Abstract
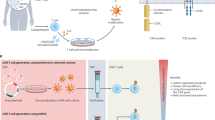
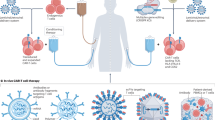
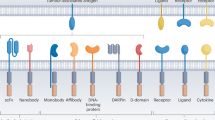
Chimeric antigen receptor (CAR)-T cell therapy has transformed the outcomes of patients with haematological malignancies, yet its use is limited by labour-intensive manufacturing, constrained production capacity and variable clinical performance. In vivo CAR-T cell engineering, in which CAR-T cells are generated directly inside the patient’s body, seeks to overcome these challenges by eliminating the need for ex vivo cell processing and complex logistics, as well as improve clinical performance. Recent advances in virology, RNA medicines and nanotechnology have catalysed a radical overhaul of this approach, which uses targeted delivery systems such as lentiviral vectors and lipid nanoparticles to introduce CAR-encoding genetic material into endogenous T cells. Early clinical studies have shown efficient transduction, sustained CAR expression and initial signs of antitumour activity, establishing proof of concept. This Review explores the underlying technologies — including RNA delivered by lipid nanoparticles and engineered viral vectors — and discusses how they are being adapted to develop more broadly applicable, scalable, safe and effective CAR-T cell therapies. By removing the need for ex vivo manipulation and chemotherapeutic conditioning, this strategy could enable the wider application of CAR-T cell therapies not just to blood cancers but to autoimmune diseases for which ex vivo CAR-T cell therapies have shown strong promise, such as systemic lupus erythematosus.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Leavy, O. The birth of monoclonal antibodies. Nat. Immunol. 17, 13 (2016).
Dumontet, C., Reichert, J. M., Senter, P. D., Lambert, J. M. & Beck, A. Antibody–drug conjugates come of age in oncology. Nat. Rev. Drug. Discov. 22, 641–661 (2023).
Rosenberg, S. A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192, 5451–5458 (2014).
Baeuerle, P. A. & Reinhardt, C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 69, 4941–4944 (2009).
Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468 (1990).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug. Discov. 17, 261–279 (2018).
June, C. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Hinrichs, C. & Rosenberg, S. A. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 257, 56–71 (2014).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
US Food and Drug Administration. KYMRIAH. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah (2017). This label corresponds to the first CAR-T cell product approved.
US Food and Drug Administration. YESCARTA. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta (2017).
US Food and Drug Administration. TECARTUS. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus (2020).
US Food and Drug Administration. BREYANZI. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/breyanzi-lisocabtagene-maraleucel (2021).
US Food and Drug Administration. ABECMA. FDA https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel (2021).
US Food and Drug Administration. CARVYKTI. FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/carvykti (2022).
US Food and Drug Administration. AUCATZYL. FDA https://www.fda.gov/vaccines-blood-biologics/aucatzyl (2024).
Joy, R., Phair, K., O’Hara, R. & Brady, D. Recent advances and current challenges in CAR-T cell therapy. Biotechnol. Lett. 46, 115–126 (2024).
Lonez, C. & Breman, E. Allogeneic CAR-T therapy technologies: has the promise been met? Cells 13, 146 (2024).
Wang, X. et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell 187, 4890–4904.e9 (2024).
Mougakiakos, D. Allogeneic CAR T cells for autoimmune diseases: a glimpse into the future. Signal. Transduct. Target. Ther. 9, 276 (2024).
Aghajanian, H. et al. Targeting cardiac fibrosis with engineered T cells. Nature 573, 430–433 (2019). This work is the first preclinical proof of concept with CAR-T cells in a fibrotic disease model.
Kansal, R. et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci. Transl. Med. 11, eaav1648 (2019). This work is the first preclinical proof of concept with CAR-T cells in an autoimmune disease model.
Müller, F. et al. CD19 CAR T-cell therapy in autoimmune disease—a case series with follow up. N. Engl. J. Med. 390, 687–700 (2024).
Mougiakakos, D. et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 385, 567–569 (2021). This work is the first clinical proof of concept with CAR-T cells in patients with autoimmune disease.
Müller, F. et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet 401, 815–818 (2023).
Haghikia, A. et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 22, 1104–1105 (2023).
Pecher, A. C. et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA 329, 2154–2162 (2023).
Zhang, W. et al. Treatment of systemic lupus erythematosus using BCMA-CD19 compound CAR. Stem Cell Rev. Rep. 17, 2120–2123 (2021).
Granit, V. et al. Safety and clinical activity of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis (MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study. Lancet Neurol. 22, 578–590 (2023).
Fischbach, F. et al. CD19-targeted chimeric antigen receptor T cell therapy in two patients with multiple sclerosis. Med 5, 550–558.e2 (2024).
Oliver-Caldes, A. First report of CART treatment in AL amyloidosis and relapsed/refractory multiple myeloma. J. Immunother. Cancer 9, e003783 (2021).
Korell, F. et al. First third-generation CAR T cell application targeting CD19 for the treatment of systemic IgM AL amyloidosis with underlying marginal zone lymphoma. Biomark. Res. 14, 11–91 (2023).
Qin, C. et al. Anti-BCMA CAR T-cell therapy CT103A in relapsed or refractory AQP4-IgG seropositive neuromyelitis optica spectrum disorders: phase 1 trial interim results. Signal. Transduct. Target. Ther. 8, 5 (2023).
Tobias, A. & Grego, A. Hematopoietic stem cell transplantation and cellular therapies for autoimmune diseases: overview and future considerations from the Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 57, 1055–1062 (2022).
Muraro, P. A. et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat. Rev. Neurol. 13, 391–405 (2017). This review discusses clinical evidence of immune reset by a radical procedure — high-dose chemotherapy followed by rescue with autologous stem cell transplantation — thus supporting similar approaches with the aim to elicit durable, drug-free responses in autoimmunity.
Brudno, J. N. & Kochenderfer, J. N. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. Mar. 34, 45–55 (2018).
Lee, D. W. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Santomasso, B. D. et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO guideline. J. Clin. Oncol. 39, 3978–3992 (2021).
Hines, M. R. et al. Immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome. Transplant. Cell. Ther. 29, 438.e1–438.e16 (2023).
Mikhael, J., Fowler, J. & Shah, N. Chimeric antigen receptor T-cell therapies: barriers and solutions to access. JCO Oncol. Pract. 18, 800–807 (2022). This review describes major hurdles to successfully deploying ex vivo engineered CAR-T cells.
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Borgert, R. Improving outcomes and mitigating costs associated with CAR T-cell therapy. Am. J. Manag. Care 27, S253–S261 (2021).
