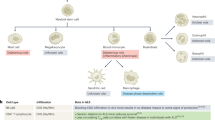
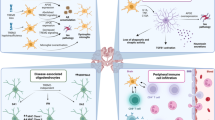
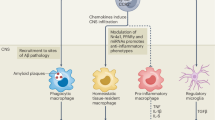
Abstract
Effective treatments for age-related chronic neurodegenerative diseases such as Alzheimer’s disease remain limited, in part because the molecular drivers of cognitive decline are still not fully understood. Human genetic studies, together with detailed analysis of disease pathology, indicate that the immune system has an important influence on disease progression. Research to date has focused largely on microglia — specialized innate immune cells that reside within the central nervous system (CNS) — as functional studies combined with deep transcriptional profiling have improved our understanding of this innate immune cell type in neurodegeneration and have identified several potential therapeutic targets. Increasing evidence now shows that microglia coordinate diverse CNS and peripheral cell populations to shape disease outcomes. In this Review, we discuss these neuroimmune interactions, which reveal a more intricate framework for how the central and peripheral immune systems may influence neurodegeneration. These insights could redirect future drug discovery efforts towards immune targets that complement existing therapies aimed at core pathological features. We also outline how this knowledge suggests new therapeutic strategies and highlight a critical need for disease-specific neuroimmune biomarkers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54, 412–436 (2022). The authors perform one of the largest AD GWAS to date, highlighting immune relevance for the disease.
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53, 1276–1282 (2021).
Nott, A. et al. Brain cell type-specific enhancer-promoter interactome maps and disease-risk association. Science 366, 1134–1139 (2019). This study maps promoter–enhancer interactomes in human brain cell types, linking noncoding AD risk variants to specific neuroimmune and glial regulatory programmes.
Corces, M. R. et al. Single-cell epigenomic analyses implicate candidate causal variants at inherited risk loci for Alzheimer’s and Parkinson’s diseases. Nat. Genet. 52, 1158–1168 (2020).
Gerhard, A. et al. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 21, 404–412 (2006).
D’Erchia, A. M. et al. Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Sci. Rep. 7, 10046 (2017).
Malpetti, M. et al. Microglial activation in the frontal cortex predicts cognitive decline in frontotemporal dementia. Brain 146, 3221–3231 (2023).
Chen, X. et al. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615, 668–677 (2023). This paper demonstrates that microglia-driven T cell infiltration into the brain parenchyma can directly promote neurodegeneration in tauopathy, cementing microglia–T cell crosstalk and adaptive immunity as a central pathogenic axis.
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020). By revealing clonally expanded, antigen-experienced CD8+ T cells patrolling CSF in AD, this study identifies an antigen-specific adaptive immune response in the CNS as an active and targetable component of neurodegeneration.
De Schepper, S. et al. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer’s disease. Nat. Neurosci. 26, 406–415 (2023). This work shows how perivascular cells instruct SPP1+ microglial phagocytic states and synaptic engulfment, mechanistically linking vascular niches to microglial neurotoxicity in AD models.
Sims, R. et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 49, 1373–1384 (2017).
Van Hove, H. et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 22, 1021–1035 (2019). This single-cell atlas defines transcriptionally distinct brain macrophage populations by ontogeny and niche, setting the stage for dissecting microglia versus border-associated macrophage functions in disease.
Kierdorf, K., Masuda, T., Jordao, M. J. C. & Prinz, M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20, 547–562 (2019).
Rustenhoven, J. & Kipnis, J. Brain borders at the central stage of neuroimmunology. Nature 612, 417–429 (2022).
Aspelund, A. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 212, 991–999 (2015).
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). This work identifies functional meningeal lymphatic vessels, which informed an updated view of CNS immune privilege and opened the modern era of brain border immunity.
Silvin, A. et al. Dual ontogeny of disease-associated microglia and disease inflammatory macrophages in aging and neurodegeneration. Immunity 55, 1448–1465.e6 (2022).
Iturria-Medina, Y. et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 7, 11934 (2016).
Yang, A. C. et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 603, 885–892 (2022). This single-cell atlas of the human brain vasculature characterizes endothelial, mural, stromal and immune cell transcriptomes, revealing blood–brain barrier changes and implicating the human brain vasculature in AD pathogenesis.
Da Mesquita, S. et al. Meningeal lymphatics affect microglia responses and anti-Aβ immunotherapy. Nature 593, 255–260 (2021).
Rustenhoven, J. et al. Age-related alterations in meningeal immunity drive impaired CNS lymphatic drainage. J. Exp. Med. https://doi.org/10.1084/jem.20221929 (2023).
Lawrence, A. R. et al. Microglia maintain structural integrity during fetal brain morphogenesis. Cell 187, 962–980.e19 (2024).
Cossart, R. & Garel, S. Step by step: cells with multiple functions in cortical circuit assembly. Nat. Rev. Neurosci. 23, 395–410 (2022).
Lawson, L. J., Perry, V. H., Dri, P. & Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170 (1990).
