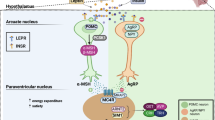
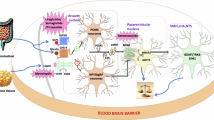
Abstract
Obesity is a multifactorial and complex disease that often manifests in early childhood with a lifelong burden. Polygenic and monogenic obesity are driven by the interaction between genetic predisposition and environmental factors. Polygenic variants are frequent and confer small effect sizes. Rare monogenic obesity syndromes are caused by defined pathogenic variants in single genes with large effect sizes. Most of these genes are involved in the central nervous regulation of body weight; for example, genes of the leptin–melanocortin pathway. Clinically, patients with monogenic obesity present with impaired satiety, hyperphagia and pronounced food-seeking behaviour in early childhood, which leads to severe early-onset obesity. With the advent of novel pharmacological treatment options emerging for monogenic obesity syndromes that target the central melanocortin pathway, genetic testing is recommended for patients with rapid weight gain in infancy and additional clinical suggestive features. Likewise, patients with obesity associated with hypothalamic damage or other forms of syndromic obesity involving energy regulatory circuits could benefit from these novel pharmacological treatment options. Early identification of patients affected by syndromic obesity will lead to appropriate treatment, thereby preventing the development of obesity sequelae, avoiding failure of conservative treatment approaches and alleviating stigmatization of patients and their families.
Key points
-
Obesity is a complex, multifactorial disease that can be classified into common polygenic obesity and rare obesity syndromes, including monogenic obesity.
-
Most monogenic obesity traits result from pathogenic variants in single genes converging in the leptin–melanocortin pathway.
-
Targeting central pathways of energy expenditure with, for example, MC4R agonists provides new and promising treatment options for patients with monogenic obesity.
-
Polygenic obesity results from an interplay among numerous genetic and environmental factors.
-
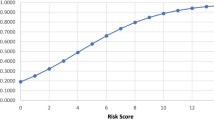
Polygenic risk scores and massively parallel sequencing approaches will help the early identification of obesity predisposition.
-
New precision medicine approaches based on genetic obesity traits might help tackle the obesity pandemic.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
World Health Organization. Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2021).
Moreno, L. A. Early severe obesity in children. Nat. Rev. Endocrinol. 14, 194–196 (2018).
Geserick, M. et al. Acceleration of BMI in early childhood and risk of sustained obesity. N. Engl. J. Med. 379, 1303–1312 (2018). This study adresses the question of whether there is a critical age window for the manifestation of childhood obesity.
Landgraf, K. et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes 64, 1249–1261 (2015).
Twig, G. et al. Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med. 374, 2430–2440 (2016).
Fastenau, J. et al. A call to action to inform patient-centred approaches to obesity management: development of a disease-illness model. Clin. Obes. 9, e12309 (2019).
Frühbeck, G. et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes. Facts 12, 131–136 (2019).
Vogel, M. et al. Age- and weight group-specific weight gain patterns in children and adolescents during the 15 years before and during the COVID-19 pandemic. Int. J. Obes. 46, 144–152 (2021).
Nogueira-de-Almeida, C. A. et al. COVID-19 and obesity in childhood and adolescence: a clinical review. J. Pediatr. 96, 546–558 (2020).
The Lancet Public Health. Childhood obesity beyond COVID-19. Lancet Public. Heal. 6, e534 (2021). A large study analysing the obesity and COVID-19 pandemics in childhood.
Hill, B. et al. Weight stigma and obesity‐related policies: a systematic review of the state of the literature. Obes. Rev. 22, e13333 (2021).
Hilbert, A. Weight stigma reduction and genetic determinism. PLoS ONE 11, e0162993 (2016).
Stunkard, A. J., Foch, T. T. & Hrubec, Z. A twin study of human obesity. JAMA 256, 51–54 (1986). A seminal study depicting heritability estimates for BMI.
Silventoinen, K. & Konttinen, H. Obesity and eating behavior from the perspective of twin and genetic research. Neurosci. Biobehav. Rev. 109, 150–165 (2020).
Maes, H. H., Neale, M. C. & Eaves, L. J. Genetic and environmental factors in relative body weight and human adiposity. Behav. Genet. 27, 325–351 (1997).