Lickefett, B. Lymphodepletion—an essential but undervalued part of the chimeric antigen receptor T-cell therapy cycle. Front. Immunol. 14, 1303935 (2023).
Bot, A. et al. Cyclophosphamide and fludarabine conditioning chemotherapy induces a key homeostatic cytokine profile in patients prior to CAR T cell therapy. Blood 126, 4426 (2015).
Warkentin, P. I. Voluntary accreditation of cellular therapies: foundation for the accreditation of cellular therapy (FACT). Cytotherapy 5, 299–305 (2003).
Locke, F. L. et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 4, 4898–4911 (2020).
Scholler, N. et al. Tumor immune contexture is a determinant of anti-CD19 CAR T cell efficacy in large B cell lymphoma. Nat. Med. 28, 1872–1882 (2022).
Locke, F. L. et al. Impact of tumor microenvironment on efficacy of anti-CD19 CAR T cell therapy or chemotherapy and transplant in large B cell lymphoma. Nat. Med. 30, 507–518 (2024).
Sotillo, E. et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 5, 1282–1295 (2015).
Plaks, V. et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood 138, 1081–1085 (2021).
Da Via, M. C. et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat. Med. 27, 616–619 (2021).
Smith, T. T. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 12, 813–820 (2017). This work is the first preclinical report of in vivo cell engineering with non-viral payload delivered via a nanoformulation.
Pfeiffer, A. et al. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol. Med. 10, e9158 (2018). This work is the first preclinical report of in vivo CAR-T cell engineering utilizing a viral vector-based approach.
Zhou, Q. et al. T-cell receptor gene transfer exclusively to human CD8+ cells enhances tumor cell killing. Blood 120, 4334–4342 (2012).
Mullin, R. Gilead to acquire Kite Pharma for $11.9 billion. Chem. Eng. N. 95, 15 (2017).
Themeli, M., Rivière, I. & Sadelain, M. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell 16, 357–366 (2015).
Jaeger, A. J. CAR-T in the courts. Genet. Eng. Biotechnol. N. 37, 31–33 (2017).
Sanofi. Sanofi R&D Day. sanofi.com https://www.sanofi.com/assets/dotcom/content-app/events/investor-presentation/2023/r-and-d-day-2023/Presentation.pdf (2023).
AstraZeneca to acquire EsoBiotec to advance cell therapy ambition. Astra Zeneca (17 March 2025); https://www.astrazeneca.com/media-centre/press-releases/2025/astrazeneca-to-acquire-esobiotec.html.
Mullard, A. In vivo CAR T cells gain traction, with AbbVie’s US$2.1 billion acquisition of Capstan therapeutics. Nature https://www.nature.com/articles/d41573-025-00131-w (2025).
Klebanoff, K. A., Khong, H. T., Antony, P. A., Palmer, D. C. & Restifo, N. P. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 26, 111–117 (2005).
Anliker, B. et al. Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat. Methods 7, 929–935 (2010).
Funke, S. et al. Targeted cell entry of lentiviral vectors. Mol. Ther. 16, 1427–1436 (2008).
Bender, R. R. et al. Receptor-targeted Nipah virus glycoproteins improve cell-type selective gene delivery and reveal a preference for membrane-proximal cell attachment. PLoS Pathog. 12, e1005641 (2016).
Charitidis, F. T. et al. CAR gene delivery by T-cell targeted lentiviral vectors is enhanced by rapamycin induced reduction of antiviral mechanisms. Adv. Sci. 10, e2302992 (2023).
Dobson, C. S. et al. Antigen identification and high-throughput interaction mapping by reprogramming viral entry. Nat. Methods 19, 449–460 (2022).
Strebinger, D. et al. Cell type-specific delivery by modular envelope design. Nat. Commun. 14, 5141 (2023).
Frank, A. M. et al. CD8-specific designed ankyrin repeat proteins improve selective gene delivery into human and primate T lymphocytes. Hum. Gene Ther. 31, 679–691 (2020).
Agarwal, S., Weidner, T., Thalheimer, F. B. & Buchholz, C. J. In vivo generated human CAR T cells eradicate tumor cells. Oncoimmunology 8, e1671761 (2019).
Agarwal, S. et al. In vivo generation of CAR T cells selectively in human CD4+ lymphocytes. Mol. Ther. 28, 1783–1794 (2020).
Frank, A. M. et al. Combining T-cell-specific activation and in vivo gene delivery through CD3-targeted lentiviral vectors. Blood Adv. 4, 5702–5715 (2020).
Huckaby, J. T. et al. Bispecific binder redirected lentiviral vector enables in vivo engineering of CAR-T cells. J. Immunother. Cancer 9, e002737 (2021).
Braun, A. H., Frank, A. M., Ho, N. & Buchholz, C. J. Dasatinib is a potent enhancer for CAR T cell generation by CD3-targeted lentiviral vectors. Mol. Ther. Methods Clin. Dev. 28, 90–98 (2022).
Kapitza, L. et al. CD62L as target receptor for specific gene delivery into less differentiated human T lymphocytes. Front. Immunol. 14, 1183698 (2023).
Ho, N. et al. In vivo generation of CAR T cells in the presence of human myeloid cells. Mol. Ther. Methods Clin. Dev. 26, 144–156 (2022).
Krug, A. et al. In vivo CAR T cell therapy against angioimmunoblastic T cell lymphoma. J. Exp. Clin. Cancer Res. 43, 262 (2024).
Cunningham, J. et al. In vivo delivery of a CD20 CAR using a CD8-targeted fusosome in southern pig-tail macaques (M. nemestrina) results in B cell depletion. Blood 138, 2769 (2021).
Mullard, A. In vivo CAR T cells move into clinical trials. Nat. Rev. Drug. Discov. 23, 727–730 (2024). This news feature reports the translation of in vivo CAR technology to the clinic.
Nicolai, C. J. et al. In vivo CAR T-cell generation in nonhuman primates using lentiviral vectors displaying a multidomain fusion ligand. Blood 144, 977–987 (2024). This work is the first peer-reviewed publication describing preclinical evidence of activity of in vivo CAR therapy in NHPs.
Andorko, J. I. et al. Targeted in vivo generation of CAR T and NK cells utilizing an engineered lentiviral vector platform. Blood 142, 763 (2023).
Andorko, J. I. et al. Targeted in vivo delivery of genetic medicines utilizing an engineered lentiviral vector platform results in CAR T and NK cell generation. Molecular Therapy https://doi.org/10.1016/j.ymthe.2025.06.036 (2025).