Grabert, K. et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 19, 504–516 (2016).
Stogsdill, J. A. et al. Pyramidal neuron subtype diversity governs microglia states in the neocortex. Nature 608, 750–756 (2022).
De Biase, L. M. et al. Local cues establish and maintain region-specific phenotypes of basal ganglia microglia. Neuron 95, 341–356.e6 (2017).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Badimon, A. et al. Negative feedback control of neuronal activity by microglia. Nature 586, 417–423 (2020). This paper elegantly demonstrates the functional link between microglia and neuronal activity.
Cserep, C. et al. Microglial control of neuronal development via somatic purinergic junctions. Cell Rep. 40, 111369 (2022).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
Liu, Y. U. et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 22, 1771–1781 (2019).
Stowell, R. D. et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat. Neurosci. 22, 1782–1792 (2019).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Vainchtein, I. D. et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 359, 1269–1273 (2018).
Sokolova, D. et al. Astrocyte-derived MFG-E8 facilitates microglial synapse elimination in Alzheimer’s disease mouse models. Preprint at bioRxiv https://doi.org/10.1101/2024.08.31.606944 (2024).
Rueda-Carrasco, J. et al. Microglia-synapse engulfment via PtdSer-TREM2 ameliorates neuronal hyperactivity in Alzheimer’s disease models. EMBO J. 42, e113246 (2023).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Wlodarczyk, A. et al. A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J. 36, 3292–3308 (2017).
McNamara, N. B. et al. Microglia regulate central nervous system myelin growth and integrity. Nature 613, 120–129 (2023).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e17 (2017).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Paolicelli, R. C. et al. Microglia states and nomenclature: a field at its crossroads. Neuron 110, 3458–3483 (2022).
Sun, N. et al. Human microglial state dynamics in Alzheimer’s disease progression. Cell 186, 4386–4403.e29 (2023).
Nugent, A. A. et al. TREM2 regulates microglial cholesterol metabolism upon chronic phagocytic challenge. Neuron 105, 837–854.e9 (2020).
van Lengerich, B. et al. A TREM2-activating antibody with a blood-brain barrier transport vehicle enhances microglial metabolism in Alzheimer’s disease models. Nat. Neurosci. 26, 416–429 (2023). This study shows that a BBB transport vehicle-enabled TREM2 agonist enhances CNS exposure and boosts microglial metabolism and function in AD models, exemplifying a translational strategy to pharmacologically tune genetically implicated microglial pathways.
Masuda, T. et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392 (2019).
Guneykaya, D. et al. Transcriptional and translational differences of microglia from male and female brains. Cell Rep. 24, 2773–2783.e6 (2018).
Qiu, Y. et al. Definition of the contribution of an osteopontin-producing CD11c+ microglial subset to Alzheimer’s disease. Proc. Natl Acad. Sci. USA 120, e2218915120 (2023). This work defines the contribution of an osteopontin-producing CD11c+ microglial subset to AD.
Weerakkody, T. N. et al. Loss of PILRA promotes microglial immunometabolism to reduce amyloid pathology in cell and mouse models of Alzheimer’s disease. Sci. Transl. Med. 17, eadw7428 (2025). This work identifies functional effects of loss of PILRA in microglia and demonstrates antibody-mediated inhibition as a potential therapeutic approach for AD.
Schlepckow, K. et al. Enhancing protective microglial activities with a dual function TREM2 antibody to the stalk region. EMBO Mol. Med. 12, e11227 (2020).
Feiten, A. F. et al. TREM2 expression level is critical for microglial state, metabolic capacity and efficacy of TREM2 agonism. Preprint at bioRxiv https://doi.org/10.1101/2024.07.18.604115 (2024).
Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015). This analysis demonstrates that drug targets supported by human genetic evidence have a significantly higher rate of success in clinical development.
Kleinberger, G. et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6, 243ra286 (2014).
Morenas-Rodriguez, E. et al. Soluble TREM2 in CSF and its association with other biomarkers and cognition in autosomal-dominant Alzheimer’s disease: a longitudinal observational study. Lancet Neurol. 21, 329–341 (2022).
Cheng, Q. et al. TREM2-activating antibodies abrogate the negative pleiotropic effects of the Alzheimer’s disease variant Trem2R47H on murine myeloid cell function. J. Biol. Chem. 293, 12620–12633 (2018).
Alector announces results from AL002 INVOKE-2 Phase 2 Trial in individuals with early Alzheimer’s disease and provides business update. Alector https://investors.alector.com/news-releases/news-release-details/alector-announces-results-al002-invoke-2-phase-2-trial (2024).
Jack, C. R. Jr. et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013).
Lee, S. et al. APOE modulates microglial immunometabolism in response to age, amyloid pathology, and inflammatory challenge. Cell Rep. 42, 112196 (2023).
Andreone, B. J. et al. Alzheimer’s-associated PLCγ2 is a signaling node required for both TREM2 function and the inflammatory response in human microglia. Nat. Neurosci. 23, 927–938 (2020).
