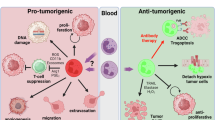
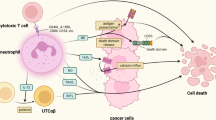
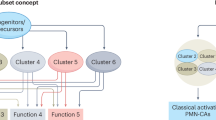
Abstract
Neutrophils are among the most abundant immune cell types in the tumour microenvironment and have been associated with poor outcomes across multiple cancer types. Yet despite mounting evidence of their role in tumour progression, therapeutic strategies targeting neutrophils have only recently gained attention and remain limited in scope. This is probably due to the increasing number of distinct neutrophil subtypes identified in cancer and the limited understanding of the mechanisms by which these subsets influence tumour progression and immune evasion. In this Review, we discuss the spectrum of neutrophil subtypes — including those with antitumour activity — and their potential to polarize towards tumour-suppressive phenotypes. We explore the molecular pathways and effector functions by which neutrophils modulate cancer progression, with an emphasis on identifying tractable therapeutic targets. Finally, we examine emerging clinical trials aimed at modulating neutrophil lineages and consider their implications for patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hidalgo, A., Chilvers, E. R., Summers, C. & Koenderman, L. The neutrophil life cycle. Trends Immunol. 40, 584–597 (2019).
Hedrick, C. C. & Malanchi, I. Neutrophils in cancer: heterogeneous and multifaceted. Nat. Rev. Immunol. 22, 173–187 (2022).
Coffelt, S. B., Wellenstein, M. D. & de Visser, K. E. Neutrophils in cancer: neutral no more. Nat. Rev. Cancer 16, 431–446 (2016).
Quail, D. F. et al. Neutrophil phenotypes and functions in cancer: a consensus statement. J. Exp. Med. 219, e20220011 (2022).
Jaillon, S. et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 20, 485–503 (2020).
Ballesteros, I. et al. Co-option of neutrophil fates by tissue environments. Cell 183, 1282–1297.e1218 (2020). This seminal article reported that tissues can co-opt neutrophil fates to serve tissue specific roles and thus demonstrated an unprecedented level of neutrophil heterogeneity.
Xie, X. et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat. Immunol. 21, 1119–1133 (2020).
Grieshaber-Bouyer, R. et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat. Commun. 12, 2856 (2021). This article provides an original description of neutrotime, a novel classification schema based on of a single, chronologically ordered neutrophil developmental spectrum.
Hackert, N. S. et al. Human and mouse neutrophils share core transcriptional programs in both homeostatic and inflamed contexts. Nat. Commun. 14, 8133 (2023).
Wigerblad, G. et al. Single-cell analysis reveals the range of transcriptional states of circulating human neutrophils. J. Immunol. 209, 772–782 (2022).
Montaldo, E. et al. Cellular and transcriptional dynamics of human neutrophils at steady state and upon stress. Nat. Immunol. 23, 1470–1483 (2022).
Matlung, H. L. et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 23, 3946–3959.e3946 (2018).
Gungabeesoon, J. et al. A neutrophil response linked to tumor control in immunotherapy. Cell 186, 1448–1464.e1420 (2023). This was the first report to show that neutrophils could be therapeutically polarized into antitumour phenotypes using immunotherapeutic approaches.
Kwok, I. et al. Combinatorial single-cell analyses of granulocyte–monocyte progenitor heterogeneity reveals an early uni-potent neutrophil progenitor. Immunity 53, 303–318.e305 (2020).
Zhu, Y. P. et al. Identification of an early unipotent neutrophil progenitor with pro-tumoral activity in mouse and human bone marrow. Cell Rep. 24, 2329–2341.e2328 (2018).
Evrard, M. et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 48, 364–379.e368 (2018).
Calzetti, F. et al. CD66b−CD64dimCD115− cells in the human bone marrow represent neutrophil-committed progenitors. Nat. Immunol. 23, 679–691 (2022).
Palomino-Segura, M., Sicilia, J., Ballesteros, I. & Hidalgo, A. Strategies of neutrophil diversification. Nat. Immunol. 24, 575–584 (2023).
Engblom, C. et al. Osteoblasts remotely supply lung tumors with cancer-promoting SiglecFhigh neutrophils. Science 358, eaal5081 (2017). This study demonstrates that adenocarcinomas induce bone marrow stromal cells to supply a unique subset of SiglecF-high tumour-promoting neutrophils to sites of tumorigenesis.
Pfirschke, C. et al. Tumor-promoting Ly-6G+ SiglecFhigh cells are mature and long-lived neutrophils. Cell Rep. 32, 108164 (2020).