Russell, R. M. et al. Rational design of a detargeted vesiculovirus fusogen to enable targeted in vivo gene delivery. In: 27th American Society of Gene & Cell Therapy (ASGCT) Annual Meeting https://doi.org/10.1016/j.ymthe.2024.04.020 (2024).
Mullen, K. F. et al. In vivo generation of both CAR T cells and CAR NK cells using a CD7 targeted lentiviral vector. In: 27th American Society of Gene & Cell Therapy (ASGCT) Annual Meeting https://doi.org/10.1016/j.ymthe.2024.04.020 (2024).
Grinstein, J. D. First patient dosed with in vivo gene therapy for CAR T. Inside Precision Medicine https://www.insideprecisionmedicine.com/topics/precision-medicine/first-patient-dosed-with-in-vivo-gene-therapy-for-car-t/ (2024).
Kite to acquire Interius BioTherapeutics to advance in vivo platform [press release]. Kite (21 August 2025); https://www.kitepharma.com/news/press-releases/2025/8/kite-to-acquire-interius-biotherapeutics-to-advance-in-vivo-platform.
Michels, K. R. et al. UB-VV100, a novel platform for in vivo engineering of therapeutic anti-CD19 CAR T cells, shows effective T cell transduction, B cell depletion, and tumor control in a humanized murine model. Umoja Biopharma https://www.umoja-biopharma.com/wp-content/uploads/2021/05/Umoja-Biopharma-ASGCT-FINAL.pdf (2021).
Nicolai, C. et al. VivoVec: a novel lentiviral-based in vivo CAR T cell generation platform with viral particle surface engineering incorporating T cell activating and co-stimulatory ligands. Umoja Biopharma https://www.umoja-biopharma.com/wp-content/uploads/2022/11/CN-ASGCT-Poster-Umoja1.pdf (2022).
Michels, K. R. et al. Preclinical proof of concept for VivoVec, a lentiviral-based platform for in vivo CAR T-cell engineering. J. Immunother. Cancer 2023 11, e006292 (2023).
AbbVie and Umoja Biopharma announce strategic collaboration to develop novel in-situ CAR-T cell therapies. Umoja Biopharma https://www.umoja-biopharma.com/news/abbvie-and-umoja-biopharma-announce-strategic-collaboration-to-develop-novel-in-situ-car-t-cell-therapies/ (2024).
Beitz, L. et al. In vivo generation of B cell depleting CAR T cell therapies for treatment of autoimmune diseases. In: ACR Convergence Annual Meeting https://acrabstracts.org/abstract/in-vivo-generation-of-b-cell-depleting-car-t-cell-therapies-for-treatment-of-autoimmune-diseases/ (2024).
Teoh, J. et al. Development of a surface engineered lentiviral vector for in vivo generation of CD22-directed CAR T cells. In: SITC Annual Meeting https://jitc.bmj.com/content/12/Suppl_2/A1267.info (2024).
Friesen, T. et al. Preclinical development of UB-VV400, a surface engineered lentiviral vector for the in vivo generation of CD22-directed CAR T cells. In: ASGCT Annual Meeting Abstract 795 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
IASO BIO announces new development partnership with umoja biopharma to develop ex vivo and in vivo cell and gene therapies. Umoja Biopharma https://www.umoja-biopharma.com/news/iaso-bio-announces-new-development-partnership-with-umoja-biopharma-to-develop-ex-vivo-and-in-vivo-cell-and-gene-therapies/ (2024).
Chen, X.-F. et al. In-vivo CAR-T cells produced by specific virus in refractory B cell lymphoma. In: SITC Annual Meeting https://doi.org/10.1136/jitc-2024-SITC2024.1482 (2024).
Chen, X. et al. Lentiviral particle-mediated generation of in-vivo CAR-T cells for treating refractory B-cell lymphoma. In: ISCT Annual Meeting https://www.isct-cytotherapy.org/article/S1465-3249%2825%2900518-3/abstract (2025).
Galvis, L. et al. ENaBL, a novel lentiviral vector platform for in vivo engineering of therapeutic anti-BCMA CAR T cells, shows effective T cell transduction and tumor control in a humanized murine model. In: ASGCT Annual Meeting Abstract 793 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Weerens, L. et al. Development of novel T cell-specific synthetic promoters for safer and more effective in vivo CAR-T cell therapies. In: ASGCT Annual Meeting Abstract 868 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Doix, B. et al. Engineering T cell-specific lentiviral vectors for safe and effective in vivo CAR-T therapy. In: ASGCT Annual Meeting Abstract 879 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
EsoBiotec doses first patient in investigator initiated trial of in vivo BCMA CAR-T candidate ESO-T01 for multiple myeloma [press release]. EsoBiotec https://www.esobiotec.com/esobiotec-doses-first-patient-in-investigator-initiated-trial-of-in-vivo-bcma-car-t-candidate-eso-t01-for-multiple-myeloma (2025).
Xu, J. et al. In-vivo B-cell maturation antigen CAR T-cell therapy for relapsed or refractory multiple myeloma. Lancet 406, 228–231 (2025). This is the first clinical proof of concept published in a peer-reviewed journal describing the efficacy and safety of an in vivo CAR technology.
Contrastano, S. G. et al. Potent in vivo transduction by iGPS particles generates CAR T cells with durable anti-tumor activity. Kelonia https://keloniatx.com/wp-content/uploads/2023/05/2023-ASGCT-Kelonia-Presentation.vFINAL.pdf (2023).
Beura, E. T. et al. T cell-specific in vivo transduction with preclinical candidate KLN-1010 generates BCMA directed CAR T cells with potent anti-multiple myeloma activity. Cancer Res. https://doi.org/10.1158/1538-7445.AM2024-48 (2024).
Bender, R. R. et al. Receptor-targeted Nipah virus glycoproteins improve cell-type selective gene delivery and reveal a preference for membrane-proximal cell attachment. PLoS Pathog. 12, 1–28 (2016).
Nakamura, T. et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotech. 23, 209–214 (2005).
Cordes, N. et al. Anti-CD19 CARs displayed at the surface of lentiviral vector particles promote transduction of target-expressing cells. Mol. Ther. Methods Clin. Dev. 21, 42–53 (2021).
Ruella, M. et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 24, 1499–1503 (2018).
Amatya, S. et al. Enabling potent T cell delivery and mitigating key off-target concerns of in vivo CAR T lentiviral vectors with the targeted paramyxovirus fusogen system. Blood 144, 2047 (2024).
Cruite, P. et al. Retargeted “fusosomes” for in vivo delivery to T cells. Mol. Ther. 30, 1081 (2022).