Marschallinger, J. et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 23, 194–208 (2020). In this work, the authors demonstrate that lipid droplet-accumulating microglia are functionally defective, which provides insights into specific microglial activities that could contribute to disease.
Guillot-Sestier, M.-V. et al. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun. Biol. 4, 711 (2021).
Kim, S. et al. Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer’s disease: a CREDOS study. Compr. Psychiatry 62, 114–122 (2015).
Hu, Y. et al. Dual roles of hexokinase 2 in shaping microglial function by gating glycolytic flux and mitochondrial activity. Nat. Metab. 4, 1756–1774 (2022).
Minhas, P. S. et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 590, 122–128 (2021).
Xiang, X. et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci. Transl. Med. 13, eabe5640 (2021).
Baik, S. H. et al. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab. 30, 493–507.e6 (2019).
Litvinchuk, A. et al. Amelioration of tau and ApoE4-linked glial lipid accumulation and neurodegeneration with an LXR agonist. Neuron 112, 384–403.e8 (2024).
Fessler, M. B. The challenges and promise of targeting the liver X receptors for treatment of inflammatory disease. Pharmacol. Ther. 181, 1–12 (2018).
Holstege, H. et al. Exome sequencing identifies rare damaging variants in ATP8B4 and ABCA1 as risk factors for Alzheimer’s disease. Nat. Genet. 54, 1786–1794 (2022).
Noveir, S. D. et al. Effect of the ABCA1 agonist CS-6253 on amyloid-β and lipoprotein metabolism in cynomolgus monkeys. Alzheimers Res. Ther. 14, 87 (2022).
Choi, H. Y., Choi, S., Iatan, I., Ruel, I. & Genest, J. Biomedical advances in ABCA1 transporter: from bench to bedside. Biomedicines https://doi.org/10.3390/biomedicines11020561 (2023).
Yang, D. et al. G protein-coupled receptors: structure- and function-based drug discovery. Signal Transduct. Target. Ther. 6, 7 (2021).
Lin, L. L. et al. GPR34 knockdown relieves cognitive deficits and suppresses neuroinflammation in Alzheimer’s disease via the ERK/NF-κB signal. Neuroscience 528, 129–139 (2023).
Raju, K. et al. GPR34 regulates microglia state and loss-of-function rescues TREM2 metabolic dysfunction. Preprint at bioRxiv https://doi.org/10.1101/2025.03.28.646038 (2025).
Rathore, N. et al. Paired immunoglobulin-like type 2 receptor alpha G78R variant alters ligand binding and confers protection to Alzheimer’s disease. PLoS Genet. 14, e1007427 (2018).
Lopatko Lindman, K. et al. PILRA polymorphism modifies the effect of APOE4 and GM17 on Alzheimer’s disease risk. Sci. Rep. 12, 13264 (2022).
Lu, Q. et al. PILRα and PILRβ have a siglec fold and provide the basis of binding to sialic acid. Proc. Natl Acad. Sci. USA 111, 8221–8226 (2014).
Selkoe, D. J. Alzheimer’s disease is a synaptic failure. Science 298, 789–791 (2002).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016). This study demonstrates that complement-tagged synapses are eliminated by microglia early in AD models, providing a mechanistic link between the classical complement cascade, immune signalling and pathological synapse loss.
Dunkelberger, J. R. & Song, W. C. Complement and its role in innate and adaptive immune responses. Cell Res. 20, 34–50 (2010).
Dejanovic, B. et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2, 837–850 (2022).
Wu, T. et al. Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 28, 2111–2123.e6 (2019).
Dejanovic, B. et al. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron 100, 1322–1336.e7 (2018).
Lui, H. et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935 (2016).
Stevens, B. et al. The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178 (2007). This foundational work shows that the classical complement cascade instructs microglial synapse elimination in development, establishing a paradigm for immune sculpting of neural circuits that underpins later disease studies.
Asavapanumas, N., Tradtrantip, L. & Verkman, A. S. Targeting the complement system in neuromyelitis optica spectrum disorder. Expert Opin. Biol. Ther. 21, 1073–1086 (2021).
Desai, D. & Dugel, P. U. Complement cascade inhibition in geographic atrophy: a review. Eye 36, 294–302 (2022).
Lansita, J. A. et al. Nonclinical development of ANX005: a humanized anti-C1q antibody for treatment of autoimmune and neurodegenerative diseases. Int. J. Toxicol. 36, 449–462 (2017).
Daskoulidou, N. et al. Complement receptor 1 is expressed on brain cells and in the human brain. Glia 71, 1522–1535 (2023).
Zelek, W. M., Bevan, R. J. & Morgan, B. P. Targeting terminal pathway reduces brain complement activation, amyloid load and synapse loss, and improves cognition in a mouse model of dementia. Brain Behav. Immun. 118, 355–363 (2024).
Filipello, F. et al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity 48, 979–991.e8 (2018).
