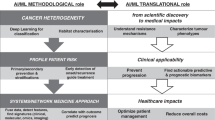
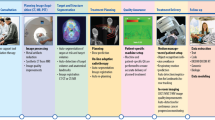
Abstract
Artificial intelligence (AI) has illuminated a clear path towards an evolving health-care system replete with enhanced precision and computing capabilities. Medical imaging analysis can be strengthened by machine learning as the multidimensional data generated by imaging naturally lends itself to hierarchical classification. In this Review, we describe the role of machine intelligence in image-based endocrine cancer diagnostics. We first provide a brief overview of AI and consider its intuitive incorporation into the clinical workflow. We then discuss how AI can be applied for the characterization of adrenal, pancreatic, pituitary and thyroid masses in order to support clinicians in their diagnostic interpretations. This Review also puts forth a number of key evaluation criteria for machine learning in medicine that physicians can use in their appraisals of these algorithms. We identify mitigation strategies to address ongoing challenges around data availability and model interpretability in the context of endocrine cancer diagnosis. Finally, we delve into frontiers in systems integration for AI, discussing automated pipelines and evolving computing platforms that leverage distributed, decentralized and quantum techniques.
Key points
-
Developments in machine intelligence have been made possible by the increase in data ubiquity and computing power and have the potential to enhance image segmentation, analysis and workflow in non-invasive endocrine cancer diagnostics.
-
Improved adherence to consensus reporting standards and evaluation criteria in artificial intelligence (AI) for medical image analysis is urgently needed in the field of endocrine cancer diagnostics as this will enable meaningful cross-study comparison.
-
A centralized inventory to track diagnostic algorithms in oncologic endocrinology that are in active clinical use would improve performance auditing and algorithm stewardship.
-
The looming risk of excessive intervention in endocrine cancers can be addressed with the improved detection facilitated by AI, possibly via correlation with prognostic data for improved risk stratification.
-
Poor data availability continues to stymie the development of robust machine learning applications, particularly in rare endocrine cancers; solutions to this problem might include database curation, pre-training techniques and workflow automation.
-
Other breakthroughs in machine intelligence will come with the exploration of alternative computing frameworks, such as decentralized, distributed and quantum networks, that might enhance model training and efficiency.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Goodfellow, I., Bengio, Y., Courville, A. & Bengio, Y. Deep learning. Vol. 1 (MIT Press, 2016).
Esteva, A. et al. A guide to deep learning in healthcare. Nat. Med. 25, 24–29 (2019). This Review provides an excellent primer on deep learning applications in medicine that covers a variety of modalities, including clinical, imaging, text and mixed data.
Hosny, A., Parmar, C., Quackenbush, J., Schwartz, L. H. & Aerts, H. J. W. L. Artificial intelligence in radiology. Nat. Rev. Cancer 18, 500–510 (2018).
Luo, Y. et al. Preoperative prediction of pancreatic neuroendocrine neoplasms grading based on enhanced computed tomography imaging: validation of deep learning with a convolutional neural network. Neuroendocrinology 110, 338–350 (2020). This paper finds the deep learning convolutional neural network approach to achieve the highest area under the curve in differentiating pancreatic NET grade 1–2 from grade 3 tumours, although convolutional neural network performance was not statistically different from that of the traditional machine learning models included in the study.
Qian, Y. et al. A novel diagnostic method for pituitary adenoma based on magnetic resonance imaging using a convolutional neural network. Pituitary 23, 246–252 (2020). A deep learning technique using convolutional neural networks to differentiate patients with pituitary adenoma from a mixed control group with both healthy and sellar lesion MRI scans.
Li, X. et al. Diagnosis of thyroid cancer using deep convolutional neural network models applied to sonographic images: a retrospective, multicohort, diagnostic study. Lancet Oncol. 20, 193–201 (2019). A large cohort study using a convolutional neural network-based approach to thyroid nodule diagnosis on ultrasound demonstrating comparable sensitivity and improved specificity when compared with a group of expert radiologists.
Wang, L. et al. Automatic thyroid nodule recognition and diagnosis in ultrasound imaging with the YOLOv2 neural network. World J. Surg. Oncol. 17, 12 (2019).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Chmielik, E. et al. Heterogeneity of thyroid cancer. Pathobiology 85, 117–129 (2018).
Topol, E. J. Individualized medicine from prewomb to tomb. Cell 157, 241–253 (2014).
Obermeyer, Z. & Emanuel, E. J. Predicting the future - big data, machine learning, and clinical medicine. N. Engl. J. Med. 375, 1216–1219 (2016).