Yvan-Charvet, L. & Ng, L. G. Granulopoiesis and neutrophil homeostasis: a metabolic, daily balancing act. Trends Immunol. 40, 598–612 (2019).
Lawrence, S. M., Corriden, R. & Nizet, V. How neutrophils meet their end. Trends Immunol. 41, 531–544 (2020).
Martin, C. et al. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19, 583–593 (2003).
Mathias, J. R. et al. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288 (2006).
Wang, J. et al. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 358, 111–116 (2017).
Wu, D. et al. Reverse-migrated neutrophils regulated by JAM-C are involved in acute pancreatitis-associated lung injury. Sci. Rep. 6, 20545 (2016).
Hind, L. E. & Huttenlocher, A. Neutrophil reverse migration and a chemokinetic resolution. Dev. Cell 47, 404–405 (2018).
Brostjan, C. & Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 6, 26 (2020).
Greenlee-Wacker, M. C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 273, 357–370 (2016).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Adrover, J. M., McDowell, S. A. C., He, X. Y., Quail, D. F. & Egeblad, M. NETworking with cancer: the bidirectional interplay between cancer and neutrophil extracellular traps. Cancer Cell 41, 505–526 (2023).
Coffelt, S. B. et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015).
Kwak, J. W. et al. CXCR1/2 antagonism inhibits neutrophil function and not recrtuitment in cancer. Oncoimmunology (in the press).
Sparmann, A. & Bar-Sagi, D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell 6, 447–458 (2004).
Karin, M. Inflammation and cancer: the long reach of Ras. Nat. Med. 11, 20–21 (2005).
Ji, H. et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 25, 2105–2112 (2006).
Nagarsheth, N., Wicha, M. S. & Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 17, 559–572 (2017).
Chang, S. H. et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc. Natl Acad. Sci. USA 111, 5664–5669 (2014). This study demonstrates that TH17-mediated immunity enhances recruitment of pro-tumour neutrophils to sites of tumorigenesis.
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Shaul, M. E. & Fridlender, Z. G. Tumour-associated neutrophils in patients with cancer. Nat. Rev. Clin. Oncol. 16, 601–620 (2019).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015). This article provides a comprehensive assessment of immune cell content across 30 cancer types showing that neutrophil content predicted mortality greater than any other immune population.
Berry, R. S. et al. High levels of tumor-associated neutrophils are associated with improved overall survival in patients with stage II colorectal cancer. PLoS ONE 12, e0188799 (2017).
Galdiero, M. R. et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int. J. Cancer 139, 446–456 (2016).
Droeser, R. A. et al. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS ONE 8, e64814 (2013).
Kuang, D. M. et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 54, 948–955 (2011).
Jensen, H. K. et al. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J. Clin. Oncol. 27, 4709–4717 (2009).
Carus, A., Ladekarl, M., Hager, H., Nedergaard, B. S. & Donskov, F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br. J. Cancer 108, 2116–2122 (2013).
Wislez, M. et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 63, 1405–1412 (2003).
Bellocq, A. et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am. J. Pathol. 152, 83–92 (1998).
Jensen, T. O. et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer 118, 2476–2485 (2012).
Shen, M. et al. Tumor-associated neutrophils as a new prognostic factor in cancer: a systematic review and meta-analysis. PLoS ONE 9, e98259 (2014).
Yamashita, J., Ogawa, M. & Shirakusa, T. Free-form neutrophil elastase is an independent marker predicting recurrence in primary breast cancer. J. Leukoc. Biol. 57, 375–378 (1995).
Kargl, J. et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat. Commun. 8, 14381 (2017).
Eruslanov, E. B. et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J. Clin. Invest. 124, 5466–5480 (2014).
Rakaee, M. et al. Prognostic effect of intratumoral neutrophils across histological subtypes of non-small cell lung cancer. Oncotarget 7, 72184–72196 (2016).
Ponzetta, A. et al. Neutrophils driving unconventional T cells mediate resistance against murine sarcomas and selected human tumors. Cell 178, 346–360.e324 (2019).
Carnevale, S. et al. Neutrophils mediate protection against colitis and carcinogenesis by controlling bacterial invasion and IL22 production by γδ T cells. Cancer Immunol. Res. 12, 413–426 (2024).
Lizotte, P. H. et al. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight 1, e89014 (2016).
Ilie, M. et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer 118, 1726–1737 (2012).
Kargl, J. et al. Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC. JCI Insight 4, e130850 (2019). This is the first article to report that neutrophil-infiltrated cancers do not respond to immune checkpoint inhibitor therapy.