Li, T. et al. A splicing isoform of GPR56 mediates microglial synaptic refinement via phosphatidylserine binding. EMBO J. 39, e104136 (2020).
Scott-Hewitt, N. et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 39, e105380 (2020).
Gunner, G. et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 22, 1075–1088 (2019).
Zhou, J. et al. The neuronal pentraxin Nptx2 regulates complement activity and restrains microglia-mediated synapse loss in neurodegeneration. Sci. Transl. Med. 15, eadf0141 (2023).
Wang, Y. et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071 (2015). By revealing that TREM2 is a lipid-sensing receptor required to sustain microglial responses in AD models, this paper mechanistically connects a major genetic risk factor to disease-relevant microglial function.
Erturk, A., Wang, Y. & Sheng, M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J. Neurosci. 34, 1672–1688 (2014).
Bader Lange, M. L. et al. Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol. Dis. 29, 456–464 (2008).
Chang, M. C. et al. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat. Neurosci. 13, 1090–1097 (2010).
Carpanini, S. M. et al. Terminal complement pathway activation drives synaptic loss in Alzheimer’s disease models. Acta Neuropathol. Commun. 10, 99 (2022).
Rua, R. & McGavern, D. B. Advances in meningeal immunity. Trends Mol. Med. 24, 542–559 (2018).
Lun, M. P., Monuki, E. S. & Lehtinen, M. K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 16, 445–457 (2015).
Profaci, C. P., Munji, R. N., Pulido, R. S. & Daneman, R. The blood-brain barrier in health and disease: important unanswered questions. J. Exp. Med. https://doi.org/10.1084/jem.20190062 (2020).
Mastorakos, P. & McGavern, D. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aav0492 (2019).
Merlini, A. et al. Distinct roles of the meningeal layers in CNS autoimmunity. Nat. Neurosci. 25, 887–899 (2022).
Xu, H. et al. The choroid plexus synergizes with immune cells during neuroinflammation. Cell 187, 4946–4963.e17 (2024).
Goldmann, T. et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805 (2016).
Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 72, 648–672 (2012).
Chow, B. W. & Gu, C. The molecular constituents of the blood-brain barrier. Trends Neurosci. 38, 598–608 (2015).
Montagne, A. et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015).
Jellinger, K. A. Pathology and pathogenesis of vascular cognitive impairment — a critical update. Front. Aging Neurosci. 5, 17 (2013).
Jellinger, K. A. & Attems, J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol. 119, 421–433 (2010).
Kuo, P. Y. et al. Differences in lobar microbleed topography in cerebral amyloid angiopathy and hypertensive arteriopathy. Sci. Rep. 14, 3774 (2024).
Kiani Shabestari, S. et al. Absence of microglia promotes diverse pathologies and early lethality in Alzheimer’s disease mice. Cell Rep. 39, 110961 (2022).
Fujita, M. et al. Cell subtype-specific effects of genetic variation in the Alzheimer’s disease brain. Nat. Genet. https://doi.org/10.1038/s41588-024-01685-y (2024).
Shade, L. M. P. et al. GWAS of multiple neuropathology endophenotypes identifies new risk loci and provides insights into the genetic risk of dementia. Nat. Genet. 56, 2407–2421 (2024).
Tayler, H., Miners, J. S., Guzel, O., MacLachlan, R. & Love, S. Mediators of cerebral hypoperfusion and blood-brain barrier leakiness in Alzheimer’s disease, vascular dementia and mixed dementia. Brain Pathol. 31, e12935 (2021).
Storck, S. E. et al. Endothelial LRP1 transports amyloid-β(1-42) across the blood-brain barrier. J. Clin. Invest. 126, 123–136 (2016).
Zhao, Z. et al. Central role for PICALM in amyloid-β blood-brain barrier transcytosis and clearance. Nat. Neurosci. 18, 978–987 (2015).
Dulken, B. W. et al. Single-cell analysis reveals T cell infiltration in old neurogenic niches. Nature 571, 205–210 (2019).
Uekawa, K. et al. Border-associated macrophages promote cerebral amyloid angiopathy and cognitive impairment through vascular oxidative stress. Mol. Neurodegen. 18, 73 (2023).
Anfray, A. et al. A cell-autonomous role of border-associated macrophages in ApoE4 neurovascular dysfunction and susceptibility to white matter injury. Nat. Neurosci. 27, 2138–2151 (2024).
Hawkes, C. A. & McLaurin, J. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 106, 1261–1266 (2009).
Solopova, E. et al. Fatal iatrogenic cerebral β-amyloid-related arteritis in a woman treated with lecanemab for Alzheimer’s disease. Nat. Commun. 14, 8220 (2023).
Schonhoff, A. M. et al. Border-associated macrophages mediate the neuroinflammatory response in an alpha-synuclein model of Parkinson disease. Nat. Commun. 14, 3754 (2023).
Carare, R. O. et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 34, 131–144 (2008).