Chavan, H. et al. In vitro and in vivo specificity and biodistribution of a novel CD8-targeted fusosome. Blood 142, 3630 (2023).
Trudeau, K. et al. Advancements in manufacturing of CD8- targeted fusosomes enhance transduction of resting T cells in vitro and in vivo. Mol. Ther. 31, 760 (2023).
Chokshi, C. et al. In vivo engineering of hematopoietic stem cells with virus-like particles to generate multi-lineage CAR immune cell therapy for cancer. In: ASGCT annual meeting Abstract 1783 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Vyriad announces strategic collaboration with Novartis to develop in vivo CAR-T cell therapies [press release]. PR Newswire https://www.prnewswire.com/news-releases/vyriad-announces-strategic-collaboration-with-novartis-to-develop-in-vivo-car-t-cell-therapies-302310717.html (2024).
In vivo delivery of CD3-directed CD19-CAR lentivectors leads to the generation of CAR-T and NK-like (CAR-TaNK) cells capable of complete ablation of B cells in the blood, bone marrow, and tissue in NSG-SGM3 CD34+ humanized mice. In: AACR Annual Meeting https://exumabio.com/wp-content/uploads/2023/04/AACR-Poster-2023-Final-2.pdf (2023).
Coillard, A. et al. A novel polymeric gene delivery platform to precisely target and reprogram cells in vivo for haematological malignancies & beyond. In: ASGCT Annual Meeting Abstract 1213 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Jaudoin, C. et al. Lentiviral vector engineering with polymer surface modification for car-T cell therapy. In: ASGCT Annual Meeting Abstract 1214 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Parayath, N. N., Stephan, S. B., Koehne, A. L., Nelson, P. S. & Stephan, M. T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080 (2020). This seminal preclinical study demonstrates in vivo CAR engineering using mRNA formulated in nanocarriers.
Cullis, P. R. & Hope, M. G. Lipid nanoparticle systems for enabling gene therapies. Mol. Ther. 25, 1467–1475 (2017). This review describes the discovery and development of current-generation LNPs comprising ionizable lipids and facilitating the design of first-in-class RNA nanomedicines.
Kularatne, R. N. The future of tissue-targeted lipid nanoparticle-mediated nucleic acid delivery. Pharmaceuticals 15, 897 (2022).
Park, M. D., Silvin, A., Ginhoux, F. & Merad, M. Macrophages in health and disease. Cell 185, 4259–4279 (2022).
Wang, Y. et al. In vivo programming of myeloid cells by mRNA mediated delivery of novel FcA fusion receptor activates anti-tumor immunity. J. Immunother. Cancer https://doi.org/10.1136/jitc-2022-SITC2022.1237 (2022).
Myeloid Therapeutics enters into agreement with Acuitas Therapeutics for lipid nanoparticle (LNP) system, enabling the first-ever delivery of mRNA-encoded CARs directly to humans [press release]. Myeloid https://myeloidtx.com/myeloid-therapeutics-enters-into-agreement-with-acuitas-therapeutics-for-lipid-nanoparticle-system/ (2022).
Argueta, S. et al. In vivo programmed myeloid cells expressing novel chimeric antigen receptors show potent anti-tumor activity in preclinical solid tumor models. Front. Immunol. https://doi.org/10.3389/fimmu.2024.1501365 (2024).
Argueta, S. et al. Preclinical development of MT-303, a novel LNP-formulated GPC3-specific CAR mRNA, for in vivo programming of monocytes to treat hepatocellular carcinoma. J. Immunother. Cancer https://doi.org/10.1136/jitc-2024-SITC2024.1125 (2024).
Myeloid Therapeutics initiates patient dosing with MT-302, a novel TROP2-targeting RNA CAR, in phase 1 study for advanced or metastatic epithelial tumors [press release]. Myeloid https://myeloidtx.com/myeloid-therapeutics-initiates-patient-dosing-with-mt-302-a-novel-trop2-targeting-rna-car-in-phase-1-study-for-advanced-or-metastatic-epithelial-tumors/ (2023).
Myeloid Therapeutics initiates patient dosing with MT-303, a novel GPC3 targeting RNA CAR, in phase 1 study for advanced hepatocellular carcinoma (HCC) [press release]. Myeloid https://myeloidtx.com/myeloid-therapeutics-initiates-patient-dosing-with-mt-303-a-novel-gpc3-targeting-rna-car-in-phase-1-study-for-advanced-hepatocellular-carcinoma-hcc/ (2024).
Myeloid therapeutics to present at 43rd Annual JP Morgan Healthcare Conference [press release]. Myeloid https://myeloidtx.com/myeloid-therapeutics-to-present-at-43rd-annual-jp-morgan-healthcare-conference/ (2025).
Wang, Y. et al. Novel innate immune receptor-based chimeric antigen receptors for in vivo programming of myeloid cells show potent anti-tumor activity in preclinical solid tumor models. In: ASGCT Annual Meeting Abstract 1727 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Parhiz, H., Atochina-Vasserman, E. N. & Weissman, D. mRNA-based therapeutics: looking beyond COVID-19 vaccines. Lancet 403, 1192–1204 (2024).
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Molecular Therapy 16, 1833–1840 (2008). This report describes the seminal discovery that incorporation of synthetic nucleotides enables the utilization of RNA.
Cullis, P. R. & Felgner, P. L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug. Discov. 23, 709–722 (2024).
Rurik, J. G. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022). This is the first preclinical report of in vivo CAR-T cell therapy in a non-oncologic indication.
Hunter et al. A novel product candidate (CPTX2309) for in vivo mRNA engineering of anti-CD19 CAR T cells utilizing novel CD8-targeted lipid nanoparticles. In: American College of Rheumatology Annual Meeting (2024).
Adams, G. et al. In vivo engineering of CAR T cells using a novel targeted LNP-mRNA technology. J. Immunother. Cancer https://doi.org/10.1136/jitc-2023-SITC2023.1202 (2023).
Zhang, Y. et al. Effective engineering of CD8+ T cells from autoimmune disease patients utilizing a CD8-targeted lipid nanoparticle encoding an anti-CD19 CAR mRNA(CPTX2309). In: American College of Rheumatology Annual Meeting (2024).
Franz, A. et al. Profound B Cell depletion and repopulation with predominantly naïve B cells in non-human primates achieved through a novel in vivo CD8-targeted lipid nanoparticle mRNA CAR. In: American College of Rheumatology Annual Meeting (2024).
Hunter, T. et al. In vivo CAR T cell generation for cancer and autoimmune disease. Science 388, 1311–1317 (2025). This work is the first preclinical report in NHPs demonstrating proof of concept with respect to in vivo CAR-T cell generation utilizing a non-viral platform.