Vlietinck, R. et al. Genetic and environmental variation in the birth weight of twins. Behav. Genet. 19, 151–161 (1989).
Hebebrand, J. Obesity. in Lewis’s Child and Adolescent Psychiatry (eds. Martin, A., Bloch, M. H. & Volkmar, F. R.) 602–614 (Lippincott Williams & Wilkins, 2007).
Plomin, R., DeFries, J., McClear, G. & Rutter, M. Behavioral Genetics (Freeman, 1997).
Kleiser, C., Schaffrath Rosario, A., Mensink, G. B. M., Prinz-Langenohl, R. & Kurth, B.-M. Potential determinants of obesity among children and adolescents in Germany: results from the cross-sectional KiGGS study. BMC Public. Health 9, 46 (2009).
Hebebrand, J. et al. Epidemic obesity: are genetic factors involved via increased rates of assortative mating? Int. J. Obes. Relat. Metab. Disord. 24, 345–353 (2000).
Magnusson, P. K. E. & Rasmussen, F. Familial resemblance of body mass index and familial risk of high and low body mass index. A study of young men in Sweden. Int. J. Obes. Relat. Metab. Disord. 26, 1225–1231 (2002).
Eichler, E. E. et al. Missing heritability and strategies for finding the underlying causes of complex disease. Nat. Rev. Genet. 11, 446–450 (2010).
Hebebrand, J., Volckmar, A.-L., Knoll, N. & Hinney, A. Chipping away the ‘missing heritability’: GIANT steps forward in the molecular elucidation of obesity – but still lots to go. Obes. Facts 3, 294–303 (2010).
Styne, D. M. et al. Pediatric obesity-assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 102, 709–757 (2017).
Loos, R. J. F. & Yeo, G. S. H. The genetics of obesity: from discovery to biology. Nat. Rev. Genet. 23, 120–133 (2022).
Khera, A. V. et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 177, 587–596.e9 (2019).
Kaur, Y., de Souza, R. J., Gibson, W. T. & Meyre, D. A systematic review of genetic syndromes with obesity. Obes. Rev. 18, 603–634 (2017).
Poitou, C., Mosbah, H. & Clément, K. Mechanisms in endocrinology update on treatments for patients with genetic obesity. Eur. J. Endocrinol. 163, R149–R166 (2020).
Yeo, G. S. H. et al. The melanocortin pathway and energy homeostasis: from discovery to obesity therapy. Mol. Metab. 48, 101206 (2021).
Kleinendorst, L. et al. Identifying underlying medical causes of pediatric obesity: results of a systematic diagnostic approach in a pediatric obesity center. PLoS ONE 15, e0244508 (2020).
Farooqi, I. S. & O’Rahilly, S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat. Clin. Pract. Endocrinol. Metab. 4, 569–577 (2008).
Saeed, S. et al. Genetic causes of severe childhood obesity: a remarkably high prevalence in an inbred population of Pakistan. Diabetes 69, 1424–1438 (2020).
Wade, K. H. et al. Loss-of-function mutations in the melanocortin 4 receptor in a UK birth cohort. Nat. Med. 27, 1088–1096 (2021).
Ling, C. & Rönn, T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 29, 1028–1044 (2019).
Rohde, K. et al. Genetics and epigenetics in obesity. Metabolism 92, 37–50 (2019).
Qiao, Y. et al. Birth weight and childhood obesity: a 12-country study. Int. J. Obes. Suppl. 5, S74–S79 (2015).
Ibáñez, L., Ong, K., Dunger, D. B. & de Zegher, F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J. Clin. Endocrinol. Metab. 91, 2153–2158 (2006).
He, Q. & Karlberg, J. BMI in childhood and its association with height gain, timing of puberty, and final height. Pediatr. Res. 49, 244–251 (2001).
Denzer, C. et al. Pubertal development in obese children and adolescents. Int. J. Obes. 31, 1509–1519 (2007).
de Groot, C. J. et al. Determinants of advanced bone age in childhood obesity. Horm. Res. Paediatr. 87, 254–263 (2017).
Kempf, E. et al. Dynamic alterations in linear growth and endocrine parameters in children with obesity and height reference values. eClinicalMedicine 37, 100977 (2021).
Kohlsdorf, K. et al. Early childhood BMI trajectories in monogenic obesity due to leptin, leptin receptor, and melanocortin 4 receptor deficiency. Int. J. Obes. 42, 1602–1609 (2018).