Brioschi, S. et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science https://doi.org/10.1126/science.abf9277 (2021).
Cugurra, A. et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science https://doi.org/10.1126/science.abf7844 (2021).
Rustenhoven, J. et al. Functional characterization of the dural sinuses as a neuroimmune interface. Cell 184, 1000–1016.e27 (2021).
Dani, N. et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell 184, 3056–3074.e21 (2021).
Lehtinen, M. K. et al. The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J. Neurosci. 33, 17553–17559 (2013).
Damkier, H. H., Brown, P. D. & Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 93, 1847–1892 (2013).
Laman, J. D. & Weller, R. O. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J. Neuroimmune Pharmacol. 8, 840–856 (2013).
Engelhardt, B., Vajkoczy, P. & Weller, R. O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 18, 123–131 (2017).
Ma, Q., Ineichen, B. V., Detmar, M. & Proulx, S. T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 8, 1434 (2017).
Goldmann, J. et al. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J. Leukoc. Biol. 80, 797–801 (2006).
Fitzpatrick, Z. et al. Venous-plexus-associated lymphoid hubs support meningeal humoral immunity. Nature https://doi.org/10.1038/s41586-024-07202-9 (2024).
Herisson, F. et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci. 21, 1209–1217 (2018).
Proulx, S. T. & Engelhardt, B. Central nervous system zoning: how brain barriers establish subdivisions for CNS immune privilege and immune surveillance. J. Intern. Med. 292, 47–67 (2022).
Smyth, L. C. D. et al. Identification of direct connections between the dura and the brain. Nature 627, 165–173 (2024).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
Smith, A. J., Yao, X., Dix, J. A., Jin, B. J. & Verkman, A. S. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife https://doi.org/10.7554/eLife.27679 (2017).
Morris, A. W. et al. Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 131, 725–736 (2016).
Szentistvanyi, I., Patlak, C. S., Ellis, R. A. & Cserr, H. F. Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol. 246, F835–F844 (1984).
Hladky, S. B. & Barrand, M. A. The glymphatic hypothesis: the theory and the evidence. Fluids Barriers CNS 19, 9 (2022).
Abbott, N. J., Pizzo, M. E., Preston, J. E., Janigro, D. & Thorne, R. G. The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol. 135, 387–407 (2018).
Hawkes, C. A. et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 121, 431–443 (2011).
Da Mesquita, S. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 560, 185–191 (2018).
Antila, S. et al. Sustained meningeal lymphatic vessel atrophy or expansion does not alter Alzheimer’s disease-related amyloid pathology. Nat. Cardiovasc. Res. https://doi.org/10.1038/s44161-024-00445-9 (2024).
Merlini, M. et al. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an Alzheimer’s disease model. Neuron 101, 1099–1108.e6 (2019).
Senatorov, V. V. Jr. et al. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFβ signaling and chronic yet reversible neural dysfunction. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaw8283 (2019).
Martin, M. et al. Engineered Wnt ligands enable blood-brain barrier repair in neurological disorders. Science 375, eabm4459 (2022).
Munji, R. N. et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain barrier dysfunction module. Nat. Neurosci. 22, 1892–1902 (2019).
Bryant, A. et al. Endothelial cells are heterogeneous in different brain regions and are dramatically altered in Alzheimer’s disease. J. Neurosci. 43, 4541–4557 (2023).
Sun, N. et al. Single-nucleus multiregion transcriptomic analysis of brain vasculature in Alzheimer’s disease. Nat. Neurosci. 26, 970–982 (2023).
Zhao, L. et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat. Commun. 11, 4413 (2020).
Yang, A. C. et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430 (2020).
Bien-Ly, N. et al. Lack of widespread BBB disruption in Alzheimer’s disease models: focus on therapeutic antibodies. Neuron 88, 289–297 (2015).
Pizzo, M. E. et al. Transferrin receptor-targeted anti-amyloid antibody enhances brain delivery and mitigates ARIA. Science 389, eads3204 (2025).
Grimm, H. P. et al. Delivery of the Brainshuttle amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans. MAbs 15, 2261509 (2023). This work describes an anti-amyloid antibody engineered to cross the BBB through engagement of Transferrin receptor, which results in improved plaque clearance.
Kariolis, M. S. et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aay1359 (2020).
Chew, K. S. et al. CD98hc is a target for brain delivery of biotherapeutics. Nat. Commun. 14, 5053 (2023).
Piehl, N. et al. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell 185, 5028–5039.e13 (2022).
Ramakrishnan, A. et al. Epigenetic dysregulation in Alzheimer’s disease peripheral immunity. Neuron https://doi.org/10.1016/j.neuron.2024.01.013 (2024).
Krogsgaard, M. & Davis, M. M. How T cells ‘see’ antigen. Nat. Immunol. 6, 239–245 (2005).
Smith-Garvin, J. E., Koretzky, G. A. & Jordan, M. S. T cell activation. Annu. Rev. Immunol. 27, 591–619 (2009).