Benguigui, M. et al. Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell 42, 253–265.e212 (2024). This article demonstrates that IFN-stimulated neutrophils express Ly6E, which acts as a biomarker for favourable treatment response to immune checkpoint blockade.
Faria, S. S. et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience 10, 702 (2016).
Grenader, T. et al. Derived neutrophil lymphocyte ratio is predictive of survival from intermittent therapy in advanced colorectal cancer: a post hoc analysis of the MRC COIN study. Br. J. Cancer 114, 612–615 (2016).
Krenn-Pilko, S. et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br. J. Cancer 110, 2524–2530 (2014).
McNamara, M. G. et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur. J. Cancer 50, 1581–1589 (2014).
Sonpavde, G. et al. Prognostic impact of the neutrophil-to-lymphocyte ratio in men with metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 12, 317–324 (2014).
Ma, J. et al. Neutrophil-to-lymphocyte ratio (NLR) as a predictor for recurrence in patients with stage III melanoma. Sci. Rep. 8, 4044 (2018).
Bar-Ad, V. et al. Neutrophil to lymphocyte ratio associated with prognosis of lung cancer. Clin. Transl. Oncol. 19, 711–717 (2017).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl Cancer Inst. 106, dju124 (2014).
Cupp, M. A. et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 18, 360 (2020).
Kim, H. R. et al. The ratio of peripheral regulatory T cells to Lox-1+ polymorphonuclear myeloid-derived suppressor cells predicts the early response to anti-PD-1 therapy in patients with non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 199, 243–246 (2019).
Yu, Y., Qian, L. & Cui, J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: a meta-analysis of 7,219 patients. Mol. Clin. Oncol. 7, 498–506 (2017).
Shao, Y. et al. Prognostic value of pretreatment neutrophil-to-lymphocyte ratio in renal cell carcinoma: a systematic review and meta-analysis. BMC Urol. 20, 90 (2020).
Cassidy, M. R. et al. Neutrophil to lymphocyte ratio is associated with outcome during ipilimumab treatment. EBioMedicine 18, 56–61 (2017).
Jiang, T. et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol. Immunother. 67, 713–727 (2018).
Valero, C. et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 12, 729 (2021).
Lalani, A. A. et al. Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J. Immunother. Cancer 6, 5 (2018).
Coussens, L. M., Tinkle, C. L., Hanahan, D. & Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103, 481–490 (2000).
Gong, L. et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol. Cancer 12, 154 (2013).
Jablonska, J., Leschner, S., Westphal, K., Lienenklaus, S. & Weiss, S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Invest. 120, 1151–1164 (2010).
Wculek, S. K., Bridgeman, V. L., Peakman, F. & Malanchi, I. Early neutrophil responses to chemical carcinogenesis shape long-term lung cancer susceptibility. iScience 23, 101277 (2020).
Fialkow, L., Wang, Y. & Downey, G. P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic. Biol. Med. 42, 153–164 (2007).
Knaapen, A. M., Gungor, N., Schins, R. P., Borm, P. J. & Van Schooten, F. J. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis 21, 225–236 (2006).
Harrison, J. E. & Schultz, J. Studies on the chlorinating activity of myeloperoxidase. J. Biol. Chem. 251, 1371–1374 (1976).
Gungor, N. et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis 25, 149–154 (2010).
Lu, Y. et al. Prognostic significance and immunological role of HPRT1 in human cancers. Biomol. Biomed. 24, 262–291 (2024).
Feyler, A. et al. Point: myeloperoxidase -463G –> a polymorphism and lung cancer risk. Cancer Epidemiol. Biomark. Prev. 11, 1550–1554 (2002).
Houghton, A. M. et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat. Med. 16, 219–223 (2010). This is one of the first studies to provide a mechanistic basis for the ability of neutrophils to promote tumour growth.
Gregory, A. D., Hale, P., Perlmutter, D. H. & Houghton, A. M. Clathrin pit-mediated endocytosis of neutrophil elastase and cathepsin G by cancer cells. J. Biol. Chem. 287, 35341–35350 (2012).
Metz, H. E. et al. Insulin receptor substrate-1 deficiency drives a proinflammatory phenotype in KRAS mutant lung adenocarcinoma. Proc. Natl Acad. Sci. USA 113, 8795–8800 (2016).
Nozawa, H., Chiu, C. & Hanahan, D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl Acad. Sci. USA 103, 12493–12498 (2006).
Ardi, V. C., Kupriyanova, T. A., Deryugina, E. I. & Quigley, J. P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl Acad. Sci. USA 104, 20262–20267 (2007).