Strobel, A., Issad, T., Camoin, L., Ozata, M. & Strosberg, A. D. A leptin missense mutation associated with hypogonadism and morbid obesity. Nat. Genet. 18, 213–215 (1998).
Farooqi, I. S. et al. Clinical and molecular genetic spectrum of congenital deficiency of the Leptin receptor. N. Engl. J. Med. 356, 237–247 (2007). A comprehensive description of genetic variants in LEPR and the relevance for obesity.
von Schnurbein, J. et al. Leptin substitution results in the induction of menstrual cycles in an adolescent with leptin deficiency and hypogonadotropic hypogonadism. Horm. Res. Paediatr. 77, 127–133 (2012).
Beghini, M. et al. Serum IGF1 and linear growth in children with congenital leptin deficiency before and after leptin substitution. Int. J. Obes. 45, 1448–1456 (2021).
Farooqi, I. S. & O’Rahilly, S. 20 years of leptin: human disorders of leptin action. J. Endocrinol. 223, T63–T70 (2014).
Farooqi, I. S. Monogenic human obesity syndromes. Handb. Clin. Neurol. 181, 301–310 (2021).
Heymsfield, S. B. et al. Hyperphagia: Current Concepts and Future Directions. Proceedings of the 2nd International Conference on Hyperphagia. Obesity 22, S1–S17 (2014).
Gibbons, C., Hopkins, M., Beaulieu, K., Oustric, P. & Blundell, J. E. Issues in measuring and interpreting human appetite (satiety/satiation) and its contribution to obesity. Curr. Obes. Rep. 8, 77–87 (2019).
Melchior, C. et al. Clinical and functional relevance of melanocortin-4 receptor variants in obese German children. Horm. Res. Paediatr. 78, 237–246 (2012).
Hinney, A., Volckmar, A.-L. & Knoll, N. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog. Mol. Biol. Transl. Sci. 114, 147–191 (2013).
Creemers, J. W. M. et al. Heterozygous mutations causing partial prohormone convertase 1 deficiency contribute to human obesity. Diabetes 61, 383–390 (2012).
Stijnen, P., Ramos-Molina, B., O’Rahilly, S. & Creemers, J. W. M. PCSK1 mutations and human endocrinopathies: from obesity to gastrointestinal disorders. Endocr. Rev. 37, 347–371 (2016).
Stijnen, P. et al. Endoplasmic reticulum-associated degradation of the mouse PC1/3-N222D hypomorph and human PCSK1 mutations contributes to obesity. Int. J. Obes. 40, 973–981 (2016).
Löffler, D. et al. Functional and clinical relevance of novel and known PCSK1 variants for childhood obesity and glucose metabolism. Mol. Metab. 6, 295–305 (2017).
Mantzoros, C. S. et al. Leptin in human physiology and pathophysiology. Am. J. Physiol. - Endocrinol. Metab. 301, E567–E584 (2011).
Park, H.-K. & Ahima, R. S. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism 64, 24–34 (2015).
Wauman, J., Zabeau, L. & Tavernier, J. The leptin receptor complex: heavier than expected? Front. Endocrinol. 8, 30 (2017).
Antunes, H., Santos, C. & Carvalho, S. Serum leptin levels in overweight children and adolescents. Br. J. Nutr. 101, 1262–1266 (2008).
Pan, W. W. & Myers, M. G. Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 19, 95–105 (2018).
Funcke, J.-B. et al. Monogenic forms of childhood obesity due to mutations in the leptin gene. Mol. Cell. Pediatr. 1, 3 (2014).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903–908 (1997). The first description of leptin deficiency in humans.
Wabitsch, M. et al. Biologically inactive leptin and early-onset extreme obesity. N. Engl. J. Med. 372, 48–54 (2015). The first description of biologically inactive variants in the leptin gene.
Wabitsch, M. et al. Severe early-onset obesity due to bioinactive leptin caused by a p.N103K mutation in the leptin gene. J. Clin. Endocrinol. Metab. 100, 3227–3230 (2015).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999). A comprehensive description of leptin treatment in severe obesity due to leptin deficiency.
Farooqi, I. S. et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 (2002).