Marchetti, L. & Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc. Biol. 2, H1–H18 (2020).
Schlager, C. et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530, 349–353 (2016).
Carrithers, M. D., Visintin, I., Kang, S. J. & Janeway, C. A. Jr. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 123, 1092–1101 (2000).
Reboldi, A. et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10, 514–523 (2009).
Comi, C., Fleetwood, T. & Dianzani, U. The role of T cell apoptosis in nervous system autoimmunity. Autoimmun. Rev. 12, 150–156 (2012).
Pappalardo, J. L. et al. Transcriptomic and clonal characterization of T cells in the human central nervous system. Sci. Immunol. https://doi.org/10.1126/sciimmunol.abb8786 (2020).
Schafflick, D. et al. Integrated single cell analysis of blood and cerebrospinal fluid leukocytes in multiple sclerosis. Nat. Commun. 11, 247 (2020).
Pasciuto, E. et al. Microglia require CD4 T cells to complete the fetal-to-adult transition. Cell 182, 625–640.e4 (2020). This study defines a functional link between T cells and microglial development, with measurable effects on brain development.
Ribeiro, M. et al. Meningeal γδ T cell-derived IL-17 controls synaptic plasticity and short-term memory. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aay5199 (2019).
Derecki, N. C. et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 207, 1067–1080 (2010).
Filiano, A. J. et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 535, 425–429 (2016).
Brynskikh, A., Warren, T., Zhu, J. & Kipnis, J. Adaptive immunity affects learning behavior in mice. Brain Behav. Immun. 22, 861–869 (2008).
Mousa, A. & Bakhiet, M. Role of cytokine signaling during nervous system development. Int. J. Mol. Sci. 14, 13931–13957 (2013).
Kaya, T. et al. CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat. Neurosci. 25, 1446–1457 (2022).
Togo, T. et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 124, 83–92 (2002).
Merlini, M., Kirabali, T., Kulic, L., Nitsch, R. M. & Ferretti, M. T. Extravascular CD3+ T cells in brains of Alzheimer disease patients correlate with tau but not with amyloid pathology: an immunohistochemical study. Neurodegener. Dis. 18, 49–56 (2018).
Sulzer, D. et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546, 656–661 (2017).
Williams, G. P. et al. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain 144, 2047–2059 (2021).
Marsh, S. E. et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl Acad. Sci. USA 113, E1316–E1325 (2016).
Perlmutter, L. S., Scott, S. A., Barron, E. & Chui, H. C. MHC class II-positive microglia in human brain: association with Alzheimer lesions. J. Neurosci. Res. 33, 549–558 (1992).
Faridar, A. et al. Restoring regulatory T-cell dysfunction in Alzheimer’s disease through ex vivo expansion. Brain Commun. 2, fcaa112 (2020).
Marin-Rodero, M. et al. The meninges host a distinct compartment of regulatory T cells that preserves brain homeostasis. Sci. Immunol. 10, eadu2910 (2025).
Dansokho, C. et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251 (2016).
Baek, H. et al. Neuroprotective effects of CD4+CD25+Foxp3+regulatory T cells in a 3xTg-AD Alzheimer’s disease model. Oncotarget 7, 69347–69357 (2016).
Baruch, K. et al. Breaking immune tolerance by targeting Foxp3+ regulatory T cells mitigates Alzheimer’s disease pathology. Nat. Commun. 6, 7967 (2015).
Su, W. et al. CXCR6 orchestrates brain CD8+ T cell residency and limits mouse Alzheimer’s disease pathology. Nat. Immunol. 24, 1735–1747 (2023).
Jorfi, M. et al. Infiltrating CD8+ T cells exacerbate Alzheimer’s disease pathology in a 3D human neuroimmune axis model. Nat. Neurosci. 26, 1489–1504 (2023).
Wu, C. T. et al. A change of PD-1/PD-L1 expression on peripheral T cell subsets correlates with the different stages of Alzheimer’s disease. Cell Biosci. 12, 162 (2022).
Thonhoff, J. R. et al. Combined regulatory T-lymphocyte and IL-2 treatment is safe, tolerable, and biologically active for 1 year in persons with amyotrophic lateral sclerosis. Neurol. Neuroimmunol. Neuroinflamm. https://doi.org/10.1212/NXI.0000000000200019 (2022).
van der Lee, S. J. et al. A nonsynonymous mutation in PLCG2 reduces the risk of Alzheimer’s disease, dementia with Lewy bodies and frontotemporal dementia, and increases the likelihood of longevity. Acta Neuropathol. 138, 237–250 (2019).
Diks, A. M. et al. Carriers of the p.P522R variant in PLCγ2 have a slightly more responsive immune system. Mol. Neurodegener. 18, 25 (2023). This study demonstrates a protective PLCG2 variant affected peripheral immune cell function, suggesting a connection between activity of the peripheral immune system and reduced AD risk.