Queen, M. M., Ryan, R. E., Holzer, R. G., Keller-Peck, C. R. & Jorcyk, C. L. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 65, 8896–8904 (2005).
Shojaei, F. et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 (2007).
Shojaei, F., Singh, M., Thompson, J. D. & Ferrara, N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc. Natl Acad. Sci. USA 105, 2640–2645 (2008).
Keane, M. P., Belperio, J. A., Xue, Y. Y., Burdick, M. D. & Strieter, R. M. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J. Immunol. 172, 2853–2860 (2004).
Pold, M. et al. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC ligand (CXCL)5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res. 64, 1853–1860 (2004).
Arenberg, D. A. et al. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J. Clin. Invest. 102, 465–472 (1998).
Mollaoglu, G. et al. The lineage-defining transcription factors SOX2 and NKX2-1 determine lung cancer cell fate and shape the tumor immune microenvironment. Immunity 49, 764–779 e769 (2018). This article demonstrates that tumour-associated neutrophils can alter cancer cell differentiation status and fate.
Zilionis, R. et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 50, 1317–1334 e1310 (2019). This is a seminal report of novel neutrophil transcriptional subtypes in cancer using single-cell RNA sequencing.
Salcher, S. et al. High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell 40, 1503–1520 e1508 (2022).
Veglia, F. et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J. Exp. Med. 218, e20201803 (2021).
Tang, K. H. et al. Combined inhibition of SHP2 and CXCR1/2 promotes antitumor T-cell response in NSCLC. Cancer Discov. 12, 47–61 (2022).
Ng, M. S. F. et al. Deterministic reprogramming of neutrophils within tumors. Science 383, eadf6493 (2024).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Kusmartsev, S., Nefedova, Y., Yoder, D. & Gabrilovich, D. I. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172, 989–999 (2004).
Bronte, V. et al. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood 96, 3838–3846 (2000).
Bronte, V., Serafini, P., Apolloni, E. & Zanovello, P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J. Immunother. 24, 431–446 (2001).
Gabrilovich, D. I., Velders, M. P., Sotomayor, E. M. & Kast, W. M. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol. 166, 5398–5406 (2001).
Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021).
Youn, J. I., Nagaraj, S., Collazo, M. & Gabrilovich, D. I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 (2008).
Schmielau, J. & Finn, O. J. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 61, 4756–4760 (2001).
Miret, J. J. et al. Suppression of myeloid cell arginase activity leads to therapeutic response in a NSCLC mouse model by activating anti-tumor immunity. J. Immunother. Cancer 7, 32 (2019).
Mazzoni, A. et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695 (2002).
Nagaraj, S. et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 13, 828–835 (2007).
Zhang, H. et al. Annexin A2/TLR2/MYD88 pathway induces arginase 1 expression in tumor-associated neutrophils. J. Clin. Invest. 132, e153643 (2022).
Davis, R. J. et al. Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid-derived suppressor cells with a selective inhibitor of PI3Kdelta/gamma. Cancer Res. 77, 2607–2619 (2017).
Clavijo, P. E. et al. Resistance to CTLA-4 checkpoint inhibition reversed through selective elimination of granulocytic myeloid cells. Oncotarget 8, 55804–55820 (2017).
Bronte, V. et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 7, 12150 (2016).
Condamine, T. et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 1, aaf8943 (2016).
Condamine, T. et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J. Clin. Invest. 124, 2626–2639 (2014).
Wang, C. et al. CD300ld on neutrophils is required for tumour-driven immune suppression. Nature 621, 830–839 (2023). This article was the first to identify CD300ld as a marker for PMN-MDSC that is required for both PMN-MDSC recruitment and immunosuppressive function in cancer.
Masucci, M. T., Minopoli, M., Del Vecchio, S. & Carriero, M. V. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front. Immunol. 11, 1749 (2020).
Alfaro, C. et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin. Cancer Res. 22, 3924–3936 (2016).
El Rayes, T. et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl Acad. Sci. USA 112, 16000–16005 (2015).
Demers, M. et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology 5, e1134073 (2016).
Demers, M. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl Acad. Sci. USA 109, 13076–13081 (2012).
Rayes, R. F. et al. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight 5, e128008 (2019).
Cools-Lartigue, J. et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 (2013).
Spiegel, A. et al. Neutrophils suppress intraluminal NK cell-mediated tumor cell clearance and enhance extravasation of disseminated carcinoma cells. Cancer Discov. 6, 630–649 (2016).
Szczerba, B. M. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 (2019).
Park, J. et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 8, 361ra138 (2016).
McDowell, S. A. C. et al. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat. Cancer 2, 545–562 (2021).