Rao, A. et al. A combinatorial radiographic phenotype may stratify patient survival and be associated with invasion and proliferation characteristics in glioblastoma. J. Neurosurg. 124, 1008–1017 (2016).
Yamamoto, S., Maki, D. D., Korn, R. L. & Kuo, M. D. Radiogenomic analysis of breast cancer using MRI: a preliminary study to define the landscape. AJR Am. J. Roentgenol. 199, 654–663 (2012).
Aerts, H. J. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 5, 4006 (2014).
Zhao, C. K. et al. A comparative analysis of two machine learning-based diagnostic patterns with thyroid imaging reporting and data system for thyroid nodules: diagnostic performance and unnecessary biopsy rate. Thyroid 31, 470–481 (2021).
Gulshan, V. et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 316, 2402–2410 (2016).
Zhou, H. et al. Machine learning reveals multimodal MRI patterns predictive of isocitrate dehydrogenase and 1p/19q status in diffuse low- and high-grade gliomas. J. Neurooncol. 142, 299–307 (2019).
Liang, W. et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern. Med. 180, 1081–1089 (2020).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Davis, R. J. et al. Pan-cancer transcriptional signatures predictive of oncogenic mutations reveal that Fbw7 regulates cancer cell oxidative metabolism. Proc. Natl Acad. Sci. USA 115, 5462–5467 (2018).
Chang, E. K. et al. Defining a patient population with cirrhosis: an automated algorithm with natural language processing. J. Clin. Gastroenterol. 50, 889–894 (2016).
Bedi, G. et al. Automated analysis of free speech predicts psychosis onset in high-risk youths. NPJ Schizophrenia 1, 15030 (2015).
Yu, P. et al. FGF-dependent metabolic control of vascular development. Nature 545, 224–228 (2017).
Samuel, A. L. Some studies in machine learning using the game of checkers. IBM J. Res. Dev. 3, 210–229 (1959).
Kumar, V. et al. Radiomics: the process and the challenges. Magn. Reson. Imaging 30, 1234–1248 (2012).
Erickson, B. J., Korfiatis, P., Akkus, Z. & Kline, T. L. Machine learning for medical imaging. Radiographics 37, 505–515 (2017).
Guo, Y., Gao, Y. & Shen, D. Deformable MR prostate segmentation via deep feature learning and sparse patch matching. IEEE Trans. Med. Imaging 35, 1077–1089 (2016).
Wu, J. et al. A deep Boltzmann machine-driven level set method for heart motion tracking using cine MRI images. Med. Image Anal. 47, 68–80 (2018).
Sutton, R. S. & Barto, A. G. Introduction to Reinforcement Learning. Vol. 135 (MIT Press, 1998).
Bengio, Y., Courville, A. & Vincent, P. Representation learning: a review and new perspectives. IEEE Trans. Pattern Anal. Mach. Intell. 35, 1798–1828 (2013).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. 42, 60–88 (2017).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542, 115–118 (2017).
Naceur, M. B., Saouli, R., Akil, M. & Kachouri, R. Fully automatic brain tumor segmentation using end-to-end incremental deep neural networks in MRI images. Comput. Methods Prog. Biomed. 166, 39–49 (2018).
Lee, M. J. et al. Benign and malignant adrenal masses: CT distinction with attenuation coefficients, size, and observer analysis. Radiology 179, 415–418 (1991).
Song, J. H., Chaudhry, F. S. & Mayo-Smith, W. W. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am. J. Roentgenol. 190, 1163–1168 (2008).
Zeiger, M. et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr. Pract. 15, 1–20 (2009).
Bae, K. T., Fuangtharnthip, P., Prasad, S. R., Joe, B. N. & Heiken, J. P. Adrenal masses: CT characterization with histogram analysis method. Radiology 228, 735–742 (2003).
Ho, L. M., Paulson, E. K., Brady, M. J., Wong, T. Z. & Schindera, S. T. Lipid-poor adenomas on unenhanced CT: does histogram analysis increase sensitivity compared with a mean attenuation threshold? Am. J. Roentgenol. 191, 234–238 (2008).
Umanodan, T. et al. ADC histogram analysis for adrenal tumor histogram analysis of apparent diffusion coefficient in differentiating adrenal adenoma from pheochromocytoma. J. Magn. Reson. Imaging 45, 1195–1203 (2017).
Tüdös, Z. & Čtvrtlík, F. Possible impact of CT histogram analysis in incidentally discovered adrenal masses. Abdom. Radiol. 45, 2937–2938 (2020).