Takalo, M. et al. The Alzheimer’s disease-associated protective Plcγ2-P522R variant promotes immune functions. Mol. Neurodegener. 15, 52 (2020).
Tsai, A. P. et al. Genetic variants of phospholipase C-γ2 alter the phenotype and function of microglia and confer differential risk for Alzheimer’s disease. Immunity 56, 2121–2136.e6 (2023).
Magno, L., Bunney, T. D., Mead, E., Svensson, F. & Bictash, M. N. TREM2/PLCγ2 signalling in immune cells: function, structural insight, and potential therapeutic modulation. Mol. Neurodegener. 16, 22 (2021).
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–1458 (2013).
Olufunmilayo, E. O. & Holsinger, R. M. D. INPP5D/SHIP1: expression, regulation and roles in Alzheimer’s disease pathophysiology. Genes https://doi.org/10.3390/genes14101845 (2023).
Chou, V. et al. INPP5D regulates inflammasome activation in human microglia. Nat. Commun. 14, 7552 (2023).
Pedicone, C. et al. Pan-SHIP1/2 inhibitors promote microglia effector functions essential for CNS homeostasis. J. Cell Sci. https://doi.org/10.1242/jcs.238030 (2020).
Pedicone, C. et al. Discovery of a novel SHIP1 agonist that promotes degradation of lipid-laden phagocytic cargo by microglia. iScience 25, 104170 (2022).
Gall, A. et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36, 120–131 (2012).
Govindarajulu, M. et al. Role of cGAS-Sting signaling in Alzheimer’s disease. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24098151 (2023).
Decout, A., Katz, J. D., Venkatraman, S. & Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Hong, Z., Mei, J., Guo, H., Zhu, J. & Wang, C. Intervention of cGAS–STING signaling in sterile inflammatory diseases. J. Mol. Cell Biol. https://doi.org/10.1093/jmcb/mjac005 (2022).
Zhu, Z. et al. Development of VHL-recruiting STING PROTACs that suppress innate immunity. Cell Mol. Life Sci. 80, 149 (2023).
Sala Frigerio, C. et al. The major risk factors for Alzheimer’s disease: age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep. 27, 1293–1306.e6 (2019).
Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat. Genet. 42, 985–990 (2010).
Fox, R. J. et al. Tolebrutinib in nonrelapsing secondary progressive multiple sclerosis. N. Engl. J. Med. 392, 1883–1892 (2025).
Tolebrutinib designated breakthrough therapy by the FDA for non-relapsing secondary progressive multiple sclerosis. Sanofi https://www.sanofi.com/en/media-room/press-releases/2024/2024-12-13-06-00-00-2996609 (2024).
Yshii, L. et al. Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation. Nat. Immunol. 23, 878–891 (2022).
Doglio, M. et al. Regulatory T cells expressing CD19-targeted chimeric antigen receptor restore homeostasis in systemic lupus erythematosus. Nat. Commun. 15, 2542 (2024).
Ossenkoppele, R., van der Kant, R. & Hansson, O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 21, 726–734 (2022).
Salvado, G. et al. Disease staging of Alzheimer’s disease using a CSF-based biomarker model. Nat. Aging https://doi.org/10.1038/s43587-024-00599-y (2024).
Johnson, E. C. B. et al. Large-scale proteomic analysis of Alzheimer’s disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat. Med. 26, 769–780 (2020).
Chai, Y. L. et al. Plasma osteopontin as a biomarker of Alzheimer’s disease and vascular cognitive impairment. Sci. Rep. 11, 4010 (2021).
Long, H. et al. Preclinical and first-in-human evaluation of AL002, a novel TREM2 agonistic antibody for Alzheimer’s disease. Alzheimers Res. Ther. 16, 235 (2024).
Pesamaa, I. et al. A microglial activity state biomarker panel differentiates FTD-granulin and Alzheimer’s disease patients from controls. Mol. Neurodegener. 18, 70 (2023). This work identifies candidate microglial biomarkers that could hold potential for monitoring the state of this key disease-relevant cell type in accessible patient biofluids.
Shojaei, M. et al. PET imaging of microglia in Alzheimer’s disease using copper-64 labeled TREM2 antibodies. Theranostics 14, 6319–6336 (2024).
Phongpreecha, T. et al. Single-cell peripheral immunoprofiling of Alzheimer’s and Parkinson’s diseases. Sci. Adv. https://doi.org/10.1126/sciadv.abd5575 (2020).
Salloway, S. et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 79, 13–21 (2022).
Cadiz, M. P. et al. Aducanumab anti-amyloid immunotherapy induces sustained microglial and immune alterations. J. Exp. Med. https://doi.org/10.1084/jem.20231363 (2024).
Wang, S. et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. https://doi.org/10.1084/jem.20200785 (2020).
Larson, K. C. et al. VGL101: an immunotherapy that enhances microglial survival for adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) (P9-9.013). Neurology 102, 2490 (2024).