Aghajanian, H. et al. A two-infusion regimen with a novel in vivo non-viral chimeric antigen receptor (CAR) achieves up to 90% CD8+ T cell engineering and tissue depletion of target cells in non-human primates (NHPs). In: ASGCT Annual Meeting Abstract 258 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Breda, L. et al. In vivo hematopoietic stem cell modification by mRNA delivery. Science 381, 436–443 (2023).
Immorna Biotherapeutics announces first clinical administration of JCXH-213, a breakthrough mRNA-based in vivo CAR therapy [press release]. Pipeline Review https://pipelinereview.com/immorna-biotherapeutics-announces-first-clinical-administration-of-jcxh-213-a-breakthrough-mrna-based-in-vivo-car-therapy/ (2025).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06801119 (2025).
Xiao, T. et al. An vivo CAR T producer (HN2301) for autoimmune disease enables B cell depletion in NHP tissues and performs therapeutic effect in an SLE mouse model. In: ASGCT Annual Meeting Abstract 794 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Wang, Q. et al. In vivo CD19 CAR T-cell therapy for refractory systemic lupus erythematosus. N. Engl. J. Med. https://doi.org/10.1056/NEJMc2509522 (2025).
Wesselhoeft, R. A., Kowalski, P. S. & Anderson, D. G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 9, 2629 (2018).
Wesselhoeft, R. A. et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell 74, 508–520.e4 (2019).
Becker, A. M. et al. In situ CAR therapy using oRNATM lipid nanoparticles regresses tumors in mice. In: SITC Annual Meeting https://www.ornatx.com/wp-content/uploads/2022/11/Orna_SITC-2022.pdf (2022).
Orna Therapeutics announces strategic acquisition of ReNAgade therapeutics to solidify leadership in circular RNA therapies. Orna https://www.ornatx.com/orna-therapeutics-announces-strategic-acquisition-of-renagade-therapeutics-to-solidify-leadership-in-circular-rna-therapies/ (2024).
Crabtree, J. et al. In vivo panCAR-mediated depletion of B cells in non-human primates using a circular (oRNA®) anti-CD20 CAR. In: ASH Annual Meeting https://ash.confex.com/ash/2024/webprogram/Paper210256.html (2024).
Beaulieu, A. et al. In vivo transient programming of T cells with Sail’s targeted nanoparticles encapsulating eRNATM-encoded hCD19 CAR achieves deep depletion of B cells in blood and lymphoid tissues in a B-cell repopulating humanized mouse model. In: ASGCT Annual Meeting Abstract 774 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Sharma, K. et al. Long-acting circular RNA CAR drives potent and durable tumor elimination invivo. In: ASGCT Annual Meeting Abstract 781 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Pan, X. et al. In vivo generation of anti-CD19 CAR T cells utilizing circular RNA encapsulated in targeted lipid nanoparticles. In: ASGCT Annual Meeting Abstract 1762 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Lemgart, V. et al. In vitro and in vivo activity of a lipid nanoparticle system for the in vivo generation of CAR T cells. In: ASH Annual Meeting https://doi.org/10.1182/blood-2024-207566 (2024).
Kamoun, W. S. et al. Antitumour activity and tolerability of an EphA2-targeted nanotherapeutic in multiple mouse models. Nat. Biomed. Eng. 3, 264–280 (2019).
Lemgart, V. et al. CD19 CAR T cells generated in vivo by T cell targeted lipid nanoparticles demonstrate robust and durable B cell depletion in non-human primates. In: ASGCT Annual Meeting Abstract 260 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38, 947–953 (2020).
Pierini, S. et al. Chimeric antigen receptor macrophages (CAR-M) sensitize solid tumors to anti-PD1 immunotherapy. J. Immunother. Cancer https://doi.org/10.1136/jitc-2022-SITC2022.0371 (2022).
Gabitova, L. et al. Pre-clinical development of a CAR monocyte platform for cancer immunotherapy. J. Immunother. Cancer https://doi.org/10.1136/jitc-2022-SITC2022.0318 (2022).
Carisma Therapeutics corporate presentation November [corporate presentation]. Carisma https://ir.carismatx.com/static-files/42a456e9-9007-4574-8aaa-e7c8db3ac852 (2024).
Abdou, Y. et al. PO2-04-08 translational insights from a phase 1, first-in-human (FIH) clinical trial of the anti-HER2 CAR macrophage CT-0508 in participants with HER2 positive metastatic breast cancer and other HER2 overexpressing solid tumors. In: SABCS (2023).
Moderna and Carisma establish collaboration to develop in vivo engineered chimeric antigen receptor monocytes (CAR-M) for oncology [press release]. Mpderna https://investors.modernatx.com/news/news-details/2022/Moderna-and-Carisma-Establish-Collaboration-to-Develop-in-vivo-Engineered-Chimeric-Antigen-Receptor-Monocytes-CAR-M-for-Oncology/default.aspx (2022).
Varghese, B. et al. In vivo CAR-M: redirecting endogenous myeloid cells with mRNA for cancer immunotherapy. J. Immunother. Cancer https://doi.org/10.1136/jitc-2023-SITC2023.1514 (2023).
Bagashev, A. et al. Pre-clinical efficacy of a novel anti-GPC3 in vivo CAR-M for hepatocellular carcinoma. J. Immunother. Cancer https://doi.org/10.1136/jitc-2024-SITC2024.0329 (2024).
Carisma and Moderna expand collaboration to develop two in vivo CAR-M therapies for autoimmune diseases [press release]. Carisma https://ir.carismatx.com/news-releases/news-release-details/carisma-and-moderna-expand-collaboration-develop-two-vivo-car-m (2024).
Carisma Therapeutics provides corporate updates [press release]. Carisma https://ir.carismatx.com/news-releases/news-release-details/carisma-therapeutics-provides-corporate-updates#:~:Text=The%20Company%20will%20assess%20a,value%20of%20all%20our%20assets (2025).
Yan, F. et al. Improved T cell delivery and expression of mRNA using CD8-targeted lipid nanoparticles with Athebody® designed ankyrin repeat proteins (DARPins). In: ASGCT Annual Meeting Abstract 667 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Fang, J. et al. Mobilize LNPs: a novel platform for in vivo targeted delivery of RNA therapeutics to T cells. In: ASGCT Annual Meeting Abstract 483 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Hashiba, K. et al. Optimizing VHH ligand density in T cell-targeted LNPs: an inverted U-shaped relationship for effective in vivo CAR T cell generation. In: ASGCT Annual Meeting Abstract 665 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Khairallah, C. et al. In: ASGCT Annual Meeting Abstract 770 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Sun, J. et al. In vivo CAR-T therapy powered by novel CLAMP technology for T cell targeted mRNA delivery. In: ASGCT Annual Meeting Abstract 1282 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Magee, M. S. et al. Deploying an RNA-based gene writer system and lipid nanoparticle (LNP) delivery to generate functional chimeric antigen receptor (CAR) T cells with in vitro and in vivo anti-tumor activity. Blood 142, 2073 (2023).