Fischer-Posovszky, P. et al. A new missense mutation in the leptin gene causes mild obesity and hypogonadism without affecting T cell responsiveness. J. Clin. Endocrinol. Metab. 95, 2836–2840 (2010).
Gruber, T. et al. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 33, 1155–1170.e10 (2021).
Von Schnurbein, J. et al. Leptin is not essential for obesity-associated hypertension. Obes. Facts 12, 460–475 (2019).
Kim, M. S. et al. The central melanocortin system affects the hypothalamo-pituitary thyroid axis and may mediate the effect of leptin. J. Clin. Invest. 105, 1005–1011 (2000).
Nillni, E. A. et al. Leptin regulates prothyrotropin-releasing hormone biosynthesis: evidence for direct and indirect pathways. J. Biol. Chem. 275, 36124–36133 (2000).
Paz-Filho, G., Delibasi, T., Erol, H. K., Wong, M.-L. & Licinio, J. Congenital leptin deficiency and thyroid function. Thyroid. Res. 2, 11 (2009).
Wabitsch, M. et al. Measurement of immunofunctional leptin to detect and monitor patients with functional leptin deficiency. Eur. J. Endocrinol. 176, 315–322 (2017).
Stanik, J. et al. Concordance of bioactive vs. total immunoreactive serum leptin levels in children with severe early onset obesity. PLoS ONE 12, e0178107 (2017).
Clément, K. et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392, 398–401 (1998). Detection of the first patients with LEPR deficiency.
Kleinendorst, L. et al. Leptin receptor deficiency: a systematic literature review and prevalence estimation based on population genetics. Eur. J. Endocrinol. 182, 47–56 (2019).
Nunziata, A. et al. Functional and phenotypic characteristics of human leptin receptor mutations. J. Endocr. Soc. 3, 27–41 (2018).
Voigtmann, F. et al. Identification of a novel leptin receptor (LEPR) variant and proof of functional relevance directing treatment decisions in patients with morbid obesity. Metabolism 116, 154438 (2021).
Li, Z., Zhou, Y., Carter-Su, C., Myers, M. G. & Rui, L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol. Endocrinol. 21, 2270–2281 (2007).
Bochukova, E. G. et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature 463, 666–670 (2010).
Doche, M. E. et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J. Clin. Invest. 122, 4732–4736 (2012).
Volckmar, A.-L. et al. Mutation screen in the GWAS derived obesity gene SH2B1 including functional analyses of detected variants. BMC Med. Genomics 5, 65 (2012).
Rui, L. SH2B1 regulation of energy balance, body weight, and glucose metabolism. World J. Diabetes 5, 511 (2014).
Argente, J. et al. Efficacy and safety results of a phase 2 trial of setmelanotide in obesity due to SH2B1 variants and 16p11.2 deletion syndrome [abstract]. ESPE Abstr. 94, FC2.1 (2021).
Krude, H. et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 19, 155–157 (1998).
Graves, L. E., Khouri, J. M., Kristidis, P. & Verge, C. F. Proopiomelanocortin deficiency diagnosed in infancy in two boys and a review of the known cases. J. Paediatr. Child Health 57, 484–490 (2021).
Kühnen, P. et al. Interindividual variation in DNA methylation at a putative POMC metastable epiallele is associated with obesity. Cell Metab. 24, 502–509 (2016).
Candler, T., Kühnen, P., Prentice, A. M. & Silver, M. Epigenetic regulation of POMC; implications for nutritional programming, obesity and metabolic disease. Front. Neuroendocrinol. 54, 100773 (2019).
O’Rahilly, S. et al. Impaired processing of prohormones associated with abnormalities of glucose homeostasis and adrenal function. N. Engl. J. Med. 333, 1386–1391 (1995).
Jackson, R. S. et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 16, 303–306 (1997).
Jackson, R. S. et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J. Clin. Invest. 112, 1550–1560 (2003).
Farooqi, I. S. et al. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J. Clin. Endocrinol. Metab. 92, 3369–3373 (2007).
Pépin, L. et al. A newcase of PCSK1 pathogenic variant with congenital proprotein convertase 1/3 deficiency and literature review. J. Clin. Endocrinol. Metab. 104, 985–993 (2019).
Benzinou, M. et al. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat. Genet. 40, 943–945 (2008).