Kumar, R. et al. A phase 2 open-label study to assess the safety, tolerability, pharmacokinetics, and pharmacodynamics of intravenous ANX005 in patients with, or at risk of, manifest Huntington’s disease (HD) (S32.009). Neurology 100, 3366 (2023).
Kantor, A. B., Akassoglou, K. & Stavenhagen, J. B. Fibrin-targeting immunotherapy for dementia. J. Prev. Alzheimers Dis. 10, 647–660 (2023).
Hincelin-Mery, A. et al. Safety, pharmacokinetics, and target engagement of a brain penetrant RIPK1 inhibitor, SAR443820 (DNL788), in healthy adult participants. Clin. Transl. Sci. 17, e13690 (2024).
Harrison, D. et al. Discovery of clinical candidate NT-0796, a brain-penetrant and highly potent NLRP3 inflammasome inhibitor for neuroinflammatory disorders. J. Med. Chem. 66, 14897–14911 (2023).
Tengesdal, I. W., Banks, M., Dinarello, C. A. & Marchetti, C. Screening NLRP3 drug candidates in clinical development: lessons from existing and emerging technologies. Front. Immunol. 15, 1422249 (2024).
Wells, R. C. et al. Dual targeting of transferrin receptor and CD98hc enhances brain exposure of large molecules. Cell Rep. 44, 116038 (2025).
An, S. et al. A brain-shuttled antibody targeting alpha synuclein aggregates for the treatment of synucleinopathies. npj Parkinsons Dis. 11, 254 (2025).
Acknowledgements
The authors thank S. V. Andrews for contributing expertise in interpreting and characterizing human genetics data. The authors also thank colleagues, especially C. Koth, for the extraordinary feedback on this manuscript during conceptualization and writing phases. S.H. is supported by the UK Dementia Research Institute (UKDRI-1011) through UK DRI Ltd, principally funded by the UK Medical Research Council, Chan Zuckerberg Initiative Neurodegeneration Challenge Network, BrightFocus Foundation (A2021032S), Alzheimer’s Association, Alzheimer’s Society UK and the Anonymous Foundation. A.C.Y. is supported by the Cure Alzheimer’s Fund, BrightFocus Foundation, Alzheimer’s Association and NIH Director’s Early Independence Award (1DP5OD033381).
Author information
Authors and Affiliations
Contributions
K.M.M. researched data for the article and contributed substantially to the discussion of the content. All authors wrote and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
K.M.M. and J.W.L. are full-time employees and shareholders of Denali Therapeutics Inc. S.H. has been a paid consultant for Eisai Ltd, Novo Nordisk and Alnylam; receives research funding from AstraZeneca and Eisai Ltd; and collaborates with Ionis Ltd.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Andrew Mendiola and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Aicardi–Goutières syndrome
-
A genetic leukodystrophy in which the immune system damages the myelin sheaths that protect neurons in the brain and spinal cord; symptoms include neurological defects such as developmental regression, seizures, microcephaly and poor feeding behaviour.
- Amyloid mouse model
-
A mouse strain that develops amyloid plaque deposition in the brain, a hallmark pathology of the Alzheimer’s disease (AD), driven by genetic modification of amyloid precursor protein (APP) with one or more mutations found in human disease.
- Amyloid-related imaging abnormalities
-
(ARIA). Defined using MRI and caused by disruption of blood vessels in the brain with amyloid deposition; the risk of ARIA is increased with some anti-amyloid antibody therapies.
- Astrocyte end-feet
-
Processes that extend from astrocytes that ensheathe blood vessels and provide structural integrity to the vasculature in the brain.
- Cerebral amyloid angiopathy
-
(CAA). A type of cerebrovascular disease characterized by amyloid deposition with cerebral blood vessels that can lead to cognitive impairment, microbleeds and other neurological symptoms.
- Glia limitans
-
The outermost barrier of the CNS parenchyma, formed by astrocyte end-feet and a basement membrane that ensheathes the brain surface and lines perivascular spaces wherein it functions as a secondary checkpoint behind the endothelial blood–brain barrier.
- Neuromyelitis optica spectrum disorder
-
An autoimmune disease in which optic nerve and spinal cord are damaged by autoantibodies; symptoms include vision loss, pain, numbness, weakness and paralysis that can worsen over time.
- Pericytes
-
Cells that wrap around blood vessel walls and contribute to blood–brain barrier integrity and regulate blood flow.
- Synaptic engulfment
-
The selective removal of synapses that mediate neuronal signalling; this process is mediated by microglia via the complement pathway during normal development of the CNS and during disease.
- Tauopathy mouse model
-
A mouse strain that recapitulates tau pathology that occurs in neurodegenerative diseases including frontotemporal dementia (FTD) and AD; tangles of tau protein form in neurons and interfere with their health and function.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Monroe, K.M., Hong, S., Lewcock, J.W. et al. Therapeutic targeting of neuroimmune mechanisms in neurodegeneration. Nat Rev Drug Discov (2026). https://doi.org/10.1038/s41573-025-01370-7
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41573-025-01370-7