Alobaidli, S. et al. The role of texture analysis in imaging as an outcome predictor and potential tool in radiotherapy treatment planning. Br. J. Radiol. 87, 20140369 (2014).
Parekh, V. S. & Jacobs, M. A. Deep learning and radiomics in precision medicine. Expert Rev. Precis. Med. Drug Dev. 4, 59–72 (2019).
Ganeshan, B. & Miles, K. A. Quantifying tumour heterogeneity with CT. Cancer Imaging 13, 140–149 (2013).
Nieman, L. K. Approach to the patient with an adrenal incidentaloma. J. Clin. Endocrinol. Metab. 95, 4106–4113 (2010).
Iñiguez-Ariza, N. M. et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clin. Proc. Innov. Qual. Outcomes 2, 30–39 (2018).
Angeli, A., Osella, G., Alì, A. & Terzolo, M. Adrenal incidentaloma: an overview of clinical and epidemiological data from the National Italian Study Group. Horm. Res. 47, 279–283 (1997).
Elmohr, M. M. et al. Machine learning-based texture analysis for differentiation of large adrenal cortical tumours on CT. Clin. Radiol. 74, 818.e1–818.e7 (2019). This study establishes a radiomics signature to differentiate large adrenal tumours using random forest-based machine learning feature extraction coupled with CT attenuation score; model performance exceeded that of two expert radiologists.
Korobkin, M. et al. Differentiation of adrenal adenomas from nonadenomas using CT attenuation values. AJR Am. J. Roentgenol. 166, 531–536 (1996).
Patel, J., Davenport, M. S., Cohan, R. H. & Caoili, E. M. Can established CT attenuation and washout criteria for adrenal adenoma accurately exclude pheochromocytoma? Am. J. Roentgenol. 201, 122–127 (2013).
Northcutt, B. G., Trakhtenbroit, M. A., Gomez, E. N., Fishman, E. K. & Johnson, P. T. Adrenal adenoma and pheochromocytoma: comparison of multidetector CT venous enhancement levels and washout characteristics. J. Comput. Assist. Tomogr. 40, 194–200 (2016).
Yi, X. et al. Adrenal incidentaloma: machine learning-based quantitative texture analysis of unenhanced CT can effectively differentiate sPHEO from lipid-poor adrenal adenoma. J. Cancer 9, 3577–3582 (2018).
Yi, X. et al. Radiomics improves efficiency for differentiating subclinical pheochromocytoma from lipid-poor adenoma: a predictive, preventive and personalized medical approach in adrenal incidentalomas. EPMA J. 9, 421–429 (2018). This study uses machine learning with a LASSO model to differentiate subclinical pheochromocytoma from lipid-poor adenomas on CT with a sensitivity of 90% and sensitivity of 99%, albeit without an expert radiologist comparison group.
Romeo, V. et al. Characterization of adrenal lesions on unenhanced MRI using texture analysis: a machine-learning approach. J. Magn. Reson. Imaging 48, 198–204 (2018).
Barstugan, M., Ceylan, R., Asoglu, S., Cebeci, H. & Koplay, M. Adrenal tumor characterization on magnetic resonance images. Int. J. Imaging Syst. Technol. 30, 252–265 (2020).
Koyuncu, H., Ceylan, R., Asoglu, S., Cebeci, H. & Koplay, M. An extensive study for binary characterisation of adrenal tumours. Med. Biol. Eng. Comput. 57, 849–862 (2019).
Henley, D. J., van Heerden, J. A., Grant, C. S., Carney, J. A. & Carpenter, P. C. Adrenal cortical carcinoma — a continuing challenge. Surgery 94, 926–931 (1983).
Dasari, A. et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 3, 1335–1342 (2017).
Halfdanarson, T. R., Rabe, K. G., Rubin, J. & Petersen, G. M. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 19, 1727–1733 (2008).
Genç, C. G. et al. A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann. Surg. 267, 1148–1154 (2018).
Modlin, I. M. et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 9, 61–72 (2008).
Zerbi, A. et al. Clinicopathological features of pancreatic endocrine tumors: a prospective multicenter study in Italy of 297 sporadic cases. Am. J. Gastroenterol. 105, 1421–1429 (2010).
Manfredi, R. et al. Non-hyperfunctioning neuroendocrine tumours of the pancreas: MR imaging appearance and correlation with their biological behaviour. Eur. Radiol. 23, 3029–3039 (2013).
Inzani, F., Petrone, G. & Rindi, G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol. Metab. Clin. North. Am. 47, 463–470 (2018).