Yeo, G. S. H. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 20, 111–112 (1998).
Vaisse, C., Clement, K., Guy-Grand, B. & Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat. Genet. 20, 113–114 (1998). This study and the study by Yeo et al. (1998) are the first studies delineating the effect of MC4R mutations on obesity.
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095 (2003).
Kühnen, P., Krude, H. & Biebermann, H. Melanocortin-4 receptor signalling: importance for weight regulation and obesity treatment. Trends Mol. Med. 25, 136–148 (2019).
Clément, K. et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 24, 551–555 (2018).
Dempfle, A. et al. Large quantitative effect of melanocortin-4 receptor gene mutations on body mass index. J. Med. Genet. 41, 795–800 (2004).
Geller, F. et al. Melanocortin-4 receptor gene variant I103 is negatively associated with obesity. Am. J. Hum. Genet. 74, 572–581 (2004). First study describing a robust polygenic effect of BMI.
Lotta, L. A. et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell 177, 597–607.e9 (2019).
Paisdzior, S. et al. Differential signaling profiles of MC4R mutations with three different ligands. Int. J. Mol. Sci. 21, 1224 (2020).
Smith, J. S., Lefkowitz, R. J. & Rajagopal, S. Biased signalling: from simple switches to allosteric microprocessors. Nat. Rev. Drug. Discov. 17, 243–260 (2018).
Akbari, P. et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 373, eabf8683 (2021).
Mendes de Oliveira, E. et al. Obesity-associated GNAS mutations and the melanocortin pathway. N. Engl. J. Med. 385, 1581–1592 (2021).
Ji, L., Wu, H. T., Qin, X. Y. & Lan, R. Dissecting carboxypeptidase E: properties, functions and pathophysiological roles in disease. Endocr. Connect. 6, R18–R38 (2017).
Alsters, S. I. M. et al. Truncating homozygous mutation of carboxypeptidase E (CPE) in a morbidly obese female with type 2 diabetes mellitus, intellectual disability and hypogonadotrophic hypogonadism. PLoS ONE 10, e0131417 (2015).
Bosch, E. et al. BDV syndrome: an emerging syndrome with profound obesity and neurodevelopmental delay resembling Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 106, 3413–3427 (2021).
York, B. & O’Malley, B. W. Steroid receptor coactivator (SRC) family: masters of systems biology. J. Biol. Chem. 285, 38743–38750 (2010).
Yang, Y. et al. Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis. Nat. Commun. 10, 1718 (2019).
Reinehr, T. et al. Lifestyle intervention in obese children with variations in the melanocortin 4 receptor gene. Obesity 17, 382–389 (2009).
Hainerová, I. et al. Melanocortin 4 receptor mutations in obese Czech children: studies of prevalence, phenotype development, weight reduction response, and functional analysis. J. Clin. Endocrinol. Metab. 92, 3689–3696 (2007).
Trier, C. et al. Obesity treatment effect in Danish children and adolescents carrying melanocortin-4 receptor mutations. Int. J. Obes. 45, 66–76 (2021).
Vos, N. et al. Bariatric surgery for monogenic non-syndromic and syndromic obesity disorders. Curr. Diab. Rep. 20, 44 (2020).
Poitou, C. et al. Long-term outcomes of bariatric surgery in patients with bi-allelic mutations in the POMC, LEPR, and MC4R genes. Surg. Obes. Relat. Dis. 17, 1449–1456 (2021).
Cooiman, M. I. et al. Long-term weight outcome after bariatric surgery in patients with melanocortin-4 receptor gene variants: a case–control study of 105 patients. Obes. Surg. 32, 837–844 (2022).
Kleinendorst, L., van Haelst, M. M. & van den Akker, E. L. T. Young girl with severe early-onset obesity and hyperphagia. BMJ Case Rep. 2017, bcr2017221067 (2017).
Zorn, S., von Schnurbein, J., Kohlsdorf, K., Denzer, C. & Wabitsch, M. Diagnostic and therapeutic odyssey of two patients with compound heterozygous leptin receptor deficiency. Mol. Cell. Pediatr. 7, 15 (2020).
Heymsfield, S. B. & Wadden, T. A. Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med. 376, 254–266 (2017).
Cardel, M. I., Jastreboff, A. M. & Kelly, A. S. Treatment of adolescent obesity in 2020. JAMA 322, 1707–1708 (2019).