Teijeira, A. et al. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity 52, 856–871.e858 (2020). This study showed that NETs form a shield around cancer cells protecting them from infiltration by immune cells, such as CD8+ T cells.
Thalin, C. et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb. Res. 139, 56–64 (2016).
Martinod, K. & Wagner, D. D. Thrombosis: tangled up in NETs. Blood 123, 2768–2776 (2014).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018). This article provides a detailed mechanistic analysis that demonstrates that NET-derived proteinases generate a novel laminin cleavage product that induces outgrowth of dormant metastases.
Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009). This article reports, for the first time, the ability of neutrophils to polarize into pro-tumour (N2) and antitumour (N1) phenotypes.
Shrestha, S. et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonist attenuate tumor growth via polarization of neutrophils toward an antitumor phenotype. Oncoimmunology 5, e1067744 (2016).
Granot, Z. et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 20, 300–314 (2011).
Gershkovitz, M. et al. Microenvironmental cues determine tumor cell susceptibility to neutrophil cytotoxicity. Cancer Res. 78, 5050–5059 (2018).
Gershkovitz, M. et al. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 78, 2680–2690 (2018).
Finisguerra, V. et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature 522, 349–353 (2015).
Dance, A. Core concept: cells nibble one another via the under-appreciated process of trogocytosis. Proc. Natl Acad. Sci. USA 116, 17608–17610 (2019).
Bouti, P. et al. Kindlin3-dependent CD11b/CD18-integrin activation is required for potentiation of neutrophil cytotoxicity by CD47-SIRPα checkpoint disruption. Cancer Immunol. Res. 9, 147–155 (2021).
Singhal, S. et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 30, 120–135 (2016). This article reports the identification of a novel subset of tumour-associated neutrophils that express HLA-DR and function as antigen presenting cells in cancer.
Linde, I. L. et al. Neutrophil-activating therapy for the treatment of cancer. Cancer Cell 41, 356–372 e310 (2023).
Hirschhorn, D. et al. T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell 186, 1432–1447.e1417 (2023). This is the first article to report that antigen-specific T cells combined with OX40 co-stimulation can induced neutrophils to eliminate antigen-escape variants.
Kruse, B. et al. CD4+ T cell-induced inflammatory cell death controls immune-evasive tumours. Nature 618, 1033–1040 (2023).
Janne, P. A. et al. Randomized, double-blind, phase II trial comparing gemcitabine-cisplatin plus the LTB4 antagonist LY293111 versus gemcitabine–cisplatin plus placebo in first-line non-small-cell lung cancer. J. Thorac. Oncol. 9, 126–131 (2014).
Di Maio, M. et al. Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol. 6, 669–677 (2005).
Shojaei, F. et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc. Natl Acad. Sci. USA 106, 6742–6747 (2009).
Kowanetz, M. et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl Acad. Sci. USA 107, 21248–21255 (2010).
Cardot-Ruffino, V. et al. G-CSF rescue of FOLFIRINOX-induced neutropenia leads to systemic immune suppression in mice and humans. J. Immunother. Cancer 11, e006589 (2023).
Kozopas, K. M., Yang, T., Buchan, H. L., Zhou, P. & Craig, R. W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl Acad. Sci. USA 90, 3516–3520 (1993).
Zhou, P., Qian, L., Kozopas, K. M. & Craig, R. W. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood 89, 630–643 (1997).
Dzhagalov, I., St John, A. & He, Y. W. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 109, 1620–1626 (2007).
Yeh, Y. Y. et al. Up-regulation of CDK9 kinase activity and Mcl-1 stability contributes to the acquired resistance to cyclin-dependent kinase inhibitors in leukemia. Oncotarget 6, 2667–2679 (2015).
Diamond, J. R. et al. First-in-human dose-escalation study of cyclin-dependent kinase 9 inhibitor VIP152 in patients with advanced malignancies shows early signs of clinical efficacy. Clin. Cancer Res. 28, 1285–1293 (2022).
Rennard, S. I. et al. CXCR2 antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 191, 1001–1011 (2015).
Kirsten, A. M. et al. The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm. Pharmacol. Ther. 31, 36–41 (2015).
Richardson, R. M., Pridgen, B. C., Haribabu, B., Ali, H. & Snyderman, R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J. Biol. Chem. 273, 23830–23836 (1998).
Baggiolini, M., Dewald, B. & Moser, B. Human chemokines: an update. Annu. Rev. Immunol. 15, 675–705 (1997).
Steele, C. W. et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29, 832–845 (2016). This is the first study to demonstrate the therapeutic potential CXCRR2 antagonists as cancer treatment.
Sun, L. et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 4, e126853 (2019).
Liao, W. et al. KRAS–IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 35, 559–572.e557 (2019).
Jamieson, T. et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Invest. 122, 3127–3144 (2012).
Highfill, S. L. et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 6, 237ra267 (2014).
Dominguez, C., McCampbell, K. K., David, J. M. & Palena, C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight 2, e94296 (2017).
Raccosta, L. et al. The oxysterol–CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J. Exp. Med. 210, 1711–1728 (2013).
Greene, S. et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-cell immunotherapy in head and neck cancer models. Clin. Cancer Res. 26, 1420–1431 (2020).
Guo, C. et al. Targeting myeloid chemotaxis to reverse prostate cancer therapy resistance. Nature 623, 1053–1061 (2023).
Schalper, K. A. et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 26, 688–692 (2020). This study identified a clear cut point in serum IL-8 content predictive of therapeutic response to immune checkpoint blockade across multiple cancer types.
Patel, S. P. et al. Safety and efficacy of first-in-class CXCR1/2 inhibitor SX-682 in combination with pembrolizumab in patients with metastatic melanoma with disease progression on anti-PD1 therapy. J. Clin. Oncol. https://doi.org/10.1200/JCO.2024.42.16_suppl.9508 (2024).
Canavan, T. N., Elmets, C. A., Cantrell, W. L., Evans, J. M. & Elewski, B. E. Anti-IL-17 medications used in the treatment of plaque psoriasis and psoriatic arthritis: a comprehensive review. Am. J. Clin. Dermatol. 17, 33–47 (2016).
Wang, W. M. & Jin, H. Z. Role of neutrophils in psoriasis. J. Immunol. Res. 2020, 3709749 (2020).
Mark, N. M. et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Am. J. Respir. Crit. Care Med. 197, 325–336 (2018).
Kwak, J. W. et al. Complement activation via a C3a receptor pathway alters CD4+ T lymphocytes and mediates lung cancer progression. Cancer Res. 78, 143–156 (2018).
Pagano, M. B. et al. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation 119, 1805–1813 (2009).
Jayne, D. R. W., Merkel, P. A., Schall, T. J., Bekker, P. & Group, A. S. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 384, 599–609 (2021).
Ariga, M. et al. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J. Immunol. 173, 7531–7538 (2004).
Jacob, C., Szilagyi, C., Allen, J. M., Bertrand, C. & Lagente, V. Role of PDE4 in superoxide anion generation through p44/42MAPK regulation: a cAMP and a PKA-independent mechanism. Br. J. Pharmacol. 143, 257–268 (2004).
Rabe, K. F., Calverley, P. M. A., Martinez, F. J. & Fabbri, L. M. Effect of roflumilast in patients with severe COPD and a history of hospitalisation. Eur. Respir. J. 50, 1700158 (2017).
Dunne, A. E. et al. Direct inhibitory effect of the PDE4 inhibitor roflumilast on neutrophil migration in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 60, 445–453 (2019).
Tsunoda, T. et al. Inhibition of phosphodiesterase-4 (PDE4) activity triggers luminal apoptosis and AKT dephosphorylation in a 3-D colonic-crypt model. Mol. Cancer 11, 46 (2012).
Bronte, V. & Zanovello, P. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5, 641–654 (2005).
Rodriguez, P. C. et al. Regulation of T cell receptor CD3ζ chain expression by l-arginine. J. Biol. Chem. 277, 21123–21129 (2002).
Munder, M. et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 105, 2549–2556 (2005).
Jacobsen, L. C., Theilgaard-Monch, K., Christensen, E. I. & Borregaard, N. Arginase 1 is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood 109, 3084–3087 (2007).
Manne, A., Woods, E., Tsung, A. & Mittra, A. Biliary tract cancers: treatment updates and future directions in the era of precision medicine and immuno-oncology. Front. Oncol. 11, 768009 (2021).
Bakker, E. M. et al. Improved treatment response to dornase alfa in cystic fibrosis patients using controlled inhalation. Eur. Respir. J. 38, 1328–1335 (2011).
Gajendran, C. et al. Alleviation of arthritis through prevention of neutrophil extracellular traps by an orally available inhibitor of protein arginine deiminase 4. Sci. Rep. 13, 3189 (2023).
Keir, H. R. et al. CXCL-8-dependent and -independent neutrophil activation in COPD: experiences from a pilot study of the CXCR2 antagonist danirixin. ERJ Open Res. 6, 00583–02020 (2020).
Elborn, J. S. et al. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur. Respir. J. 40, 969–976 (2012).
Scannevin, R. H. et al. Discovery of a highly selective chemical inhibitor of matrix metalloproteinase-9 (MMP-9) that allosterically inhibits zymogen activation. J. Biol. Chem. 292, 17963–17974 (2017).
Korkmaz, B., Horwitz, M. S., Jenne, D. E. & Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 62, 726–759 (2010).
Inada, M., Yamashita, J. & Ogawa, M. Neutrophil elastase inhibitor (ONO-5046-Na) inhibits the growth of human lung cancer cell lines transplanted into severe combined immunodeficiency (scid) mice. Res. Commun. Mol. Pathol. Pharmacol. 97, 229–232 (1997).
Kawabata, K. et al. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem. Biophys. Res. Commun. 177, 814–820 (1991).
Pu, S. et al. Effect of sivelestat sodium in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. BMC Pulm. Med. 17, 148 (2017).
Stockley, R. et al. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir. Med. 107, 524–533 (2013).
Cui, C. et al. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell 184, 3163–3177.e3121 (2021).
Demkow, U. & van Overveld, F. J. Role of elastases in the pathogenesis of chronic obstructive pulmonary disease: implications for treatment. Eur. J. Med. Res. 15, 27–35 (2010).
Lee, W. L. & Downey, G. P. Leukocyte elastase: physiological functions and role in acute lung injury. Am. J. Respir. Crit. Care Med. 164, 896–904 (2001).
Wakeham, D. J. & New, K. J. Neutrophil elastase: a key factor in the development of aortic aneurysm. Am. J. Hypertens. 37, 321–322 (2024).
Dallegri, F. et al. Tumor cell lysis by activated human neutrophils: analysis of neutrophil-delivered oxidative attack and role of leukocyte function-associated antigen 1. Inflammation 15, 15–30 (1991).
Logtenberg, M. E. W., Scheeren, F. A. & Schumacher, T. N. The CD47-SIRPα immune checkpoint. Immunity 52, 742–752 (2020).
Ring, N. G. et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc. Natl Acad. Sci. USA 114, E10578–E10585 (2017).
Jiang, Z., Sun, H., Yu, J., Tian, W. & Song, Y. Targeting CD47 for cancer immunotherapy. J. Hematol. Oncol. 14, 180 (2021).
Kohrt, H. E. et al. Combination strategies to enhance antitumor ADCC. Immunotherapy 4, 511–527 (2012).
Singhal, S. et al. Human tumor-associated macrophages and neutrophils regulate antitumor antibody efficacy through lethal and sublethal trogocytosis. Cancer Res. 84, 1029–1047 (2024).
Behrens, L. M., van Egmond, M. & van den Berg, T. K. Neutrophils as immune effector cells in antibody therapy in cancer. Immunol. Rev. 314, 280–301 (2023).
Pascal, V. et al. Anti-CD20 IgA can protect mice against lymphoma development: evaluation of the direct impact of IgA and cytotoxic effector recruitment on CD20 target cells. Haematologica 97, 1686–1694 (2012).
Leusen, J. H. IgA as therapeutic antibody. Mol. Immunol. 68, 35–39 (2015).
Bohlander, F. A new hope? Possibilities of therapeutic IgA antibodies in the treatment of inflammatory lung diseases. Front. Immunol. 14, 1127339 (2023).
Derynck, R., Akhurst, R. J. & Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 29, 117–129 (2001).
Pang, Y. et al. TGF-β signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 3, 936–951 (2013).
Lichtenstein, A. K., Berek, J., Kahle, J. & Zighelboim, J. Role of inflammatory neutrophils in antitumor effects induced by intraperitoneal administration of Corynebacterium parvum in mice. Cancer Res. 44, 5118–5123 (1984).
Lichtenstein, A. K., Kahle, J., Berek, J. & Zighelboim, J. Successful immunotherapy with intraperitoneal Corynebacterium parvum in a murine ovarian cancer model is associated with the recruitment of tumor-lytic neutrophils into the peritoneal cavity. J. Immunol. 133, 519–526 (1984).
Yam, A. O. et al. Neutrophil conversion to a tumor-killing phenotype underpins effective microbial therapy. Cancer Res. 83, 1315–1328 (2023).
Hong, F. et al. β-Glucan functions as an adjuvant for monoclonal antibody immunotherapy by recruiting tumoricidal granulocytes as killer cells. Cancer Res. 63, 9023–9031 (2003).
Kalafati, L. et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183, 771–785.e712 (2020).
He, G. et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 34, 141 (2015).
Ravi, A. et al. Genomic and transcriptomic analysis of checkpoint blockade response in advanced non-small cell lung cancer. Nat. Genet. 55, 807–819 (2023).
Gauchat, J. F. et al. Human CD40-ligand: molecular cloning, cellular distribution and regulation of expression by factors controlling IgE production. FEBS Lett. 315, 259–266 (1993).
Grewal, I. S. & Flavell, R. A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16, 111–135 (1998).
Lum, H. D. et al. In vivo CD40 ligation can induce T-cell-independent antitumor effects that involve macrophages. J. Leukoc. Biol. 79, 1181–1192 (2006).
Li, D. K. & Wang, W. Characteristics and clinical trial results of agonistic anti-CD40 antibodies in the treatment of malignancies. Oncol. Lett. 20, 176 (2020).
Padron, L. J. et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat. Med. 28, 1167–1177 (2022).
Weiss, S. A. et al. A phase II trial of the CD40 agonistic antibody sotigalimab (APX005M) in combination with nivolumab in subjects with metastatic melanoma with confirmed disease progression on anti-PD-1 therapy. Clin. Cancer Res. 30, 74–81 (2024).
ElTanbouly, M. A., Croteau, W., Noelle, R. J. & Lines, J. L. VISTA: a novel immunotherapy target for normalizing innate and adaptive immunity. Semin. Immunol. 42, 101308 (2019).
Lines, J. L. et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 74, 1924–1932 (2014).
Lee, W. L. & Downey, G. P. Neutrophil activation and acute lung injury. Curr. Opin. Crit. Care 7, 1–7 (2001).
Dancey, J. T., Deubelbeiss, K. A., Harker, L. A. & Finch, C. A. Neutrophil kinetics in man. J. Clin. Invest. 58, 705–715 (1976).
Fouret, P. et al. Expression of the neutrophil elastase gene during human bone marrow cell differentiation. J. Exp. Med. 169, 833–845 (1989).
Rorvig, S., Ostergaard, O., Heegaard, N. H. & Borregaard, N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. J. Leukoc. Biol. 94, 711–721 (2013).
Manley, H. R., Keightley, M. C. & Lieschke, G. J. The neutrophil nucleus: an important influence on neutrophil migration and function. Front. Immunol. 9, 2867 (2018).
Huh, S. J., Liang, S., Sharma, A., Dong, C. & Robertson, G. P. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 70, 6071–6082 (2010).
Wculek, S. K. & Malanchi, I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature 528, 413–417 (2015).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Lyden, D. et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 7, 1194–1201 (2001).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Spicer, J. D. et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 72, 3919–3927 (2012). This is a seminal report of how neutrophils can engage circulating tumour cells and promote subsequent metastasis formation.
Li, R. et al. Chronic IL-1β-induced inflammation regulates epithelial-to-mesenchymal transition memory phenotypes via epigenetic modifications in non-small cell lung cancer. Sci. Rep. 10, 377 (2020).
Harris, E. S., Weyrich, A. S. & Zimmerman, G. A. Lessons from rare maladies: leukocyte adhesion deficiency syndromes. Curr. Opin. Hematol. 20, 16–25 (2013).
Kaplan, J., De Domenico, I. & Ward, D. M. Chediak–Higashi syndrome. Curr. Opin. Hematol. 15, 22–29 (2008).
Song, E. et al. Chronic granulomatous disease: a review of the infectious and inflammatory complications. Clin. Mol. Allergy 9, 10 (2011).
Abraham, E. Neutrophils and acute lung injury. Crit. Care Med. 31, S195–S199 (2003).
Herrero-Cervera, A., Soehnlein, O. & Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 19, 177–191 (2022).
Acknowledgements
This work was supported by grants to A.M.H. from the NIH (nos. R01CA282465 and P50CA228944).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Paul Kubes, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Citrullination
-
A post-translational modification whereby the amino acid arginine is converted to a unique amino acid termed citrulline. In neutrophils, this process is catalysed by the enzyme peptidyl arginine deiminase-4 (PAD4).
- Cross-dressing
-
The ability of some antigen presenting cells to display an exogenous peptide/MHC complex originating from a neighbouring cell or extracellular vesicle.
- Engineered bioparticles
-
Heat-killed or chemically killed bacteria (such as S. aureus) that can be engineered to induce an inflammatory response.
- HLA-DR+ neutrophils
-
A subset of neutrophils that display the MHC class II cell surface marker, HLA-DR. This affords the ability of this neutrophil subset to present antigen in a similar fashion to macrophages and dendritic cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwak, J.W., Houghton, A.M. Targeting neutrophils for cancer therapy. Nat Rev Drug Discov 24, 666–684 (2025). https://doi.org/10.1038/s41573-025-01210-8
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41573-025-01210-8