Rindi, G. & Wiedenmann, B. Neuroendocrine neoplasms of the gut and pancreas: new insights. Nat. Rev. Endocrinol. 8, 54 (2012).
Oronsky, B., Ma, P. C., Morgensztern, D. & Carter, C. A. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia 19, 991–1002 (2017).
Lee, N. J., Hruban, R. H. & Fishman, E. K. Pancreatic neuroendocrine tumor: review of heterogeneous spectrum of CT appearance. Abdom. Radiol. 43, 3025–3034 (2018).
Karmazanovsky, G. et al. Nonhypervascular pancreatic neuroendocrine tumors: Spectrum of MDCT imaging findings and differentiation from pancreatic ductal adenocarcinoma. Eur. J. Radiol. 110, 66–73 (2019).
Rösch, T. et al. Localization of pancreatic endocrine tumors by endoscopic ultrasonography. N. Engl. J. Med. 326, 1721–1726 (1992).
Song, Y. et al. Multiple machine learnings revealed similar predictive accuracy for prognosis of PNETs from the surveillance, epidemiology, and end result database. J. Cancer 9, 3971–3978 (2018).
Saleh, M. et al. New frontiers in imaging including radiomics updates for pancreatic neuroendocrine neoplasms. Abdom. Radiol. https://doi.org/10.1007/s00261-020-02833-8 (2020).
Zhao, Z. et al. CT-radiomic approach to predict G1/2 nonfunctional pancreatic neuroendocrine tumor. Acad. Radiol. 27, e272–e281 (2020).
Liang, W. et al. A combined nomogram model to preoperatively predict histologic grade in pancreatic neuroendocrine tumors. Clin. Cancer Res. 25, 584–594 (2019).
Gu, D. et al. CT radiomics may predict the grade of pancreatic neuroendocrine tumors: a multicenter study. Eur. Radiol. 29, 6880–6890 (2019).
Gao, X. & Wang, X. Deep learning for World Health Organization grades of pancreatic neuroendocrine tumors on contrast-enhanced magnetic resonance images: a preliminary study. Int. J. Comput. Assist. Radiol. Surg. 14, 1981–1991 (2019).
Choi, T. W., Kim, J. H., Yu, M. H., Park, S. J. & Han, J. K. Pancreatic neuroendocrine tumor: prediction of the tumor grade using CT findings and computerized texture analysis. Acta Radiol. 59, 383–392 (2018).
Duan, H., Baratto, L. & Iagaru, A. The role of PET/CT in the imaging of pancreatic neoplasms. Semin. Ultrasound CT MR 40, 500–508 (2019).
Zaharchuk, G. Next generation research applications for hybrid PET/MR and PET/CT imaging using deep learning. Eur. J. Nucl. Med. Mol. Imaging 46, 2700–2707 (2019).
Wei, L., Osman, S., Hatt, M. & El Naqa, I. Machine learning for radiomics-based multimodality and multiparametric modeling. Q. J. Nucl. Med. Mol. Imaging 63, 323–338 (2019).
Hidalgo, M. Pancreatic cancer. N. Engl. J. Med. 362, 1605–1617 (2010).
Cameron, J. L. et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am. J. Surg. 161, 120–124 (1991).
Guo, C. et al. The differentiation of pancreatic neuroendocrine carcinoma from pancreatic ductal adenocarcinoma: the values of CT imaging features and texture analysis. Cancer Imaging 18, 37 (2018).
Li, J. et al. Differentiation of atypical pancreatic neuroendocrine tumors from pancreatic ductal adenocarcinomas: Using whole-tumor CT texture analysis as quantitative biomarkers. Cancer Med. 7, 4924–4931 (2018).
Fu, M. et al. Hierarchical combinatorial deep learning architecture for pancreas segmentation of medical computed tomography cancer images. BMC Syst. Biol. 12, 56 (2018).
Man, Y., Huang, Y., Feng, J., Li, X. & Wu, F. Deep Q learning driven CT pancreas segmentation with geometry-aware U-net. IEEE Trans. Med. Imaging 38, 1971–1980 (2019).
Heinrich, M. P., Blendowski, M. & Oktay, O. TernaryNet: faster deep model inference without GPUs for medical 3D segmentation using sparse and binary convolutions. Int. J. Comput. Assist. Radiol. Surg. 13, 1311–1320 (2018).
Gibson, E. et al. Automatic multi-organ segmentation on abdominal CT with dense V-networks. IEEE Trans. Med. Imaging 37, 1822–1834 (2018).
Liang, Y. et al. Auto-segmentation of pancreatic tumor in multi-parametric MRI using deep convolutional neural networks. Radiother. Oncol. 145, 193–200 (2020).