Viner, R. M., Hsia, Y., Tomsic, T. & Wong, I. C. K. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obes. Rev. 11, 593–602 (2009).
Sherafat-Kazemzadeh, R., Yanovski, S. Z. & Yanovski, J. A. Pharmacotherapy for childhood obesity: present and future prospects. Int. J. Obes. 37, 1–15 (2013).
Peirson, L. et al. Treatment of overweight and obesity in children and youth: a systematic review and meta-analysis. CMAJ Open 3, E35–E46 (2015).
von Schnurbein, J. et al. Rapid improvement of hepatic steatosis after initiation of leptin substitution in a leptin-deficient girl. Horm. Res. Paediatr. 79, 310–317 (2013).
Farr, O. M., Gavrieli, A. & Mantzoros, C. S. Leptin applications in 2015: what have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes. 22, 353–359 (2015).
Heymsfield, S. B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282, 1568–1575 (1999).
Tsoukas, M. A., Farr, O. M. & Mantzoros, C. S. Leptin in congenital and HIV-associated lipodystrophy. Metabolism 64, 47–59 (2015).
Milos, G. et al. Short-term metreleptin treatment of patients with anorexia nervosa: rapid on-set of beneficial cognitive, emotional, and behavioral effects. Transl. Psychiatry 10, 303 (2020). The first clinical use of metreleptin in the treatment of patients with the eating disorder anorexia nervosa.
Antel, J. et al. Rapid amelioration of anorexia nervosa in a male adolescent during metreleptin treatment including recovery from hypogonadotropic hypogonadism. Eur. Child. Adolesc. Psychiatry https://doi.org/10.1007/s00787-021-01778-7 (2021).
de Candia, P. et al. The pleiotropic roles of leptin in metabolism, immunity, and cancer. J. Exp. Med. 218, e20191593 (2021).
Kühnen, P. et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 375, 240–246 (2016). The first report on the weight-lowering effects of the MC4R agonist setmelanotide in patients with POMC deficiency.
Greenfield, J. R. et al. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 360, 44–52 (2009).
Kanti, V. et al. A melanocortin-4 receptor agonist induces skin and hair pigmentation in patients with monogenic mutations in the leptin-melanocortin pathway. Ski. Pharmacol. Physiol. 34, 307–316 (2021).
Tagliabue, E. et al. MC1R variants as melanoma risk factors independent of at-risk phenotypic characteristics: a pooled analysis from the M-SKIP project. Cancer Manag. Res. 10, 1143–1154 (2018).
Collet, T. H. et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol. Metab. 6, 1321–1329 (2017).
Clément, K. et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 8, 960–970 (2020).
Markham, A. Setmelanotide: first approval. Drugs 81, 397–403 (2021).
Haws, R. et al. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes. Metab. 22, 2133–2140 (2020).
Argente, J. et al. Phase 3 trial of setmelanotide in participants with Bardet-Biedl syndrome: placebo-controlled results [abstract]. ESPE Abstr. 94, FC2.2 (2021).
Ryan, D. H. Setmelanotide: what does it mean for clinical care of patients with obesity? Lancet Diabetes Endocrinol. 8, 933–935 (2020).
Akinci, B. et al. The complicated clinical course in a case of atypical lipodystrophy after development of neutralizing antibody to metreleptin: treatment with setmelanotide. Endocrinol. Diabetes Metab. Case Rep. 2020, 19–0139 (2020).
Kamermans, A. et al. Setmelanotide, a novel, selective melanocortin receptor-4 agonist exerts anti-inflammatory actions in astrocytes and promotes an anti-inflammatory macrophage phenotype. Front. Immunol. 10, 2312 (2019).
Kelly, A. S. et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N. Engl. J. Med. 382, 2117–2128 (2020).
Iepsen, E. W. et al. Patients with obesity caused by melanocortin-4 receptor mutations can be treated with a glucagon-like peptide-1 receptor agonist. Cell Metab. 28, 23–32.e3 (2018).
Iepsen, E. W. et al. GLP-1 receptor agonist treatment in morbid obesity and type 2 diabetes due to pathogenic homozygous melanocortin-4 receptor mutation: a case report. Cell Rep. Med. 1, 100006 (2020).
