netw. Open. 7, e243604 (2024).
Tinsley, G. M. & Heymsfield, S. B. Fundamental body composition principles provide context for fat-free and skeletal muscle loss with GLP-1 RA treatments. J. Endocr. Soc. 8, bvae164 (2024).
Conte, C., Hall, K. D. & Klein, S. Is weight loss-induced muscle mass loss clinically relevant? Jama 332, 9–10 (2024).
Jensen, S. B. K. et al. Healthy weight loss maintenance with exercise, GLP-1 receptor agonist, or both combined followed by one year without treatment: a post-treatment analysis of a randomised placebo-controlled trial. EClinicalMedicine 69, 102475 (2024).
Trivedi, A. et al. Evaluation of the pharmacokinetics and safety of AMG 986 tablet and capsule formulations in healthy adult subjects: a phase I, open-label, randomized study. Drugs R. D. 22, 147–154 (2022).
Rodriguez, P. J. et al. Discontinuation and reinitiation of dual-labeled GLP-1 receptor agonists among us adults with overweight or obesity. JAMA Netw. Open. 8, e2457349 (2025).
Locatelli, J. C. et al. Incretin-based weight loss pharmacotherapy: can resistance exercise optimize changes in body composition? Diabetes Care 47, 1718–1730 (2024).
Linge, J., Birkenfeld, A .L. & Neeland, I. J. Muscle mass and glucagon-like peptide-1 receptor agonists: adaptive or maladaptive response to weight loss? Circulation 150, 1216–1218 (2024).
Halberg, I. B. et al. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial. Lancet Diabetes Endocrinol. 7, 179–188 (2019).
Aroda, V. R. et al. Efficacy and safety of once-daily oral semaglutide 25 mg and 50 mg compared with 14 mg in adults with type 2 diabetes (PIONEER PLUS): a multicentre, randomised, phase 3b trial. Lancet 402, 693–704 (2023).
Knop, F. K. et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 402, 705–719 (2023).
Acknowledgements
D.J.D. is supported, in part, by a Banting and Best Diabetes Centre–Novo Nordisk Chair in Incretin Biology, a Sinai Health–Novo Nordisk Foundation Fund in Regulatory Peptides, CIHR grants 154321 and 19204 and Diabetes Canada-Canadian Cancer Society grant (OG-3- 24-5819-DD).
Author information
Authors and Affiliations
Contributions
D.J.D. conceptualized, researched, wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.J.D. has received consulting fees from Amgen, AstraZeneca Inc., Insulet, Kallyope and Pfizer Inc. and speaking fees from Boehringer Ingelheim and Novo Nordisk Inc. Mount Sinai Hospital has received investigator-initiated grant support from Amgen, Eli Lilly Inc., Novo Nordisk, Pfizer and Zealand Pharmaceuticals Inc. to support preclinical studies in the Drucker laboratory.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Timo Müller and the other anonymous reviewer/s for their contribution to the peer review of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ACCG-2671: https://ir.structuretx.com/static-files/ca239943-dfac-45e0-8ec8-6e4f3ba06e42
Amycretin: https://www.novonordisk.com/content/dam/nncorp/global/en/investors/irmaterial/cmd/2024/P5-Obesity-Care.pdf and https://www.novonordisk.com/news-and-media/news-and-ir-materials/news-details.html?id=915251 and https://www.novonordisk.com/investors/capital-markets-day-2024.html
ASC30: https://www.ascletis.com/news_detail/192/id/1146.html
Bioglutide: https://www.biomedind.com/news-NA-931-world-obesity.html
Bofanglutide: https://onlinelibrary.wiley.com/doi/full/10.1002/oby.24194 and https://www.ganlee.com/detail/765.html
CT-868: https://assets.roche.com/f/176343/x/f513f69de2/pharma_day_20240930_final_online_v02.pdf
CT-996: https://assets.roche.com/f/176343/x/f513f69de2/pharma_day_20240930_final_online_v02.pdf and https://www.roche.com/media/releases/med-cor-2024-07-17