Corral, J. E. et al. Deep learning to classify intraductal papillary mucinous neoplasms using magnetic resonance imaging. Pancreas 48, 805–810 (2019).
Kuwahara, T. et al. Usefulness of deep learning analysis for the diagnosis of malignancy in intraductal papillary mucinous neoplasms of the pancreas. Clin. Transl. Gastroenterol. 10, 1–8 (2019).
Hussein, S., Kandel, P., Bolan, C. W., Wallace, M. B. & Bagci, U. Lung and pancreatic tumor characterization in the deep learning era: novel supervised and unsupervised learning approaches. IEEE Trans. Med. Imaging 38, 1777–1787 (2019).
Molitch, M. E. Diagnosis and treatment of pituitary adenomas: a review. JAMA 317, 516–524 (2017).
Melmed, S. Pituitary-tumor endocrinopathies. N. Engl. J. Med. 382, 937–950 (2020).
Chahal, J. & Schlechte, J. Hyperprolactinemia. Pituitary 11, 141–146 (2008).
Vilar, L., Vilar, C. F., Lyra, R., Lyra, R. & Naves, L. A. Acromegaly: clinical features at diagnosis. Pituitary 20, 22–32 (2017).
Ntali, G. & Wass, J. A. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 21, 111–118 (2018).
Amlashi, F. G. & Tritos, N. A. Thyrotropin-secreting pituitary adenomas: epidemiology, diagnosis, and management. Endocrine 52, 427–440 (2016).
Varlamov, E. V., McCartney, S. & Fleseriu, M. Functioning pituitary adenomas — current treatment options and emerging medical therapies. Eur. Endocrinol. 15, 30–40 (2019).
Zamora, C. & Castillo, M. Sellar and parasellar imaging. Neurosurgery 80, 17–38 (2017).
Connor, S. E. & Penney, C. C. MRI in the differential diagnosis of a sellar mass. Clin. Radiol. 58, 20–31 (2003).
Kitajima, M. et al. Differentiation of common large sellar-suprasellar masses effect of artificial neural network on radiologists’ diagnosis performance. Acad. Radiol. 16, 313–320 (2009).
Zhang, S. et al. Non-invasive radiomics approach potentially predicts non-functioning pituitary adenomas subtypes before surgery. Eur. Radiol. 28, 3692–3701 (2018).
Zeynalova, A. et al. Preoperative evaluation of tumour consistency in pituitary macroadenomas: a machine learning-based histogram analysis on conventional T2-weighted MRI. Neuroradiology 61, 767–774 (2019).
Fan, Y. et al. Preoperative noninvasive radiomics approach predicts tumor consistency in patients with acromegaly: development and multicenter prospective validation. Front. Endocrinol. 10, 403 (2019). This prospective, multi-institutional machine learning study evaluates pituitary adenoma consistency in patients with acromegaly using a support vector machine-derived radiomics signature found to have a higher diagnostic accuracy than clinical characteristics alone.
Zhu, H., Fang, Q., Huang, Y. & Xu, K. Semi-supervised method for image texture classification of pituitary tumors via CycleGAN and optimized feature extraction. BMC Med. Inf. Decis. Mak. 20, 215 (2020). This study uses multiple deep learning techniques for pituitary texture analysis including a generative adversarial network for data augmentation followed by unsupervised feature extraction with a convolutional neural network-based auto-encoder framework that is then fed into a convolutional recurrent neural network for classification.
Yamamoto, J. et al. Tumor consistency of pituitary macroadenomas: predictive analysis on the basis of imaging features with contrast-enhanced 3D FIESTA at 3T. Am. J. Neuroradiol. 35, 297–303 (2014).
Iuchi, T., Saeki, N., Tanaka, M., Sunami, K. & Yamaura, A. MRI prediction of fibrous pituitary adenomas. Acta Neurochir. 140, 779–786 (1998).
Niu, J. et al. Preoperative prediction of cavernous sinus invasion by pituitary adenomas using a radiomics method based on magnetic resonance images. Eur. Radiol. 29, 1625–1634 (2019).
Staartjes, V. E. et al. Neural network-based identification of patients at high risk for intraoperative cerebrospinal fluid leaks in endoscopic pituitary surgery. J. Neurosurg. 133, 329–335 (2019).
Kitahara, C. M. & Sosa, J. A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 12, 646–653 (2016).
Cabanillas, M. E., McFadden, D. G. & Durante, C. Thyroid cancer. Lancet 388, 2783–2795 (2016).