Rodriguez, J. et al. Targeted lipid nanoparticle delivery of an RNA gene writer in vivo enables generation of CAR-T cells in a humanized mouse model. Blood 144, 4799 (2024).
De Iaco, A. et al. In: ASGCT Annual Meeting Abstract 153 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Monte, M. et al. In vivo RNA delivery to T cells and hematopoietic stem cells in humanized mice and non-human primates using targeting lipid nanoparticles. In: ASGCT Annual Meeting Abstract 668 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Wang, Z. et al. Efficient and effective in vivo CAR T generation using recombinase-based, precise, high-capacity, off-the-shelf, LNP-based in vivo genomic engineering. In: ASGCT Annual Meeting Abstract 797 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Petazzi, P. et al. Precise gene writing system for CAR-T cell therapy generation. In: ASGCT Annual Meeting Abstract 1140 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Bimbo, J. et al. Novel non-viral DNA-based gene therapy vector for CAR T engineering in vivo. In: ASGCT Annual Meeting Abstract 213 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Urits, I. et al. A review of patisiran (ONPATTRO®) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol. Ther. 9, 301–315 (2020).
Patil, H. et al. CAR-T cell therapy in rheumatic diseases: a review article. Clin Rheumatol. https://doi.org/10.1007/s10067-025-07451-7 (2025).
Mueller, F. et al. Comparison of the safety profiles of CD19-targeting CAR T-cell therapy in patients with SLE and B-cell lymphoma. Blood https://doi.org/10.1182/blood.2025028375 (2025).
Pecher, A. C. et al. CD19-targeting CAR T cell therapy in five patients with systemic sclerosis unsuitable for autologous stem cell transplantation. EULAR Rheumatologyhttps://doi.org/10.1016/j.ero.2025.03.005 (2025).
Hagen, M. et al. Local immune effector cell-associated toxicity syndrome in CAR T-cell treated patients with autoimmune disease: an observational study. Lancet Rheumatol. https://doi.org/10.1016/S2665-9913(25)00091-8 (2025).
Gust, J. BCMA-CAR T-cell treatment-associated parkinsonism. Blood 142, 1181–1183 (2023).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy kills switches. N. Engl. J. Med. 365, 1673–1683 (2011).
Hu, J. & Dunbar, C. E. T-cell lymphomas in recipients of CAR-T cells: assessing risks and causalities. Blood 144, 2473–2481 (2024).
Hamilton, M. P. et al. Risk of second tumors and T-cell lymphoma after CAR T-cell therapy. N. Engl. J. Med. 390, 2047–2060 (2024).
Ruella, M. & June, C. H. CAR T-cell resistance to oncogenic transformation. Blood Cancer Discov. 5, 229–233 (2024).
Fraietta, J. A. et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T-cells. Nature 558, 307–312 (2018).
US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry: long term follow-up after administration of human gene therapy products. FDA https://www.fda.gov/media/113768/download (2020).
Szebeni, J., Simberg, D., González-Fernández, A., Barenholz, Y. & Dobrovolskaia, M. A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 13, 1100–1108 (2018).
Uehata, T. & Takeuchi, O. RNA recognition and immunity—innate immune sensing and its posttranscriptional regulation mechanisms. Cells 9, 1701 (2020).
Hosseini-Kharat, M., Bremmell, K. E. & Prestidge, C. A. Why do lipid nanoparticles target the liver? Understanding of biodistribution and liver-specific tropism. Mol. Ther. Methods Clin. Dev. https://doi.org/10.1016/j.omtm.2025.101436 (2025).
Szebeni, J. et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat. Nanotechnol. 17, 337–346 (2022).
FDA Center for Biologics Evaluation and Research (CBER) Office of Therapeutic Products (OTP). Guidance document: considerations for the development of chimeric antigen receptor (CAR) T cell products. FDA https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-development-chimeric-antigen-receptor-car-t-cell-products (2024).
US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH), Office of Good Clinical Practice (OGCP), Office of Regulatory Affairs (ORA). Guidance for industry: oversight of clinical investigations—a risk-based approach to monitoring. FDA https://www.fda.gov/media/116754/download (2013).
Rejeski, K. et al. Immune effector cell-associated haematotoxicity after CAR T-cell therapy: from mechanism to management. Lancet Haematol. 11, e459–e470 (2024).
Neelapu, S. S. et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat. Med. 28, 735–742 (2022).
Swan, D., Madduri, D. & Hocking, J. CAR-T cell therapy in multiple myeloma: current status and future challenges. Blood Cancer J. 14, 206 (2024).
Costa, L. J. MRD accelerating myeloma drug development. Blood 144, 345–347 (2024).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Fuchs, E. J. Transplantation tolerance: from theory to clinic. Immunol. Rev. 258, 64–79 (2014).
Su, Y., Yuan, C. & Shi, M. Editorial: screening and verification of new targets for CAR-T immunotherapy in cancer. Front. Immunol. 14, 1189773 (2023).
Lee, J. et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J. Clin. Invest. 130, 6317–6324 (2020).
Reincke, S. M. et al. Chimeric autoantibody receptor T cells deplete NMDA receptor-specific B cells. Cell 186, 5084–5097.e18 (2023).
Yang, J. et al. Dual-targeted CAR T-cell immunotherapies for hematological malignancies: latest updates from the 2023 ASH annual meeting. Exp. Hematol. Oncol. 13, 25 (2024).
Ramishetti, S. et al. Systemic gene silencing in primary T lymphocytes using targeted lipid nanoparticles. ACS Nano 9, 6706–6716 (2015). This article provides evidence for the first time of in vivo delivery to a T cell subset using targeted nanomedicine.
Inamdar, V. V., Hao, S., Stephan, S. B. & Stephan, M. T. Biomaterial-based scaffolds for direct in situ programming of tumor-infiltrating T lymphocytes. J. Control. Rel. 370, 310–317 (2024).
Nyberg, W. A. et al. An evolved AAV variant enables efficient genetic engineering of murine T cells. Cell 186, 446–460.e19 (2023).