Danuglipron: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-advances-development-once-daily-formulation-oral-glp and https://www.pfizer.com/news/press-release/press-release-detail/pfizer-provides-update-oral-glp-1-receptor-agonist
GSBR-1290: https://ir.structuretx.com/static-files/775ca535-9bd3-455e-a41a-44dea273c90d
KAI-7535: https://www.kailera.com/wp-content/uploads/2024/09/95-LB_2023_ADA_2023_7535_LBA_poster_final.pdf
KAI-9531: https://www.kailera.com/wp-content/uploads/2024/09/LBA66_Hong-Chen_HRS9531-in-T2DM_HRS9531-202_EASD2024.pdf and https://www.kailera.com/wp-content/uploads/2024/09/HRS9531-201_ADA2024_Poster.pdf and https://www.kailera.com/press-release/jiangsu-hengrui-pharmaceuticals-and-kailera-therapeutics-report-positive-topline-data-from-8-mg-dose-of-phase-2-obesity-trial-of-glp-1-gip-receptor-dual-agonist-hrs9531/
MET-097: https://www.businesswire.com/news/home/20240924023447/en/Metsera-Reports-Highly-Competitive-Results-from-Ongoing-Trial-of-Novel-Potential-Once-Monthly-Injectable-GLP-1-Receptor-Agonist-MET-097 and https://investors.metsera.com/news-releases/news-release-details/metsera-announces-positive-topline-phase-2a-clinical-data-its
Orforglipron: https://investor.lilly.com/static-files/cdedba2e-c4a7-4047-9e61-4ac3b1e2cd73 and https://investor.lilly.com/news-releases/news-release-details/lillys-oral-glp-1-orforglipron-demonstrated-statistically
Pemvidutide: https://ir.altimmune.com/news-releases/news-release-details/altimmune-presents-data-phase-2-momentum-trial-pemvidutide and https://ir.altimmune.com/news-releases/news-release-details/altimmune-announces-successful-completion-end-phase-2-meeting
Petrelintide: https://www.zealandpharma.com/pipeline/petrelintide/
REDEFINE trials Cagri-Sema: https://www.novonordisk.com/content/dam/nncorp/global/en/investors/irmaterial/cmd/2024/P5-Obesity-Care.pdf and https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=915082 and https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/newsdetails.html?id=915294
Retatrutide: https://investor.lilly.com/static-files/cdedba2e-c4a7-4047-9e61-4ac3b1e2cd73
SURMOUNT-5 trial: https://investor.lilly.com/news-releases/news-release-details/lillys-zepboundr-tirzepatide-superior-wegovyr-semaglutide-head
The ESSENCE trial: https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=171971
The SOUL Trial: https://www.novonordisk.com/content/nncorp/global/en/news-and-media/news-and-ir-materials/news-details.html?id=171480
VK2735: https://vikingtherapeutics.com/pipeline/metabolic-disease-program/vk2735/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Drucker, D.J. GLP-1-based therapies for diabetes, obesity and beyond. Nat Rev Drug Discov 24, 631–650 (2025). https://doi.org/10.1038/s41573-025-01183-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-025-01183-8
This article is cited by
-
The expanding landscape of GLP-1 medicines
Nature Medicine (2026)
-
Human liver and pancreas innervation: resolving 3D neurohistological challenges and advancing insights
Journal of Biomedical Science (2025)
-
Neurodegeneration onset with glucagon-like peptide-1 receptor agonists in people with type 2 diabetes: a real-world multinational cohort study
Cardiovascular Diabetology (2025)
-
Repurposing glucagon-like peptide-1 receptor agonists for the treatment of neurodegenerative disorders
Nature Aging (2025)
-
Oromucosal films for peptide delivery: formulation strategies using permeation enhancers and polymers
Drug Delivery and Translational Research (2025)