Song, W. et al. Multitask cascade convolution neural networks for automatic thyroid nodule detection and recognition. IEEE J. Biomed. Health Inf. 23, 1215–1224 (2019).
Li, H. et al. An improved deep learning approach for detection of thyroid papillary cancer in ultrasound images. Sci. Rep. 8, 6600 (2018).
Ma, J., Wu, F., Zhu, J., Xu, D. & Kong, D. A pre-trained convolutional neural network based method for thyroid nodule diagnosis. Ultrasonics 73, 221–230 (2017).
Lim, K. J. et al. Computer-aided diagnosis for the differentiation of malignant from benign thyroid nodules on ultrasonography. Acad. Radiol. 15, 853–858 (2008).
Chi, J. et al. Thyroid nodule classification in ultrasound images by fine-tuning deep convolutional neural network. J. Digit. Imaging 30, 477–486 (2017).
Acharya, U. R. et al. Non-invasive automated 3D thyroid lesion classification in ultrasound: a class of ThyroScan™ systems. Ultrasonics 52, 508–520 (2012).
Tessler, F. N. et al. ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 14, 587–595 (2017).
Zhang, B. et al. Machine learning-assisted system for thyroid nodule diagnosis. Thyroid 29, 858–867 (2019).
Zhu, L. C. et al. A model to discriminate malignant from benign thyroid nodules using artificial neural network. PLoS One 8, e82211 (2013).
Song, G., Xue, F. & Zhang, C. A model using texture features to differentiate the nature of thyroid nodules on sonography. J. Ultrasound Med. 34, 1753–1760 (2015).
Xu, L. et al. Computer-aided diagnosis systems in diagnosing malignant thyroid nodules on ultrasonography: a systematic review and meta-analysis. Eur. Thyroid. J. 9, 186–193 (2020).
Shi, G. et al. Knowledge-guided synthetic medical image adversarial augmentation for ultrasonography thyroid nodule classification. Comput. Methods Prog. Biomed. 196, 105611 (2020).
Zhao, W. J., Fu, L. R., Huang, Z. M., Zhu, J. Q. & Ma, B. Y. Effectiveness evaluation of computer-aided diagnosis system for the diagnosis of thyroid nodules on ultrasound: a systematic review and meta-analysis. Medicine 98, e16379 (2019).
Buda, M. et al. Management of thyroid nodules seen on US images: deep learning may match performance of radiologists. Radiology 292, 695–701 (2019).
Jeong, E. Y. et al. Computer-aided diagnosis system for thyroid nodules on ultrasonography: diagnostic performance and reproducibility based on the experience level of operators. Eur. Radiol. 29, 1978–1985 (2019).
Sollini, M., Cozzi, L., Chiti, A. & Kirienko, M. Texture analysis and machine learning to characterize suspected thyroid nodules and differentiated thyroid cancer: where do we stand? Eur. J. Radiol. 99, 1–8 (2018).
Choi, Y. J. et al. A computer-aided diagnosis system using artificial intelligence for the diagnosis and characterization of thyroid nodules on ultrasound: initial clinical assessment. Thyroid 27, 546–552 (2017).
Daniels, K. et al. Machine learning by ultrasonography for genetic risk stratification of thyroid nodules. JAMA Otolaryngol. Head Neck Surg. 146, 36–41 (2020).
Kosilek, R. P. et al. Diagnostic use of facial image analysis software in endocrine and genetic disorders: review, current results and future perspectives. Eur. J. Endocrinol. 173, M39–44 (2015).
Kong, X., Gong, S., Su, L., Howard, N. & Kong, Y. Automatic detection of acromegaly from facial photographs using machine learning methods. EBioMedicine 27, 94–102 (2018). This study evaluates multiple machine learning and deep learning models to differentiate patients with acromegaly from facial photographs, with the top-performing ensemble model achieving a diagnostic accuracy that was on par with that of specialists and superior to that of primary care physicians.
Schneider, H. J. et al. A novel approach to the detection of acromegaly: accuracy of diagnosis by automatic face classification. J. Clin. Endocrinol. Metab. 96, 2074–2080 (2011).
Kosilek, R. P. et al. Automatic face classification of Cushing’s syndrome in women — a novel screening approach. Exp. Clin. Endocrinol. Diabetes 121, 561–564 (2013).
Popp, K. H. et al. Computer vision technology in the differential diagnosis of Cushing’s syndrome. Exp. Clin. Endocrinol. Diabetes 127, 685–690 (2019).
Dal, J. et al. Disease control and gender predict the socioeconomic effects of acromegaly: a nationwide cohort study. J. Clin. Endocrinol. Metab. 105, 2975–2982 (2020).