Hale, M. et al. Homology-directed recombination for enhanced engineering of chimeric antigen receptor T cells. Mol. Ther. Methods Clin. Dev. 4, 192–203 (2017).
Demircan, M. B. et al. T-cell specific in vivo gene delivery with DART-AAVs targeted to CD8. Mol. Ther. 32, 3470–3484 (2024).
Theuerkauf, S. A. et al. AAV vectors displaying bispecific DARPins enable dual-control targeted gene delivery. Biomaterials 303, 122399 (2023).
Chen, H. et al. In vivo CAR-T cell therapy using modified AAV capsids targeting human T cells. In: ASGCT Annual Meeting Abstract 1739 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Decker, C. et al. In vivo VLP-based engineering generates multi-lineage CAR-immune cells that mediate potent and durable anti-tumor activity. In: SITC Annual Meeting https://ensoma.com/wp-content/uploads/SITC-2024-HER2-poster-final.pdf (2024).
Tessera Therapeutics to present new data across preclinical programs for its gene writing™ and delivery platforms at the American Ssociety of Gene and Cell Therapy 28th Annual Meeting [press release]. Tessera https://www.tesseratherapeutics.com/news/tessera-therapeutics-to-present-new-data-across-preclinical-programs-for-its-gene-writing-and-delivery-platforms-at-the-american-society-of-gene-and-cell-therapy-28th-annual-meeting (2025).
Li, Y.-R., Zhu, Y., Halladay, T. & Yang, L. In vivo CAR engineering for immunotherapy. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-025-01174-1 (2025).
Lu, L., Xie, M., Yang, B., Zhao, W. B. & Cao, J. Enhancing the safety of CAR-T cell therapy: synthetic genetic switch for spatiotemporal control. Sci. Adv. 10, eadj6251 (2024).
Dannenfelser, R. et al. Discriminatory power of combinatorial antigen recognition in cancer T cell therapies. Cell Syst. 11, 215–228.e5 (2020).
Parrett, B. J. et al. Reducing off-target expression of mRNA therapeutics and vaccines in the liver with microRNA binding sites. Mol. Ther. Methods Clin. Dev. 33, 1101402 (2025).
Xue, L. et al. High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models. Nat. Commun. 15, 1884 (2024).
Rhym, L. H. et al. Peptide-encoding mRNA barcodes for the high-throughput in vivo screening of libraries of lipid nanoparticles for mRNA delivery. Nat. Biomed. Eng. 7, 901–910 (2023).
Da Silva Sanchez, A. J. et al. Universal barcoding predicts in vivo ApoE-independent lipid nanoparticle delivery. Nano Lett. 22, 4822–4830 (2022).
Das, K. P. & Chandra, J. Nanoparticles and convergence of artificial intelligence for targeted drug delivery for cancer therapy: current progress and challenges. Front. Med. Technol. 4, 1067144 (2023).
Zhou, J. et al. Lipid nanoparticles produce chimeric antigen receptor T cells with interleukin-6 knockdown in vivo. J. Controlled Rel. 350, 298–307 (2022).
Kath, J. et al. PASTA: CRISPR knock-in paired with integrase for simple, precise, and efficient large transgene insertion in T cells. In: ASGCT Annual Meeting Abstract 649 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Amabile, A. et al. Bispecific antibody targeting of lipid nanoparticles. Preprint at bioRxiv https://doi.org/10.1101/2024.12.20.629467 (2024).
Kim, M. et al. Development of in vivo CAR T cell therapy using nanoparticle and RNA-based gene delivery systems. In: ASGCT Annual Meeting Abstract 796 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Divita, G. et al. In vivo mRNA CAR-T cell engineering utilizing a novel CD3/CD5 peptide based targeted nanoparticles. In: ASGCT Annual Meeting Abstract 1283 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Lee, C. et al. In vivo CAR delivery as supercoiled DNA with lymphatic tropism polyasparagine nanoparticles. In: ASGCT Annual Meeting Abstract 699 https://www.sciencedirect.com/journal/molecular-therapy/vol/33/issue/4/suppl/S1 (2025).
Lian, H. et al. Cytokine therapy combined with nanomaterials participates in cancer immunotherapy. Pharmaceutics 14, 2606 (2022).
Huang, C. et al. Lipid nanoparticle delivery system for mRNA encoding B7H3-redirected bispecific antibody displays potent antitumor effects on malignant tumors. Adv. Sci. https://doi.org/10.1002/advs.202205532 (2022).
Hamilton, A. G. et al. Ionizable lipid nanoparticles with integrated immune checkpoint inhibition for mRNA CAR T cell engineering. Adv. Healthc. Mater. 12, e2301515 (2023).
Danko, I. & Wolff, J. A. Direct gene transfer into muscle. Vaccine 12, 1499–1502 (1994).
University Of Pittsburgh Medical Center. University of Pittsburgh Medical Center performs first in vivo gene therapy for hemophilia A. Science Daily https://www.sciencedaily.com/releases/1999/06/990604081001.htm (1999).
Kuwana, Y. et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived c regions. Biochem. Biophys. Res. Commun. 149, 960–968 (1987). This work is the first report of design and in vitro activity of a construct comparable with current CARs.
Gross, G., Waks, T. & Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993).
Finney, H. M., Lawson, A. D., Bebbington, C. R. & Weir, A. N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 161, 2791–2797 (1998).
Maher, J., Brentjens, R. J., Gunset, G., Rivière, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Kochenderfer, J. N. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010). This article reports the first evidence of objective clinical response afforded by CAR-T cell intervention in a patient with B cell lymphoma.
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. J. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Umoja Biopharma announces FDA clearance of IND application for UB-VV111, a CD19 directed in situ CAR T for hematologic malignancies press release. Umoja Biopharma https://www.umoja-biopharma.com/news/umoja-biopharma-announces-fda-clearance-of-ind-application-for-ub-vv111-a-cd19-directed-in-situ-car-t-for-hematologic-malignancies/ (2024).
Papahadjopoulos, D., Vail, W. J., Jacobson, K. & Poste, G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim. Biophys. Acta 394, 483–491 (1975).
Dimitriadis, G. J. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature 274, 923–924 (1978).
Malone, R. W., Felgner, P. L. & Verma, I. M. Cationic liposome-mediated RNA transfection. Proc. Natl Acad. Sci. USA 86, 6077–6081 (1989). This seminal report describes the use of mRNA to directly transfect human, rat, mouse, Xenopus and Drosophila cells.
Conry, R. M. et al. Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res. 55, 1397–1400 (1995).