Welling, M. S. et al. Effects of glucagon-like peptide-1 analogue treatment in genetic obesity: a case series. Clin. Obes. 11, e12481 (2021).
Müller, T. D., Clemmensen, C., Finan, B., DiMarchi, R. D. & Tschöp, M. H. Anti-obesity therapy: from rainbow pills to polyagonists. Pharmacol. Rev. 70, 712–746 (2018).
Day, J. W. et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 5, 749–757 (2009).
Clemmensen, C. et al. Emerging hormonal-based combination pharmacotherapies for the treatment of metabolic diseases. Nat. Rev. Endocrinol. 15, 90–104 (2019).
Frías, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515 (2021).
Rosenstock, J. et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 398, 143–155 (2021).
Ludvik, B. et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 398, 583–598 (2021).
Del Prato, S. et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 398, 1811–1824 (2021).
Dahl, D. et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes. JAMA 327, 534–545 (2022).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2206038 (2022).
Brandt, S. J., Götz, A., Tschöp, M. H. & Müller, T. D. Gut hormone polyagonists for the treatment of type 2 diabetes. Peptides 100, 190–201 (2018).
Müller, T. D., Blüher, M., Tschöp, M. H. & DiMarchi, R. D. Anti-obesity drug discovery: advances and challenges. Nat. Rev. Drug Discov. 21, 201–223 (2022). Comprehensive review of the state of the art in anti-obesity drug discovery.
Angelidi, A. M., Belanger, M. J., Kokkinos, A., Koliaki, C. C. & Mantzoros, C. S. Novel noninvasive approaches to the treatment of obesity: from pharmacotherapy to gene therapy. Endocr. Rev. 43, 507–557 (2022).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yamanaka, S. Pluripotent stem cell-based cell therapy—promise and challenges. Cell Stem Cell 27, 523–531 (2020).
Rajamani, U. et al. Super-obese patient-derived iPSC hypothalamic neurons exhibit obesogenic signatures and hormone responses. Cell Stem Cell 22, 698–712.e9 (2018).
Uddin, F., Rudin, C. M. & Sen, T. CRISPR gene therapy: applications, limitations, and implications for the future. Front. Oncol. 10, 1387 (2020).
Zhu, L. et al. Leptin gene-targeted editing in ob/ob mouse adipose tissue based on the CRISPR/Cas9 system. J. Genet. Genomics 48, 134–146 (2021).
Keller, E. et al. Auxological computer based network for early detection of disorders of growth and weight attainment. J. Pediatr. Endocrinol. Metab. 15, 149–156 (2002).
Ayers, K. L. et al. Melanocortin 4 receptor pathway dysfunction in obesity: patient stratification aimed at MC4R agonist treatment. J. Clin. Endocrinol. Metab. 103, 2601–2612 (2018).
Dayton, K. & Miller, J. Finding treatable genetic obesity: strategies for success. Curr. Opin. Pediatr. 30, 526–531 (2018).
Brennan, L. & de Roos, B. Nutrigenomics: lessons learned and future perspectives. Am. J. Clin. Nutr. 113, 503–516 (2021).
Bouchard, C. Exercise genomics — a paradigm shift is needed: a commentary. Br. J. Sports Med. 49, 1492–1496 (2015).
Riveros-McKay, F. et al. Genetic architecture of human thinness compared to severe obesity. PLoS Genet. 15, e1007603 (2019).
Hinney, A. et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE 2, e1361 (2007).
Orthofer, M. et al. Identification of ALK in thinness. Cell 181, 1246–1262.e22 (2020).
Watson, H. J. et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 51, 1207–1214 (2019).
Zheng, Y. et al. PTBP2 - a gene with relevance for both anorexia nervosa and body weight regulation. Transl. Psychiatry. 12, 241 (2022).
Hinney, A. et al. Evidence for three genetic loci involved in both anorexia nervosa risk and variation of body mass index. Mol. Psychiatry 22, 192–201 (2017).
Hinney, A. & Hebebrand, J. Polygenic obesity in humans. Obes. Facts 1, 35–42 (2008).
Felix, J. F. et al. Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum. Mol. Genet. 25, 389–403 (2016).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649 (2018).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28, 166–174 (2019).
Loos, R. J. F. & Yeo, G. S. H. The bigger picture of FTO – the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 10, 51–61 (2014).
Zeggini, E., Gloyn, A. L., Barton, A. C. & Wain, L. V. Translational genomics and precision medicine: moving from the lab to the clinic. Science 365, 1409–1413 (2019).
Dina, C. et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat. Genet. 39, 724–726 (2007).
Frayling, T. M. et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316, 889–894 (2007).
Wang, J.-Y., Chen, L.-J. & Qiang, P. The potential role of N6-methyladenosine (m6A) demethylase fat mass and obesity-associated gene (FTO) in human cancers. Onco. Targets Ther. 13, 12845–12856 (2020).
Lan, N. et al. FTO – a common genetic basis for obesity and cancer. Front. Genet. 11, 559138 (2020).
Annapoorna, P. K. et al. FTO: an emerging molecular player in neuropsychiatric diseases. Neuroscience 418, 15–24 (2019).
Landgraf, K. et al. The obesity-susceptibility gene TMEM18 promotes adipogenesis through activation of PPARG. Cell Rep. 33, 108295 (2020).
Han, Y. et al. Mesenchymal stem cells for regenerative medicine. Cells 8, 886 (2019).
Zuk, P. A. et al. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13, 4279–4295 (2002).
Shukla, L., Yuan, Y., Shayan, R., Greening, D. W. & Karnezis, T. Fat therapeutics: the clinical capacity of adipose-derived stem cells and exosomes for human disease and tissue regeneration. Front. Pharmacol. 11, 158 (2020).
Wang, C.-H. et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. 12, eaaz8664 (2020).
Chouchani, E. T., Kazak, L. & Spiegelman, B. M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37 (2019).
Acknowledgements
The authors thank L.S. Rajcsanyi (University of Duisburg-Essen, Essen, Germany) for her help with the first version of the figure. A.H. acknowledges the support of funding from Deutsche Forschungsgemeinschaft (DFG; HI 865/2-1), the BMBF (01GS0820, PALGER2017-33: 01DH19010) and the Stiftung Universitätsmedizin Essen. A.K. acknowledges the support of grants from the DFG, KO3512/3-1 and the DFG-funded Collaborative Research Center “ObesityMechanisms” CRC1052 (no. 209933838), and the German Diabetes Association (DDG). P.F.-P. acknowledges the support of funding from Deutsche Forschungsgemeinschaft (Heisenberg professorship, project number 398707781).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
A.K. provided unpaid advice to Rhythm Pharmaceuticals. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks ELT van den Akker who co-reviewed the manuscript with Ozair Abawi, Jesus Argente and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CDC growth charts: https://www.cdc.gov/growthcharts/clinical_charts.htm
Clinical trials: https://clinicaltrials.gov/ct2/home
Confirmed genes for different complex traits: https://www.ebi.ac.uk/gwas/
FDA approval: https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes
OMIM database: https://www.omim.org/
WHO child growth standards: https://www.who.int/tools/child-growth-standards
Glossary
- Heritability estimates
-
The proportion of variation that is attributable to genetic as opposed to environmental factors for a given phenotype.
- Polygenic predictor
-
An estimation of the genetic liability to a complex human trait using genome-wide genetic variants.
- Barker hypothesis
-
This hypothesis postulates that the origins of chronic diseases in adult life lie in fetal responses to the intrauterine environment.
- CRISPR-mediated gene editing
-
CRISPR–Cas9 enzymes were developed from a naturally occurring genome editing system, important for immune defence in bacteria, and can be used to edit parts of the genome.
Rights and permissions
About this article
Cite this article
Hinney, A., Körner, A. & Fischer-Posovszky, P. The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat Rev Endocrinol 18, 623–637 (2022). https://doi.org/10.1038/s41574-022-00716-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41574-022-00716-0
This article is cited by
-
NCOA1 is a gatekeeper of the sexually dimorphic thermogenic activity of white adipose tissue
Nature Communications (2026)
-
Obesity: exploring its connection to brain function through genetic and genomic perspectives
Molecular Psychiatry (2025)
-
The expanding landscape of genetic causes of obesity
Pediatric Research (2025)
-
Detecting monogenic obesity: a systematic exome-wide workup of over 500 individuals
International Journal of Obesity (2025)
-
The Interplay of UCP3 and PCSK1 Variants in Severe Obesity
Current Obesity Reports (2025)