Gkourogianni, A. et al. Pediatric Cushing disease: disparities in disease severity and outcomes in the Hispanic and African-American populations. Pediatr. Res. 82, 272–277 (2017).
Lambin, P. et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 14, 749–762 (2017).
Mongan, J., Moy, L. & Kahn, C. E. Jr Checklist for artificial intelligence in medical imaging (CLAIM): a guide for authors and reviewers. Radiol. Artif. Intell. 2, e200029 (2020).
Liu, X., Cruz Rivera, S., Moher, D., Calvert, M. J. & Denniston, A. K. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat. Med. 26, 1364–1374 (2020).
Miotto, R., Wang, F., Wang, S., Jiang, X. & Dudley, J. T. Deep learning for healthcare: review, opportunities and challenges. Brief. Bioinform. 19, 1236–1246 (2018).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56 (2019).
Wang, F., Kaushal, R. & Khullar, D. Should health care demand interpretable artificial intelligence or accept “Black Box” Medicine? Ann. Intern. Med. 172, 59–60 (2019).
Murdoch, W. J., Singh, C., Kumbier, K., Abbasi-Asl, R. & Yu, B. Definitions, methods, and applications in interpretable machine learning. Proc. Natl Acad. Sci. USA 116, 22071–22080 (2019).
Reyes, M. et al. On the interpretability of artificial intelligence in radiology: challenges and opportunities. Radiol. Artif. Intell. 2, e190043 (2020).
Chattopadhay, A., Sarkar, A., Howlader, P. & Balasubramanian, V. N. Grad-CAM++: generalized gradient-based visual explanations for deep convolutional networks. IEEE Winter Conf. Appl. Comput. Vis. https://doi.org/10.1109/WACV.2018.00097 (2018).
Ribeiro, M. T., Singh, S. & Guestrin, C. Why Should I Trust You?: Explaining the Predictions of Any Classifier. in Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 1135-1144 (Association for Computing Machinery, 2016).
Akkus, Z. et al. Reduction of Unnecessary Thyroid Biopsies using Deep Learning. Vol. 10949 MI (SPIE, 2019).
Pereira, S. et al. Enhancing interpretability of automatically extracted machine learning features: application to a RBM-Random Forest system on brain lesion segmentation. Med. Image Anal. 44, 228–244 (2018).
Natekar, P., Kori, A. & Krishnamurthi, G. Demystifying brain tumor segmentation networks: interpretability and uncertainty analysis. Front. Comput. Neurosci. 14, 6 (2020).
Philbrick, K. A. et al. What does deep learning see? Insights from a classifier trained to predict contrast enhancement phase from CT images. Am. J. Roentgenol. 211, 1184–1193 (2018).
Thomas, J. & Haertling, T. AIBx, artificial intelligence model to risk stratify thyroid nodules. Thyroid 30, 878–884 (2020).
Gallego-Ortiz, C. & Martel, A. L. Using quantitative features extracted from T2-weighted MRI to improve breast MRI computer-aided diagnosis (CAD). PLoS One 12, e0187501 (2017).
Gale, W., Oakden-Rayner, L., Carneiro, G., Palmer, L. J. & Bradley, A. P. Producing radiologist-quality reports for interpretable deep learning. IEEE Int. Symp. Biomed. Imaging https://doi.org/10.1109/ISBI.2019.8759236 (2019).
Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020).
Shuja, J., Alanazi, E., Alasmary, W. & Alashaikh, A. COVID-19 open source data sets: a comprehensive survey. Appl. Intell. 51, 1296–1325 (2021).
Bai, H. X. & Thomasian, N. M. RICORD: a precedent for open AI in COVID-19 image analytics. Radiology 299, E219–E220 (2021).
Marcus, D. S., Olsen, T. R., Ramaratnam, M. & Buckner, R. L. The extensible neuroimaging archive toolkit: an informatics platform for managing, exploring, and sharing neuroimaging data. Neuroinformatics 5, 11–34 (2007).
Dao, T. et al. A kernel theory of modern data augmentation. Proc. Mach. Learn. Res. 97, 1528–1537 (2019).
Hussain, Z., Gimenez, F., Yi, D. & Rubin, D. Differential data augmentation techniques for medical imaging classification tasks. AMIA Annu. Symp. Proc. 2017, 979–984 (2017).
Deepak, S. & Ameer, P. M. Brain tumor classification using deep CNN features via transfer learning. Comput. Biol. Med. 111, 103345 (2019).
Shin, H. C. et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans. Med. Imaging 35, 1285–1298 (2016).
Sheller, M. J. et al. Federated learning in medicine: facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 10, 12598 (2020).
Chang, K. et al. Distributed deep learning networks among institutions for medical imaging. J. Am. Med. Inf. Assoc. 25, 945–954 (2018). This paper provides a helpful overview and empirical demonstration of distributed learning techniques for multi-institutional collaborative deep learning model training.
Sheller, M. J., Reina, G. A., Edwards, B., Martin, J. & Bakas, S. Multi-institutional deep learning modeling without sharing patient data: a feasibility study on brain tumor segmentation. Brainlesion 11383, 92–104 (2019).
Biamonte, J. et al. Quantum machine learning. Nature 549, 195–202 (2017).
Arute, F. et al. Quantum supremacy using a programmable superconducting processor. Nature 574, 505–510 (2019).
Princeton University Center for Information Technology Policy. Implications of quantum computing for encryption policy. CEIP. https://carnegieendowment.org/2019/04/25/implications-of-quantum-computing-for-encryption-policy-pub-78985 (2019).
Smets, E. et al. Large-scale wearable data reveal digital phenotypes for daily-life stress detection. NPJ Digital Med. 1, 67 (2018).
Tuncer, S. A. & Alkan, A. Segmentation of thyroid nodules with K-means algorithm on mobile devices. IEEE Int. Symp. Biomed. Imaging https://doi.org/10.1109/CINTI.2015.7382947 (2015).
Ma, J. et al. Efficient deep learning architecture for detection and recognition of thyroid nodules. Comput. Intell. Neurosci. 2020, 1242781 (2020).
Poudel, P., Illanes, A., Sheet, D. & Friebe, M. Evaluation of commonly used algorithms for thyroid ultrasound images segmentation and improvement using machine learning approaches. J. Healthc. Eng. 2018, 8087624 (2018).
Shin, H. C., Orton, M. R., Collins, D. J., Doran, S. J. & Leach, M. O. Stacked autoencoders for unsupervised feature learning and multiple organ detection in a pilot study using 4D patient data. IEEE Trans. Pattern Anal. Mach. Intell. 35, 1930–1943 (2013).
Song, J. et al. Ultrasound image analysis using deep learning algorithm for the diagnosis of thyroid nodules. Medicine 98, e15133 (2019).
Wang, H. et al. Machine learning-based multiparametric MRI radiomics for predicting the aggressiveness of papillary thyroid carcinoma. Eur. J. Radiol. 122, 108755 (2020). This study found a machine learning pipeline with LASSO for feature selection with a Gradient Boosting Classifier for classification that was superior to clinical characteristics in terms of preoperatively differentiating aggressive versus non-aggressive papillary thyroid carcinoma.
Haugen, B. R. et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26, 1–133 (2016).
Yi, T. et al. DICOM Image Analysis and Archive (DIANA): an open-source system for clinical AI applications. J. Digit. Imaging https://doi.org/10.1007/s10278-021-00488-5 (2021).
Perseguers, S., Lewenstein, M., Acín, A. & Cirac, J. I. Quantum random networks. Nat. Phys. 6, 539–543 (2010).
Biamonte, J., Faccin, M. & De Domenico, M. Complex networks from classical to quantum. Commun. Phys. 2, 53 (2019).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks J. Thomas and J. Wang for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
International Agency for Research on Cancer: https://gco.iarc.fr/
UK Biobank: https://www.ukbiobank.ac.uk/imaging-data/
US National Institute of Health Cancer Imaging Archive: https://www.cancerimagingarchive.net
Rights and permissions
About this article
Cite this article
Thomasian, N.M., Kamel, I.R. & Bai, H.X. Machine intelligence in non-invasive endocrine cancer diagnostics. Nat Rev Endocrinol 18, 81–95 (2022). https://doi.org/10.1038/s41574-021-00543-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41574-021-00543-9
This article is cited by
-
Patient classification and attribute assessment based on machine learning techniques in the qualification process for surgical treatment of adrenal tumours
Scientific Reports (2024)
-
Hardware-efficient quantum principal component analysis for medical image recognition
Frontiers of Physics (2024)
-
RETRACTED ARTICLE: Enhanced image diagnosing approach in medicine using quantum adaptive machine learning techniques
Optical and Quantum Electronics (2024)
-
DNA-framework-based multidimensional molecular classifiers for cancer diagnosis
Nature Nanotechnology (2023)
-
The Driver Role of Pathologists in Endocrine Oncology: What Clinicians Seek in Pathology Reports
Endocrine Pathology (2023)