Hafez, I. M., Ansell, S. & Cullis, P. R. Tunable pH-sensitive liposomes composed of mixtures of cationic and anionic lipids. Biophysical J. 79, 1438–1446 (2000).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Barrett, D. M. et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Hum. Gene Ther. 22, 1575–1586 (2011).
US Food and Drug Administration. COMIRNATY FDA approval. FDA https://www.fda.gov/media/151710/download?attachment (2021).
US Food and Drug Administration. SPIKEVAX FDA approval. FDA https://www.fda.gov/media/155815/download?attachment (2022).
Uslu, U. & June, C. H. Beyond the blood: expanding CAR T cell therapy to solid tumors. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02446-2 (2024).
Aghajanian, H., Rurik, J. G. & Epstein, J. A. CAR-based therapies: opportunities for immuno-medicine beyond cancer. Nat. Metab. 4, 163–169 (2022).
Wat, J. & Barmettler, S. Hypogammaglobulinemia after chimeric antigen receptor (CAR) T-cell therapy: characteristics, management, and future directions. J. Allergy Clin. Immunol. Pract. 10, 460–466 (2022).
Acknowledgements
The authors acknowledge the contribution of P. Johnson and D. Fontana to the section ‘Viral vectors’. The authors apologize to those authors whose work was not cited directly owing to space limitations.
Author information
Authors and Affiliations
Contributions
J.I.A., A.B., A.S., K.F., L.G., R.H., M.K., F.N., J.V.S., A.J.S. and K.T. generated the sections ‘Viral vectors’ and ‘RNA-based platforms’. A.B. conceived the article layout. M.T.S., C.J.B., D.W. and C.H.J. contributed to the Abstract, Introduction and Outlook. All authors reviewed and edited the manuscript before submission. C.J.B.’s contribution to the manuscript represents his own perspective and not the official view of the Paul-Erlich Institute.
Corresponding author
Ethics declarations
Competing interests
J.I.A. is a paid employee of Interius BioTherapeutics, Inc., holds equity in the company, and is an inventor on patents issued and/or pending related to this work. A.B. is a shareholder and employee of Capstan Therapeutics, a company developing in vivo chimeric antigen receptor (CAR) therapies, and inventor or co-inventor on multiple relevant patents in the CAR and related fields. C.J.B. is a co-inventor on patents covering T cell-targeted lentiviral vectors. K.F. is a shareholder and member of the Board of Directors of Kelonia Therapeutics, and is also an inventor on patent applications assigned to Kelonia. L.G. is a shareholder and employee of Moderna, and a shareholder of Tessera. A.S. is a shareholder and employee of Umoja Therapeutics, a company developing in vivo CAR therapies. R.H. is a shareholder and employee of Myeloid Therapeutics, a company developing in vivo CAR therapies. C.H.J. is an inventor of multiple CAR patents and shareholder of several companies developing CAR products. M.K. is a founder, employee and shareholder of Carisma Therapeutics; a founder, shareholder and board director of Chymal Therapeutics; and an inventor of multiple patents related to CAR macrophages and monocytes (CAR-M) that have been licensed by Carisma Therapeutics. F.N. is a shareholder and employee of Orna Therapeutics, a company developing in vivo CAR therapies. A.J.S. is a shareholder and current employee of Sanofi. J.V.S. is an employee and shareholder in Mirai Bio, and a consultant and shareholder in Sana Biotechnology. M.T.S. is co-founder of and has received stock options from Persistence Therapeutics (Jupiter Bioventures); and has IP Licensing with Sanofi, Juno Therapeutics (now Bristol Myers Squibb) and Jupiter Bioventures. K.T. is a shareholder and employee of Sana Therapeutics, a company developing in vivo CAR and cell gene therapies. D.W. has filed patent applications based on some aspects of this work; those interests were fully disclosed to the University of Pennsylvania, with an approved plan in place for managing any potential conflicts arising from licensing of these patents.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Melgious Ang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Circular RNA
-
A single-stranded RNA molecule that forms a continuous loop. Although naturally a type of non-coding RNA that is found in many species, circular RNA can be adapted for cell engineering.
- Designed ankyrin repeat protein
-
(DARPin). A genetically engineered antibody mimetic protein typically exhibiting highly specific and high-affinity target protein binding, derived from natural ankyrin repeat proteins.
- Fusogen
-
A protein on the surface of a virus that helps the virus enter a host cell by fusing the viral membrane with the host cell membrane. This process releases the genetic material of the virus into the host cell’s cytoplasm.
- Hypogammaglobulinaemia
-
A condition characterized by abnormally low levels of immunoglobulins in the blood, making individuals more susceptible to infections.
- Internal ribosomal entry site
-
A specific RNA sequence that allows ribosomes to bind to mRNA and initiate protein synthesis at an internal location, bypassing the typical cap-dependent initiation process.
- Ionizable lipids
-
Lipid molecules that can change their charge depending on the pH level of their environment. They are a key component of lipid nanoparticles (LNPs), which are used to deliver RNA therapeutics.
- Lymphodepletion conditioning
-
A treatment that prepares the body for chimeric antigen receptor (CAR)-T cell therapy or adoptive cell transfer in general. It involves using chemotherapy drugs (most frequently cyclophosphamide and fludarabine) to deplete endogenous immune cells.
- Minimal residual disease
-
A term used for a small number of cancer cells that remain in the body after treatment. Minimal residual disease can occur in blood cancers such as leukaemia and lymphoma, as well as solid tumours.
- N 1-Methylpseudouridine
-
A chemical compound found in tRNA and mRNA vaccines. Although a natural component of archaea, it is presently utilized in biochemistry and molecular biology.
- Nipah virus (NiV) glycoproteins
-
NiV uses two key glycoproteins on its surface: the attachment glycoprotein (G) and the fusion glycoprotein (F), which are crucial for viral entry into host cells. The G protein facilitates attachment to the host cell, whereas the F protein triggers membrane fusion, allowing the virus to enter the cell.
- PiggyBac transposon system
-
A genetic engineering tool used to introduce and integrate DNA sequences into a genome, often in a stable and reproducible manner. It utilizes a transposase enzyme to ‘cut and paste’ a transposon (a DNA sequence) into a new location in the genome.
- Pseudotype
-
A virus particle that has been engineered to display a foreign viral envelope protein on its surface.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bot, A., Scharenberg, A., Friedman, K. et al. In vivo chimeric antigen receptor (CAR)-T cell therapy. Nat Rev Drug Discov 25, 116–137 (2026). https://doi.org/10.1038/s41573-025-01291-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41573-025-01291-5
This article is cited by
-
The dawn of in vivo immune cell engineering in oncology
Nature Biotechnology (2026)
-
2025: research in review
Nature Biotechnology (2025)