Abstract
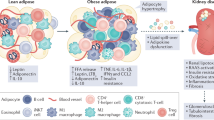
Inflammation is an essential physiological defence mechanism, but prolonged or excessive inflammation can cause disease. Indeed, unresolved systemic and adipose tissue inflammation drives obesity-related cardiovascular disease and type 2 diabetes mellitus. Drugs targeting pro-inflammatory cytokine pathways or inflammasome activation have been approved for clinical use for the past two decades. However, potentially serious adverse effects, such as drug-induced weight gain and increased susceptibility to infections, prevented their wider clinical implementation. Furthermore, these drugs do not modulate the resolution phase of inflammation. This phase is an active process orchestrated by specialized pro-resolving mediators, such as lipoxins, and other endogenous resolution mechanisms. Pro-resolving mediators mitigate inflammation and development of obesity-related disease, for instance, alleviating insulin resistance and atherosclerosis in experimental disease models, so mechanisms to modulate their activity are, therefore, of great therapeutic interest. Here, we review current clinical attempts to either target pro-inflammatory mediators (IL-1β, NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome, tumour necrosis factor (TNF) and IL-6) or utilize endogenous resolution pathways to reduce obesity-related inflammation and improve cardiometabolic outcomes. A remaining challenge in the field is to establish more precise biomarkers that can differentiate between acute and chronic inflammation and to assess the functionality of individual leukocyte populations. Such advancements would improve the monitoring of drug effects and support personalized treatment strategies that battle obesity-related inflammation and cardiometabolic disease.
Key points
-
Chronic unresolved systemic and adipose tissue inflammation drives obesity-related cardiometabolic disease.
-
Drugs targeting pro-inflammatory cytokines, or inflammasome activation, are approved for clinical use but can elicit serious adverse effects (such as weight gain and increased susceptibility to infections), hampering their clinical implementation.
-
The resolution phase of inflammation is an active process regulated by specialized pro-resolving mediators. These mediators mitigate obesity-related inflammation and systemic disease in experimental models and are of therapeutic interest.
-
The field lacks biomarkers that can differentiate between acute and chronic inflammation and assess the functionality of individual leukocyte populations, which would improve personalized treatment strategies and support drug monitoring.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Furman, D. et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832 (2019). This manuscript offers a comprehensive review of the role of chronic low-grade inflammation in systemic disease, including obesity-related pathophysiologies.
Bradley, D., Deng, T., Shantaram, D. & Hsueh, W. A. Orchestration of the adipose tissue immune landscape by adipocytes. Annu. Rev. Physiol. 86, 199–223 (2024).
Hagberg, C. E. & Spalding, K. L. White adipocyte dysfunction and obesity-associated pathologies in humans. Nat. Rev. Mol. Cell Biol. 25, 270–289 (2024).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Shaikh, S. R., Beck, M. A., Alwarawrah, Y. & MacIver, N. J. Emerging mechanisms of obesity-associated immune dysfunction. Nat. Rev. Endocrinol. 20, 136–148 (2024).
Xourafa, G., Korbmacher, M. & Roden, M. Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 20, 27–49 (2024).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J. Am. Coll. Cardiol. 71, 2392–2401 (2018). This study shows that although inhibiting IL-1β in patients who have had a myocardial infarction and CRP levels ≥2 mg/l prevents further cardiovascular events, it has no effect on reducing their risk of developing diabetes mellitus.
Li, D., Zhong, J., Zhang, Q. & Zhang, J. Effects of anti-inflammatory therapies on glycemic control in type 2 diabetes mellitus. Front. Immunol. 14, 1125116 (2023).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017). This study provides definitive evidence that inhibiting IL-1β in patients who have had a myocardial infarction and CRP levels ≥2 mg/l prevents further cardiovascular events.
Antonelli, M. & Kushner, I. It’s time to redefine inflammation. FASEB J. 31, 1787–1791 (2017).
Medzhitov, R. The spectrum of inflammatory responses. Science 374, 1070–1075 (2021).
Meizlish, M. L., Franklin, R. A., Zhou, X. & Medzhitov, R. Tissue homeostasis and inflammation. Annu. Rev. Immunol. 39, 557–581 (2021).
Sugimoto, M. A., Sousa, L. P., Pinho, V., Perretti, M. & Teixeira, M. M. Resolution of inflammation: what controls its onset? Front. Immunol. 7, 160 (2016).
Serhan, C. N. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol. Asp. Med. 58, 1–11 (2017).
Wallace, J. L., Ianaro, A., Flannigan, K. L. & Cirino, G. Gaseous mediators in resolution of inflammation. Semin. Immunol. 27, 227–233 (2015).
Perretti, M. & D’Acquisto, F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 9, 62–70 (2009).
Schett, G. & Neurath, M. F. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat. Commun. 9, 3261 (2018).
Brennan, E., Kantharidis, P., Cooper, M. E. & Godson, C. Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 17, 725–739 (2021).
Chiang, N. & Serhan, C. N. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Asp. Med. 58, 114–129 (2017). This review provides an elegant and comprehensive overview of the synthesis, receptors and function of SPMs.
Fredman, G. & Serhan, C. N. Specialized pro-resolving mediators in vascular inflammation and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-023-00984-x (2024).
Panigrahy, D., Gilligan, M. M., Serhan, C. N. & Kashfi, K. Resolution of inflammation: an organizing principle in biology and medicine. Pharmacol. Ther. 227, 107879 (2021).
Pirault, J. & Back, M. Lipoxin and resolvin receptors transducing the resolution of inflammation in cardiovascular disease. Front. Pharmacol. 9, 1273 (2018).
Dalli, J. & Serhan, C. N. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 (2012).
Doran, A. C., Yurdagul, A. Jr. & Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 20, 254–267 (2020).
Julliard, W. A. et al. Specialized pro-resolving mediators as modulators of immune responses. Semin. Immunol. 59, 101605 (2022).
Borgeson, E. et al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metab. 22, 125–137 (2015). This study shows that lipoxin treatment of mice alleviates obesity-related adipose tissue inflammation, as well as kidney and liver disease.
Borgeson, E. et al. Lipoxin A4 attenuates adipose inflammation. FASEB J. 26, 4287–4294 (2012).
Cheng, T. et al. Resolvin D1 improves the Treg/Th17 imbalance in systemic lupus erythematosus through miR-30e-5p. Front. Immunol. 12, 668760 (2021).
Perez-Hernandez, J., Chiurchiu, V., Perruche, S. & You, S. Regulation of T-cell immune responses by pro-resolving lipid mediators. Front. Immunol. 12, 768133 (2021).
Ramon, S., Bancos, S., Serhan, C. N. & Phipps, R. P. Lipoxin A4 modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. Eur. J. Immunol. 44, 357–369 (2014).
Kim, N. et al. Lipoxin B4 enhances human memory B cell antibody production via upregulating cyclooxygenase-2 expression. J. Immunol. 201, 3343–3351 (2018).
Ramon, S., Gao, F., Serhan, C. N. & Phipps, R. P. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 189, 1036–1042 (2012).
Kraft, J. D. et al. Specialized pro-resolving mediators and the lymphatic system. Int. J. Mol. Sci. 22, 2750 (2021).
Nathan, C. Nonresolving inflammation redux. Immunity 55, 592–605 (2022).
Ridker, P. M. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J. Am. Coll. Cardiol. 49, 2129–2138 (2007).
Yang, S., Pan, Y. & Zheng, W. Baseline high-sensitivity C-reactive protein as a predictor of adverse clinical events in patients with coronary artery disease undergoing percutaneous coronary intervention: a meta-analysis. Cardiol. Rev. https://doi.org/10.1097/CRD.0000000000000604 (2023).
Rohm, T. V., Meier, D. T., Olefsky, J. M. & Donath, M. Y. Inflammation in obesity, diabetes, and related disorders. Immunity 55, 31–55 (2022).
Rattarasarn, C. Dysregulated lipid storage and its relationship with insulin resistance and cardiovascular risk factors in non-obese Asian patients with type 2 diabetes. Adipocyte 7, 71–80 (2018).
Fandriks, L. Roles of the gut in the metabolic syndrome: an overview. J. Intern. Med. 281, 319–336 (2017).
Violi, F. et al. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 20, 24–37 (2023).
Sanz, M. et al. Periodontitis and cardiovascular diseases: consensus report. J. Clin. Periodontol. 47, 268–288 (2020).
Mishra, S. P. et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 72, 1848–1865 (2023).
Scheithauer, T. P. M. et al. Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front. Immunol. 11, 571731 (2020).
Camilleri, M. & Vella, A. What to do about the leaky gut. Gut 71, 424–435 (2022).
Fasano, A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res 9, 69 (2020).
An, L. et al. The role of gut-derived lipopolysaccharides and the intestinal barrier in fatty liver diseases. J. Gastrointest. Surg. 26, 671–683 (2022).
Boren, J., Taskinen, M. R., Olofsson, S. O. & Levin, M. Ectopic lipid storage and insulin resistance: a harmful relationship. J. Intern. Med. 274, 25–40 (2013).
Docherty, S. et al. The effect of exercise on cytokines: implications for musculoskeletal health: a narrative review. BMC Sports Sci. Med. Rehabil. 14, 5 (2022).
Kawai, T., Autieri, M. V. & Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 320, C375–C391 (2021).
Kolb, H. Obese visceral fat tissue inflammation: from protective to detrimental? BMC Med. 20, 494 (2022).
de Baat, A., Trinh, B., Ellingsgaard, H. & Donath, M. Y. Physiological role of cytokines in the regulation of mammalian metabolism. Trends Immunol. 44, 613–627 (2023).
Lempesis, I. G., van Meijel, R. L. J., Manolopoulos, K. N. & Goossens, G. H. Oxygenation of adipose tissue: a human perspective. Acta Physiol. 228, e13298 (2020).
Sun, K., Li, X. & Scherer, P. E. Extracellular matrix (ECM) and fibrosis in adipose tissue: overview and perspectives. Compr. Physiol. 13, 4387–4407 (2023).
AlZaim, I., de Rooij, L., Sheikh, B. N., Borgeson, E. & Kalucka, J. The evolving functions of the vasculature in regulating adipose tissue biology in health and obesity. Nat. Rev. Endocrinol. 19, 691–707 (2023).
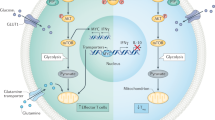
Maqdasy, S. et al. Impaired phosphocreatine metabolism in white adipocytes promotes inflammation. Nat. Metab. 4, 190–202 (2022).
Bradley, D. et al. Interferon gamma mediates the reduction of adipose tissue regulatory T cells in human obesity. Nat. Commun. 13, 5606 (2022).
Li, Q. et al. Obesity and hyperinsulinemia drive adipocytes to activate a cell cycle program and senesce. Nat. Med. 27, 1941–1953 (2021).
Hildebrandt, X., Ibrahim, M. & