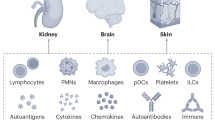
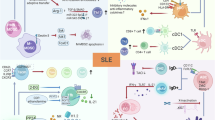
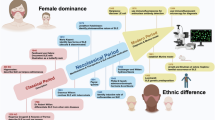
Abstract
Systemic lupus erythematous is a clinically and pathogenetically heterogeneous disease that has long challenged researchers and clinicians aiming to improve its treatment. Advances over the past 75 years have revealed a number of key immune mechanisms that drive clinical manifestations, paving the way for the development of therapies that go beyond broad immunosuppression to improve clinical efficacy and reduce side effects. These include approaches aimed at specific immune pathways, and emerging efforts to restore immune homeostasis, such as chimeric antigen receptor T cell therapies to eliminate pathogenic B cells, low-dose interleukin 2 or regulatory T cell therapies. Although hopes for durable remissions or cure are rising, major obstacles remain owing to the complex nature of the disease. In this Review, we discuss emerging therapeutic strategies designed to address these challenges.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 12, ITC81–ITC96 (2011).
Moe, S. R. et al. Persisting mortality gap in systemic lupus erythematosus; a population-based study on juvenile- and adult-onset SLE in Norway 1999–2022. Rheumatology https://doi.org/10.1093/rheumatology/kead519 (2023).
Utset, T. O. et al. Work disability, lost productivity and associated risk factors in patients diagnosed with systemic lupus erythematosus. Lupus Sci. Med. 2, e000058 (2015).
Tsokos, G. C. The immunology of systemic lupus erythematosus. Nat. Immunol. 25, 1332–1343 (2024). This is a comprehensive overview on recent advances on the pathogenesis of SLE immunopathology.
Morand, E. F. & Golder, V. Treat-to-target endpoint definitions in systemic lupus erythematosus: more is less? J. Rheumatol. 46, 1256–1258 (2019).
Stull, C., Sprow, G. & Werth, V. P. Cutaneous involvement in systemic lupus erythematous: a review for the rheumatologist. J. Rheumatol. https://doi.org/10.3899/jrheum.220089 (2022).
Ceccarelli, F. et al. Arthritis in systemic lupus erythematosus: from 2022 international GISEA/OEG symposium. J. Clin. Med. 11, 6016 (2022).
Cervera, R. et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European working party on systemic lupus erythematosus. Medicine 72, 113–124 (1993).
Bajema, I. M. et al. Revision of the international society of nephrology/renal pathology society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 93, 789–796 (2018).
Hanly, J. G. et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 69, 529–535 (2010).
Tsokos, G. C. et al. The immunoregulatory roles of non-haematopoietic cells in the kidney. Nat. Rev. Nephrol. 20, 206–217 (2024).
Bhargava, R. et al. Aberrantly glycosylated IgG elicits pathogenic signaling in podocytes and signifies lupus nephritis. JCI Insight 6, e147789 (2021).
Mohan, C., Zhang, T. & Putterman, C. Pathogenic cellular and molecular mediators in lupus nephritis. Nat. Rev. Nephrol. 19, 491–508 (2023).
Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725 (1997).
Aringer, M. et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 71, 1400–1412 (2019).
Kolios, A. G. A., Tsokos, G. C. & Klatzmann, D. Interleukin-2 and regulatory T cells in rheumatic diseases. Nat. Rev. Rheumatol. 17, 749–766 (2021). This is a comprehensive review on the use of IL-2 in autoimmune diseases.
Rajendiran, A. & Tenbrock, K. Regulatory T cell function in autoimmune disease. J. Transl. Autoimmun. 4, 100130 (2021).
Caielli, S. et al. An unconventional mechanism of IL-1β secretion that requires type I IFN in lupus monocytes (Cold Spring Harbor Laboratory, 2023).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016).
Scherlinger, M. et al. Selectins impair regulatory T cell function and contribute to systemic lupus erythematosus pathogenesis. Sci. Transl. Med. 13, eabi4994 (2021).
Scherlinger, M., Richez, C., Tsokos, G. C., Boilard, E. & Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 23, 495–510 (2023).
Duffau, P. et al. Platelet CD154 potentiates interferon-α secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2, 47ra63–47ra63 (2010).
Ulff-Møller, C. J., Simonsen, J., Kyvik, K. O., Jacobsen, S. & Frisch, M. Family history of systemic lupus erythematosus and risk of autoimmune disease: nationwide cohort study in Denmark 1977–2013. Rheumatology 56, 957–964 (2017).
Harley, I. T. W. & Sawalha, A. H. Systemic lupus erythematosus as a genetic disease. Clin. Immunol. 236, 108953 (2022).
Sisirak, V. et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166, 88–101 (2016).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022). This article reports the discovery of a new TLR7 gain-of-function mutation that causes monogenic lupus, along with the development of a lupus mouse model that recapitulates human lupus accurately.
Skopelja-Gardner, S. et al. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc. Natl Acad. Sci. USA 118, e2019097118 (2021). This report demonstrates that ultraviolet can activate peripheral neutrophils which migrate to SLE target organs to promote inflammation.
Harley, J. B. et al. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat. Genet. 50, 699–707 (2018).
Silverman, G. J., Azzouz, D. F., Gisch, N. & Amarnani, A. The gut microbiome in systemic lupus erythematosus: lessons from rheumatic fever. Nat. Rev. Rheumatol. 20, 143–157 (2024).
Arnaud, L. et al. Drug-induced systemic lupus: revisiting the ever-changing spectrum of the disease using the WHO pharmacovigilance database. Ann. Rheum. Dis. 78, 504–508 (2019).
Xing, M. et al. Air pollution, genetic susceptibility, and risk of incident systemic lupus erythematosus: a prospective cohort study. Arthritis Rheumatol. 76, 1530–1537 (2024). This paper demonstrates the impact of air pollution on SLE development along with the importance of genetic susceptibility: a host–environment interaction leading to the onset of SLE.
Souyris, M. et al. TLR7 escapes X chromosome inactivation in immune cells. Sci. Immunol. 3, eaap8855 (2018). This paper explains how immune cells from women fail to inactive X chromosome (which encodes for TLR7 and other immune molecules), and explains, at least in part, the female predominance in SLE.
Dou, D. R. et al. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell 187, 733–749.e716 (2024).
Al Sawah, S. et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus — the Hopkins lupus cohort. Lupus Sci. Med. 2, e000066 (2015).
Frodlund, M. et al. Glucocorticoid treatment in SLE is associated with infections, comorbidities and mortality — a national cohort study. Rheumatology 63, 1104–1112 (2024).
Fanouriakis, A. et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. https://doi.org/10.1136/ard-2023-224762 (2023). This article describe the most recent international recommendation on the treatment of SLE.
Askanase, A. D. et al. Disease-modifying therapies in systemic lupus erythematosus for extrarenal manifestations. Lupus Sci. Med. 11, e001124 (2024).
Alarcon, G. S. et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann. Rheum. Dis. 66, 1168–1172 (2007).
Crow, M. K. & Kirou, K. A. Hydroxychloroquine and lupus flare: a good drug, but we need to do better. Ann. Rheum. Dis. 81, 303–305 (2022).
Marmor, M. F. et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 123, 1386–1394 (2016).
Almeida-Brasil, C. C. et al. Retinal toxicity in a multinational inception cohort of patients with systemic lupus on hydroxychloroquine. Lupus Sci. Med. 9, e000789 (2022).
Sakthiswary, R. & Suresh, E. Methotrexate in systemic lupus erythematosus: a systematic review of its efficacy. Lupus 23, 225–235 (2014).
Houssiau, F. A. et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann. Rheum. Dis. 69, 2083–2089 (2010).
Gourley, M. F. et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann. Intern. Med. 125, 549–557 (1996).
Steinberg, A. D. et al. Cyclophosphamide in lupus nephritis: a controlled trial. Ann. Intern. Med. 75, 165–171 (1971).
Illei, G. G. et al. Renal flares are common in patients with severe proliferative lupus nephritis treated with pulse immunosuppressive therapy: long-term follow up of a cohort of 145 patients participating in randomized controlled studies. Arthritis Rheum. 46, 995–1002 (2002).
Hows, J. M. et al. Comparison of mesna with forced diuresis to prevent cyclophosphamide induced haemorrhagic cystitis in marrow transplantation: a prospective randomised study. Br. J. Cancer 50, 753–756 (1984).
Boumpas, D. T. et al. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann. Intern. Med. 119, 366–369 (1993).
Gupta, D., Zachariah, A., Roppelt, H., Patel, A. M. & Gruber, B. L. Prophylactic antibiotic usage for Pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus on cyclophosphamide: a survey of US rheumatologists and the review of literature. J. Clin. Rheumatol. 14, 267–272 (2008).
Ginzler, E. M. et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N. Engl. J. Med. 353, 2219–2228 (2005).
Appel, G. B. et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J. Am. Soc. Nephrol. 20, 1103–1112 (2009).
Terrier, B. et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French autoimmunity and rituximab registry. Arthritis Rheum. 62, 2458–2466 (2010).
Merrill, J. T. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 62, 222–233 (2010).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. 64, 1215–1226 (2012).
Kamburova, E. G. et al. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am. J. Transpl. 13, 1503–1511 (2013).
Vital, E. M. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 63, 3038–3047 (2011).
Bologna, L. et al. Mechanism of action of type II, glycoengineered, anti-CD20 monoclonal antibody GA101 in B-chronic lymphocytic leukemia whole blood assays in comparison with rituximab and alemtuzumab. J. Immunol. 186, 3762–3769 (2011).
Mysler, E. F. et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. 65, 2368–2379 (2013).
Furie, R. A. et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 81, 100–107 (2022).
Furie, R. A. et al. Efficacy and safety of obinutuzumab in active lupus nephritis. N. Engl. J. Med. https://doi.org/10.1056/nejmoa2410965 (2025). This article the positive outcomes from a phase III trial that demonstrated the efficacy of anti-CD20 obinutuzumab in lupus nephritis.
Szelinski, F. et al. Plasmablast-like phenotype among antigen-experienced CXCR5–CD19low B cells in systemic lupus erythematosus. Arthritis Rheumatol. 74, 1556–1568 (2022).
Clowse, M. E. B. et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase III randomized, double‐blind, placebo‐controlled trials. Arthritis Rheumatol. 69, 362–375 (2017).
Zhang, Y. et al. B cell-activating factor and its targeted therapy in autoimmune diseases. Cytokine Growth Factor. Rev. 64, 57–70 (2022).
Dörner, T. et al. Treatment of primary Sjögren’s syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann. Rheum. Dis. 78, 641–647 (2019).
Agmon-Levin, N. et al. OP0089 phase 2 safety and efficacy of subcutaneous (S.C.) dose ianalumab (VAY736; anti-BAFFR mAb) administered every 4 weeks up to 48 weeks in patients with systemic lupus erythematosus (SLE). Ann. Rheum. Dis. 83, 140–142 (2024).
Cohen, S. et al. A phase 1, randomized, double-blind, placebo-controlled, single- and multiple-dose escalation study to evaluate the safety and pharmacokinetics/pharmacodynamics of PF-06835375, a C-X-C chemokine receptor type 5 directed antibody, in patients with systemic lupus erythematosus or rheumatoid arthritis. Arthritis Res. Ther. 26, 117 (2024).
Ostendorf, L. et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N. Engl. J. Med. 383, 1149–1155 (2020).
McDonnell, S. R. P. et al. Mezagitamab in systemic lupus erythematosus: clinical and mechanistic findings of CD38 inhibition in an autoimmune disease. Lupus Sci. Med. 11, e001112 (2024).
Werth, V. P. et al. Trial of anti-BDCA2 antibody litifilimab for cutaneous lupus erythematosus. N. Engl. J. Med. 387, 321–331 (2022).
Furie, R. A. et al. Trial of anti-BDCA2 antibody litifilimab for systemic lupus erythematosus. N. Engl. J. Med. 387, 894–904 (2022).
Robles-Carrillo, L. et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J. Immunol. 185, 1577–1583 (2010).
Robert, M. & Scherlinger, M. Platelets are a major player and represent a therapeutic opportunity in systemic lupus erythematosus. Jt. Bone Spine 91, 105622 (2024).
St. Clair, E. W. et al. CD40 ligand antagonist dazodalibep in Sjögren’s disease: a randomized, double-blinded, placebo-controlled, phase 2 trial. Nat. Med. 30, 1583–1592 (2024).
Tocoian, A. et al. First-in-human trial of the safety, pharmacokinetics and immunogenicity of a PEGylated anti-CD40L antibody fragment (CDP7657) in healthy individuals and patients with systemic lupus erythematosus. Lupus 24, 1045–1056 (2015).
Furie, R. A. et al. Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus. Rheumatology 60, 5397–5407 (2021).
Biogen. (2024).
Jayne, D. et al. Pos0687 a randomised dose ranging, placebo-controlled, phase II study assessing the efficacy and safety of Bi 655064, an antagonistic anti-Cd40 antibody, in patients with lupus nephritis. Ann. Rheum. Dis. 80, 589–590 (2021).
The efficacy and safety of abatacept in patients with non–life‐threatening manifestations of systemic lupus erythematosus: results of a twelve‐month, multicenter, exploratory, phase IIb, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheumatol. https://doi.org/10.1002/art.27601 (2010).
Wilhelm, M. et al. Lupus regulator peptide P140 represses B cell differentiation by reducing HLA class II molecule overexpression. Arthritis Rheumatol. 70, 1077–1088 (2018).
Zimmer, R., Scherbarth, H. R., Rillo, O. L., Gomez-Reino, J. J. & Muller, S. Lupuzor/P140 peptide in patients with systemic lupus erythematosus: a randomised, double-blind, placebo-controlled phase IIb clinical trial. Ann. Rheum. Dis. 72, 1830–1835 (2013).
Felten, R. et al. Efficacy of BAFF inhibition and B-cell depletion in non-obese diabetic mice as a spontaneous model for Sjögren’s disease. RMD Open 10, e004112 (2024).
Carter, L. M., Isenberg, D. A. & Ehrenstein, M. R. Elevated serum BAFF levels are associated with rising anti–double‐stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. 65, 2672–2679 (2013).
Manzi, S. et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann. Rheum. Dis. 71, 1833–1838 (2012).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Furie, R. et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N. Engl. J. Med. 383, 1117–1128 (2020). This article reports the results from the phase III trial that led to the approval of belimumab in lupus nephritis.
Mian, A. et al. Suppl 1 edn 1454–1455 (BMJ Publishing Group Ltd and European League Against Rheumatism).
Urowitz, M. B. et al. Organ damage in patients treated with belimumab versus standard of care: a propensity score-matched comparative analysis. Ann. Rheum. Dis. 78, 372–379 (2019).
Aranow, C. et al. Efficacy and safety of sequential therapy with subcutaneous belimumab and one cycle of rituximab in patients with systemic lupus erythematosus: the phase 3, randomised, placebo-controlled BLISS-BELIEVE study. Ann. Rheum. Dis. 83, 1502–1512 (2024). This article reports the negative outcome in the phase III trial assessing the use of the combination therapy rituximab plus belimumab in SLE.
Petri, M. et al. Belimumab use during pregnancy: a summary of birth defects and pregnancy loss from belimumab clinical trials, a pregnancy registry and postmarketing reports. Ann. Rheum. Dis. 82, 217–225 (2023).
Wu, D. et al. Telitacicept in patients with active systemic lupus erythematosus: results of a phase 2b, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 83, 475–487 (2024).
Rodríguez-Carrio, J. et al. Association between type I interferon pathway activation and clinical outcomes in rheumatic and musculoskeletal diseases: a systematic literature review informing EULAR points to consider. RMD Open 9, e002864 (2023).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Rodríguez-Carrio, J. et al. 2022 EULAR points to consider for the measurement, reporting and application of IFN-I pathway activation assays in clinical research and practice. Ann. Rheum. Dis. 82, 754–762 (2023).
Kalunian, K. C. et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 75, 196–202 (2016).
Khamashta, M. et al. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 75, 1909–1916 (2016).
Furie, R. A. et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. 1, e208–e219 (2019). This paper reports the negative results from the phase III trial assessing anifrolumab in non-renal SLE, with many secondary end points favouring anifrolumab.
Morand, E. F. et al. Trial of anifrolumab in active systemic lupus erythematosus. N. Engl. J. Med. 382, 211–221 (2020). This report describe the results from the phase III trial assessing anifrolumab in non-renal SLE, which led to its approval.
Bruce, I. N. et al. Concordance and discordance in SLE clinical trial outcome measures: analysis of three anifrolumab phase 2/3 trials. Ann. Rheum. Dis. 81, 962–969 (2022).
Morand, E. F., Abreu, G., Furie, R. A., Golder, V. & Tummala, R. Lupus low disease activity state attainment in the phase 3 TULIP trials of anifrolumab in active systemic lupus erythematosus. Ann. Rheum. Dis. 82, 639–645 (2023).
Morand, E. F. et al. Efficacy of anifrolumab across organ domains in patients with moderate-to-severe systemic lupus erythematosus: a post-hoc analysis of pooled data from the TULIP-1 and TULIP-2 trials. Lancet Rheumatol. 4, e282–e292 (2022).
Trefond, L. et al. Efficacy of valaciclovir in preventing herpes zoster in patients receiving anifrolumab. RMD Open. 11, e005076 (2025).
Moulton, V. R. & Tsokos, G. C. Abnormalities of T cell signaling in systemic lupus erythematosus. Arthritis Res. Ther. 13, 207 (2011).
Li, H., Boulougoura, A., Endo, Y. & Tsokos, G. C. Abnormalities of T cells in systemic lupus erythematosus: new insights in pathogenesis and therapeutic strategies. J. Autoimmun. 132, 102870 (2022).
Van Vollenhoven, R. F. et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392, 1330–1339 (2018).
Van Vollenhoven, R. F. et al. Phase 3, multicentre, randomised, placebo-controlled study evaluating the efficacy and safety of ustekinumab in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 81, 1556–1563 (2022).
Wallace, D. J. et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann. Rheum. Dis. 76, 534–542 (2017).
Lin, C. M., Cooles, F. A. & Isaacs, J. D. Basic mechanisms of JAK inhibition. Mediterr. J. Rheumatol. 31, 100–104 (2020).
Kawasaki, M. et al. Possible role of the JAK/STAT pathways in the regulation of T cell–interferon related genes in systemic lupus erythematosus. Lupus 20, 1231–1239 (2011).
de la Varga Martinez, R. et al. Clinical relevance of circulating anti-ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur. J. Immunol. 47, 1211–1219 (2017).
Remmers, E. F. et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 357, 977–986 (2007).
Hasni, S. A. et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat. Commun. 12, 3391 (2021).
Wallace, D. J. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 392, 222–231 (2018).
Dorner, T. et al. Baricitinib decreases anti-dsDNA in patients with systemic lupus erythematosus: results from a phase II double-blind, randomized, placebo-controlled trial. Arthritis Res. Ther. 24, 112 (2022).
Dorner, T. et al. Mechanism of action of baricitinib and identification of biomarkers and key immune pathways in patients with active systemic lupus erythematosus. Ann. Rheum. Dis. 81, 1267–1272 (2022).
Morand, E. F. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-I). Lancet 401, 1001–1010 (2023).
Petri, M. et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet 401, 1011–1019 (2023).
Baker, M. et al. Phase II, randomised, double-blind, multicentre study evaluating the safety and efficacy of filgotinib and lanraplenib in patients with lupus membranous nephropathy. RMD Open 6, e001490 (2020).
Merrill, J. T. et al. Efficacy and safety of upadacitinib or elsubrutinib alone or in combination for patients with systemic lupus erythematosus: a phase 2 randomized controlled trial. Arthritis Rheumatol. https://doi.org/10.1002/art.42926 (2024). This article describes the phase II trial of upadacitinib in non-renal SLE, which supported an ongoing phase III trial.
Ytterberg, S. R. et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 386, 316–326 (2022).
Morand, E. et al. Deucravacitinib, a tyrosine kinase 2 inhibitor, in systemic lupus erythematosus: a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 75, 242–252 (2023).
Mammoliti, O. et al. Discovery of GLPG3667, a selective ATP competitive tyrosine kinase 2 inhibitor for the treatment of autoimmune diseases. J. Med. Chem. 67, 8545–8568 (2024).
Haselmayer, P. et al. Efficacy and pharmacodynamic modeling of the BTK inhibitor evobrutinib in autoimmune disease models. J. Immunol. 202, 2888–2906 (2019).
Goess, C. et al. ABBV-105, a selective and irreversible inhibitor of Bruton’s tyrosine kinase, is efficacious in multiple preclinical models of inflammation. Mod. Rheumatol. 29, 510–522 (2019).
Wallace, D. J. et al. Efficacy and safety of the Bruton’s tyrosine kinase inhibitor evobrutinib in systemic lupus erythematosus: results of a phase II, randomized, double-blind, placebo-controlled dose-ranging trial. ACR Open. Rheumatol. 5, 38–48 (2023).
Isenberg, D. et al. Efficacy, safety, and pharmacodynamic effects of the Bruton’s tyrosine kinase inhibitor fenebrutinib (GDC-0853) in systemic lupus erythematosus: results of a phase II, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 73, 1835–1846 (2021).
Li, R. et al. LB0005 orelabrutinib, an irreversible inhibitor of bruton’s tyrosine kinase (BTK), for the treatment of systemic lupus erythematosus (SLE): results of a randomized, double-blind, placebo-controlled, phase Ib/IIa dose-finding study. Ann. Rheum. Dis. 81, 210.212–210 (2022).
He, J., Ma, J., Ren, B. & Liu, A. Advances in systemic lupus erythematosus pathogenesis via mTOR signaling pathway. Semin. Arthritis Rheum. 50, 314–320 (2020).
Ji, L., Xie, W. & Zhang, Z. Efficacy and safety of sirolimus in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Semin. Arthritis Rheum. 50, 1073–1080 (2020).
Peng, L. et al. Clinical efficacy and safety of sirolimus in systemic lupus erythematosus: a real-world study and meta-analysis. Ther. Adv. Musculoskelet. Dis. 12, 1759720X20953336 (2020).
Shi, G. et al. mTOR inhibitor INK128 attenuates systemic lupus erythematosus by regulating inflammation-induced CD11b+Gr1+ cells. Biochim. Biophys. Acta Mol. Basis Dis. 1865, 1–13 (2019).
Rafael-Vidal, C. et al. Calcineurin and systemic lupus erythematosus: the rationale for using calcineurin inhibitors in the treatment of lupus nephritis. Int. J. Mol. Sci. 22, 1263 (2021).
Moroni, G. et al. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin. J. Am. Soc. Nephrol. 1, 925–932 (2006).
Yang, T. H. et al. Cyclosporine for the treatment of lupus nephritis in patients with systemic lupus erythematosus. Clin. Nephrol. 89, 277–285 (2018).
Rihova, Z. et al. Treatment of lupus nephritis with cyclosporine — an outcome analysis. Kidney Blood Press. Res. 30, 124–128 (2007).
Mok, C. C., To, C. H., Yu, K. L. & Ho, L. Y. Combined low-dose mycophenolate mofetil and tacrolimus for lupus nephritis with suboptimal response to standard therapy: a 12-month prospective study. Lupus 22, 1135–1141 (2013).
Chen, W. et al. Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: a multicenter randomized clinical trial. Am. J. Kidney Dis. 57, 235–244 (2011).
Zheng, Z. et al. Effect of tacrolimus vs intravenous cyclophosphamide on complete or partial response in patients with lupus nephritis: a randomized clinical trial. JAMA Netw. Open 5, e224492 (2022).
Kamanamool, N. et al. Comparison of disease activity between tacrolimus and mycophenolate mofetil in lupus nephritis: a randomized controlled trial. Lupus 27, 647–656 (2018).
Mok, C. C. et al. Long-term outcome of a randomised controlled trial comparing tacrolimus with mycophenolate mofetil as induction therapy for active lupus nephritis. Ann. Rheum. Dis. 79, 1070–1076 (2020).
Bao, H. et al. Successful treatment of class V + IV lupus nephritis with multitarget therapy. J. Am. Soc. Nephrol. 19, 2001–2010 (2008).
Liu, Z. et al. Multitarget therapy for induction treatment of lupus nephritis: a randomized trial. Ann. Intern. Med. 162, 18–26 (2015).
Szeto, C. C. et al. Tacrolimus for the treatment of systemic lupus erythematosus with pure class V nephritis. Rheumatology 47, 1678–1681 (2008).
Takeuchi, T., Wakasugi, N., Uno, S. & Makino, H. Long-term safety and effectiveness of tacrolimus in patients with lupus nephritis: 5-year interim postmarketing surveillance study in Japan (TRUST). J. Rheumatol. 48, 74–81 (2021).
Suzuki, K., Uno, S. & Wakasugi, N. Tacrolimus use and renal function in pregnancy with lupus nephritis: analysis of post-marketing surveillance data in Japan. Mod. Rheumatol. 33, 944–952 (2023).
Rovin, B. H. et al. A randomized, controlled double-blind study comparing the efficacy and safety of dose-ranging voclosporin with placebo in achieving remission in patients with active lupus nephritis. Kidney Int. 95, 219–231 (2019).
Rovin, B. H. et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 397, 2070–2080 (2021).
S, P. Arthritis Rheumatol. 75 (2023).
Arriens, C. et al. Update on the efficacy and safety profile of voclosporin: an integrated analysis of clinical trials in lupus nephritis. Arthritis Care Res. 75, 1399–1408 (2023).
Saxena, A. et al. Safety and efficacy of long-term voclosporin treatment for lupus nephritis in the phase 3 AURORA 2 clinical trial. Arthritis Rheumatol. 76, 59–67 (2024).
Fuchs, A. et al. Minimum information about T regulatory cells: a step toward reproducibility and standardization. Front. Immunol. 8, 1844 (2017).
Miyara, M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009).
Yuan, Y. et al. Therapeutic potential of interleukin-2 in autoimmune diseases. Trends Mol. Med. 28, 596–612 (2022).
von Spee-Mayer, C. et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 75, 1407–1415 (2016).
He, J. et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 22, 991–993 (2016).
Humrich, J. Y. et al. Low-dose interleukin-2 therapy in refractory systemic lupus erythematosus: an investigator-initiated, single-centre phase 1 and 2a clinical trial. Lancet Rheumatol. 1, e44–e54 (2019).
He, J. et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 79, 141–149 (2020).
Raeber, M. E. et al. Interleukin-2 immunotherapy reveals human regulatory T cell subsets with distinct functional and tissue-homing characteristics. Immunity 57, 2232–2250 e2210 (2024).
Lorenzon, R. et al. The universal effects of low-dose interleukin-2 across 13 autoimmune diseases in a basket clinical trial. J. Autoimmun. 144, 103172 (2024).
Rosenzwajg, M. et al. Immunological and clinical effects of low-dose interleukin-2 across 11 autoimmune diseases in a single, open clinical trial. Ann. Rheum. Dis. 78, 209–217 (2019).
Humrich, J. Y. et al. Low-dose interleukin-2 therapy in active systemic lupus erythematosus (LUPIL-2): a multicentre, double-blind, randomised and placebo-controlled phase II trial. Ann. Rheum. Dis. 81, 1685–1694 (2022).
Shao, M. et al. Interleukin-2 deficiency associated with renal impairment in systemic lupus erythematosus. J. Interferon Cytokine Res. 39, 117–124 (2019).
Zhao, C. et al. Low dose of IL-2 combined with rapamycin restores and maintains the long-term balance of Th17/Treg cells in refractory SLE patients. BMC Immunol. 20, 32 (2019).
Rosenzwajg, M. et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J. Autoimmun. 58, 48–58 (2015).
Konrad, M. W. et al. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 50, 2009–2017 (1990).
Klatzmann, D. & Abbas, A. K. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat. Rev. Immunol. 15, 283–294 (2015).
Wu, J. et al. A PD-1-targeted, receptor-masked IL-2 immunocytokine that engages IL-2Rα strengthens T cell-mediated anti-tumor therapies. Cell Rep. Med. 5, 101747 (2024).
Klein, C. et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology 6, e1277306 (2017).
van Brummelen, E. M. J. et al. (89)Zr-labeled CEA-targeted IL-2 variant immunocytokine in patients with solid tumors: CEA-mediated tumor accumulation and role of IL-2 receptor-binding. Oncotarget 9, 24737–24749 (2018).
Weide, B. et al. A phase II study of the L19IL2 immunocytokine in combination with dacarbazine in advanced metastatic melanoma patients. Cancer Immunol. Immunother. 68, 1547–1559 (2019).
Neri, D. & Sondel, P. M. Immunocytokines for cancer treatment: past, present and future. Curr. Opin. Immunol. 40, 96–102 (2016).
Horwitz, D. A., Bickerton, S., Koss, M., Fahmy, T. M. & La Cava, A. Suppression of murine lupus by CD4+ and CD8+ Treg cells induced by T cell-targeted nanoparticles loaded with interleukin-2 and transforming growth factor beta. Arthritis Rheumatol. 71, 632–640 (2019).
Sarkar N, H. X. et al. Regulatory T cell defects in SLE and therapy with a novel IL-2 mutein: phase 1 clinical results with efavaleukin alfa. Arthritis Rheumatol. 74, abstr. 0989 (2022).
Dong, S. et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 6, e147474 (2021).
Zhang, S.-X. et al. The efficacy and safety of short-term and low-dose IL-2 combined with tocilizumab to treat rheumatoid arthritis. Front. Immunol. 15, 1359041 (2024).
Wang, J. et al. Low-dose IL-2 improved clinical symptoms by restoring reduced regulatory T cells in patients with refractory rheumatoid arthritis: a randomized controlled trial. Front. Immunol. 13, 947341 (2022).
Geetha, D. et al. Efficacy and safety of avacopan in patients with ANCA-associated vasculitis receiving rituximab in a randomised trial. Ann. Rheum. Dis. 83, 223–232 (2024).
Jayne, D. R. W., Merkel, P. A., Schall, T. J. & Bekker, P. Avacopan for the treatment of ANCA-associated vasculitis. N. Engl. J. Med. 384, 599–609 (2021).
McKeage, K. Ravulizumab: first global approval. Drugs 79, 347–352 (2019).
Scherlinger, M. et al. CAR T-cell therapy in autoimmune diseases: where are we and where are we going? Lancet Rheumatol. https://doi.org/10.1016/s2665-9913(24)00377-1 (2025). This is a comprehensive review on the current and future strategies on CAR-T cell therapies in autoimmune diseases including SLE.
Schett, G., Mackensen, A. & Mougiakakos, D. CAR T-cell therapy in autoimmune diseases. Lancet https://doi.org/10.1016/S0140-6736(23)01126-1 (2023).
Müller, F. et al. CD19 CAR T-cell therapy in autoimmune disease — a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024). This article presents a clinical report from the first 8 SLE patients treated with anti-CD19 CAR-T cells in Erlangen.
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28, 2124–2132 (2022).
Tian, D.-S. et al. B cell lineage reconstitution underlies CAR-T cell therapeutic efficacy in patients with refractory myasthenia gravis. EMBO Mol. Med. 16, 966–987 (2024).
Wang, W. et al. BCMA-CD19 compound CAR T cells for systemic lupus erythematosus: a phase 1 open-label clinical trial. Ann. Rheum. Dis. https://doi.org/10.1136/ard-2024-225785 (2024). This artocle presents the data from the phase I trial showing the promissing clinical and biological impact of dual CD19/BCMA targeting in SLE using CAR-T cells.
Ma, Y. et al. OP0017 BCMA CD19 compound chimeric antigen receptor T cells (cCAR) provides complete humoral reset, eliminates all elevated autoantibodies, improves symptoms and renal function in lupus nephritis (LN) patients. Ann. Rheum. Dis. 83, 136–136 (2024).
Wang, X. et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell https://doi.org/10.1016/j.cell.2024.06.027 (2024).
Ledford, H. Cancer-fighting CAR T cells could be made inside body with viral injection. Nature 625, 225–226 (2024).
Harrison, S. J. et al. CAR+ T-cell lymphoma after cilta-cel therapy for relapsed or refractory myeloma. N. Engl. J. Med. 392, 677–685 (2025).
Perica, K. et al. CD4+ T-cell lymphoma harboring a chimeric antigen receptor integration in TP53. N. Engl. J. Med. 392, 577–TP583 (2025).
Dall’Era, M. et al. Adoptive Treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol. 71, 431–440 (2019).
Eggenhuizen, P. J. et al. Smith-specific regulatory T cells halt the progression of lupus nephritis. Nat. Commun. 15, 899 (2024).
Doglio, M. et al. Regulatory T cells expressing CD19-targeted chimeric antigen receptor restore homeostasis in systemic lupus erythematosus. Nat. Commun. 15, 2542 (2024).
Zhou, T., Li, H.-Y., Liao, C., Lin, W. & Lin, S. Clinical efficacy and safety of mesenchymal stem cells for systemic lupus erythematosus. Stem Cell Int. 2020, 1–11 (2020).
Houssiau, F. A. et al. IFN-α kinoid in systemic lupus erythematosus: results from a phase IIb, randomised, placebo-controlled study. Ann. Rheum. Dis. 79, 347–355 (2020).
Brilland, B. et al. Platelets and IgE: shaping the innate immune response in systemic lupus erythematosus. Clin. Rev. Allergy Immunol. https://doi.org/10.1007/s12016-019-08744-x (2019).
Hasni, S. et al. Safety and tolerability of omalizumab: a randomized clinical trial of humanized anti‐IgE monoclonal antibody in systemic lupus erythematosus. Arthritis Rheumatol. 71, 1135–1140 (2019).
Burge, D. J., Werth, V. P., Boackle, S. A. & Posada, J. Evaluation of RNase therapy in systemic lupus erythematosus: a randomised phase 2a clinical trial of RSLV-132. Lupus Sci. Med. 11, e001113 (2024).
Merrill, J. T. et al. Phase 2 trial of iberdomide in systemic lupus erythematosus. N. Engl. J. Med. 386, 1034–1045 (2022).
Field-Smith, A., Morgan, G. J. & Davies, F. E. Bortezomib (Velcade?) in the treatment of multiple myeloma. Ther. Clin. Risk Manag. 2, 271–279 (2006).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74, 1474–1478 (2015).
Baribaud, F. et al. (BMJ Publishing Group Ltd and European League Against Rheumatism).
Morand, E. et al. LB0004 randomised, placebo-controlled phase II study of oral enpatoran, a first-in-class Toll-like receptor 7/8 inhibitor, in systemic lupus erythematosus. Ann. Rheum. Dis. 84, 316–317 (2025).
Vial, G. et al. The impact of clopidogrel on plasma-soluble CD40 ligand levels in systemic lupus erythematosus patients: the CLOPUS phase I/II pilot study. Jt. Bone Spine 88, 105097 (2021).
Ataga, K. I. et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N. Engl. J. Med. 376, 429–439 (2017).
Mayer, C. L. et al. A phase 1 study in healthy participants to characterize the safety and pharmacology of inclacumab, a fully human anti-P-selectin antibody, in development for treatment of sickle cell disease. Eur. J. Clin. Pharmacol. 79, 1219–1228 (2023).
Scherlinger, M. et al. Phosphofructokinase P fine-tunes T regulatory cell metabolism, function, and stability in systemic autoimmunity. Sci. Adv. 8, eadc9657 (2022).
Sun, F. et al. Effects of metformin on disease flares in patients with systemic lupus erythematosus: post hoc analyses from two randomised trials. Lupus Sci. Med. 7, e000429 (2020).
Sun, F. et al. Safety and efficacy of metformin in systemic lupus erythematosus: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Rheumatol. 2, e210–e216 (2020).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Yen, F.-S., Wang, S.-I., Hsu, C.-C., Hwu, C.-M. & Wei, J. C.-C. Sodium-glucose cotransporter-2 inhibitors and nephritis among patients with systemic lupus erythematosus. JAMA Netw. Open. 7, e2416578 (2024).
Jorge, A et al. Glucagon-like peptide-1 receptor agonist use and the risk of adverse cardiac and kidney outcomes among patients with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol. 76, abstr. 2576 (2024).
Scherlinger, M. & Tsokos, G. C. Reactive oxygen species: the Yin and Yang in (auto-)immunity. Autoimmun. Rev. 20, 102869 (2021).
Lai, Z.-W. et al. N-Acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 64, 2937–2946 (2012).
Alexander, T., Krönke, J., Cheng, Q., Keller, U. & Krönke, G. Teclistamab-induced remission in refractory systemic lupus erythematosus. N. Engl. J. Med. 391, 864–866 (2024). This is the first article to report the use of an anti-BCMA bsAb in SLE with good efficacy.
Hagen, M. et al. BCMA-targeted T-cell-engager therapy for autoimmune disease. N. Engl. J. Med. 391, 867–869 (2024).
Budde, L. E. et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 23, 1055–1065 (2022).
Subklewe, M. et al. Application of blinatumomab, a bispecific anti-CD3/CD19 T-cell engager, in treating severe systemic sclerosis: a case study. Eur. J. Cancer 204, 114071 (2024).
Bucci, L. et al. Bispecific T cell engager therapy for refractory rheumatoid arthritis. Nat. Med. https://doi.org/10.1038/s41591-024-02964-1 (2024).
Scherlinger, M. et al. Rituximab in moderate to severe non-renal systemic lupus erythematosus: a reanalysis of the EXPLORER study. Ann. Rheum. Dis. https://doi.org/10.1136/annrheumdis-2018-214833 (2019).
Rossi, E. A., Chang, C.-H. & Goldenberg, D. M. Anti-CD22/CD20 bispecific antibody with enhanced trogocytosis for treatment of lupus. PLoS ONE 9, e98315 (2014).
Abuqayyas, L. et al. Safety and biological activity of rozibafusp alfa, a bispecific inhibitor of inducible costimulator ligand and B cell activating factor, in patients with rheumatoid arthritis: results of a phase 1b, randomized, double‐blind, placebo‐controlled, multiple ascending dose study. ACR Open Rheumatol. 4, 903–911 (2022).
Merrill, J. T. et al. Obexelimab in systemic lupus erythematosus with exploration of response based on gene pathway co-expression patterns: a double-blind, randomized, placebo-controlled, phase 2 trial. Arthritis Rheumatol. 75, 2185–2194 (2023).
Scherlinger, M. et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun. Rev. 19, 102531 (2020).
Kandane-Rathnayake, R. et al. Lupus low disease activity state and remission and risk of mortality in patients with systemic lupus erythematosus: a prospective, multinational, longitudinal cohort study. Lancet Rheumatol. 4, e822–e830 (2022).
Mathian, A, et al. Withdrawal of low-dose prednisone in SLE patients with a clinically quiescent disease for more than 1 year: a randomised clinical trial. Ann. Rheum. Dis. 79, 339–346 (2020).
Carter, L. M. et al. Gene expression and autoantibody analysis revealing distinct ancestry‐specific profiles associated with response to rituximab in refractory systemic lupus erythematosus. Arthritis Rheumatol. 75, 697–710 (2023).
van Vollenhoven, R. F. et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann. Rheum. Dis. 73, 958–967 (2014).
Bombardier, C., Gladman, D. D., Urowitz, M. B., Caron, D. & Chang, C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 35, 630–640 (1992).
Isenberg, D. A. et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group’s disease activity index for patients with systemic lupus erythematosus. Rheumatology 44, 902–906 (2005).
Franklyn, K. et al. Definition and initial validation of a lupus low disease activity state (LLDAS). Ann. Rheum. Dis. 75, 1615–1621 (2016).
van Vollenhoven, R. F. et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force. Lupus Sci. Med. 8, e000538 (2021).
Mahmoud, K. et al. Responsiveness of clinical and ultrasound outcome measures in musculoskeletal systemic lupus erythematosus. Rheumatology 58, 1353–1360 (2019).
Klein, R. et al. Development of the CLASI as a tool to measure disease severity and responsiveness to therapy in cutaneous lupus erythematosus. Arch. Dermatol. 147, 203–208 (2011).
Mok, C. C., Teng, Y. K. O., Saxena, R. & Tanaka, Y. Treatment of lupus nephritis: consensus, evidence and perspectives. Nat. Rev. Rheumatol. 19, 227–238 (2023).
Renaudineau Y. et al. Urinary soluble CD163 is useful as “liquid biopsy” marker in lupus nephritis at both diagnosis and follow-up to predict impending flares. J. Transl. Autoimmun 20, 100244 (2024).
Wobma H, R. X. et al. Human chimeric antigen receptor (CAR)-Tregs targeting OX40L for treatment of systemic lupus erythematosus. Arthritis Rheumatol. 75, abstr. 0799 (2023).
Bittner, S., Hehlgans, T. & Feuerer, M. Engineered Treg cells as putative therapeutics against inflammatory diseases and beyond. Trends Immunol. 44, 468–483 (2023).
Bittner, S. et al. Biosensors for inflammation as a strategy to engineer regulatory T cells for cell therapy. Proc. Natl Acad. Sci. USA 119, e2208436119 (2022).
Hippen, K. L. et al. Massive ex vivo expansion of human natural regulatory T cells (T regs) with minimal loss of in vivo functional activity. Sci. Transl. Med. 3, 83ra41–83ra41 (2011).
Battaglia, M. et al. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 177, 8338–8347 (2006).
Acknowledgements
M.S. is supported by the Bettencourt-Schueller Foundation, the INSERM (ATIP-Avenir), Ecole de l’INSERM Bettencourt-Schueller, the FOREUM foundation and the Arthritis Pierre Coubertin foundation. Work in the Tsokos Lab was supported by grants from NIAID, NIH.
Author information
Authors and Affiliations
Contributions
G.C.T., M.S., A.G.K. and V.C.K. researched data for the article and provided substantial contribution to discussion of the content. All authors contributed to writing the article and reviewed and edited it before submission.
Corresponding authors
Ethics declarations
Competing interests
M.S. is a consultant for Abbvie, Amgen, AstraZeneca, Biogen, BMS, Fresenius, Galapagos, GSK, Innate Pharma, Nordic Pharma, Novartis, Roche, Sandoz. G.C.T. is on the scientific advisory boards of CUGENE, A2 Biotherapeutics, Biolojic and CorEvitas. The other authors declare no additional competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Betty Diamond and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- British Islands Lupus Assessment Group-based Combined Lupus Assessment (BICLA)
-
A composite SLE trial end point relying on the absence on new severe BILAG organ involvement and improvement of disease base on the physician evaluation.
- Cytapheresis
-
A procedure similar to haemodialysis during which circulating cells are collected from a patient often to use them as a therapeutic compound.
- Cytokine release syndrome (CRS)
-
A condition during which a large amount of inflammatory cytokines are released, often following T cell activation. Its severity ranges from isolated fever to hypotension and multi-organ failure.
- Definition of remission in SLE (DORIS)
-
A disease state characterized by the absence of clinical symptoms and a daily prednisone dose of ≤5 mg. Sustained DORIS remission is associated with improved outcomes and is now a key end point in clinical trials.
- IgG4-related disease
-
An inflammatory and fibrotic disorder characterized by a non-clonal expansion of IgG4 + B cells in various tissues.
- Lupus low disease activity state (LLDAS)
-
A disease state characterized by minimal clinical activity and a daily prednisone dose of ≤7.5 mg. It is less stringent than DORIS remission, associated with better SLE outcome, and an important end point in trials.
- Lymphodepletion
-
A cytotoxic chemotherapy (cyclophosphamide ± fludarabine), which allows a better cellular graft expansion and efficacy.
- Molecular mimicry
-
The structural proximity between an exogenous antigen (for instance, viral) and an autoantigen, which may result in the induction of autoimmunity.
- NETosis
-
Immunogenic programmed neutrophil death during which neutrophils release a net of DNA, histones and other molecules in the extracellular milieu to trap pathogens and elicit an immune response.
- Organic brain syndrome
-
A general term referring to the impairment of brain function.
- Self-tolerance
-
Mechanisms underlying the prevention of immune system activation against oneself.
- Sjögren disease
-
A systemic autoimmune disease characterized by salivary glands immune infiltration along with B cell activation, which may lead to complications such as cryoglobulinemia and lymphoma.
- SLE disease activity index (SLEDAI-2K)
-
SLE disease activity index 2000: an imperfect but easy tool to measure SLE disease activity in clinical settings and in trials.
- SLE responder index 4 (SRI-4)
-
A composite clinical end point that encompass a decrease ≥4 points in the SLE disease activity (SLEDAI) index, the absence of new severe organ involvement (BILAG score A/B) and no disease worsening in the physician global assessment.
- Type I interferon (IFN-I) signature
-
A transcriptomic signature of circulating cell exposed to IFN-I characterized by the upregulation of IFN-stimulated genes. The IFN-I signature is used in the clinics because its measurement is easier and cheaper than IFN-I level evaluation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scherlinger, M., Kolios, A.G.A., Kyttaris, V.C. et al. Advances in the treatment of systemic lupus erythematosus. Nat Rev Drug Discov (2025). https://doi.org/10.1038/s41573-025-01242-0
Accepted:
Published:
DOI: https://doi.org/10.1038/s41573-025-01242-0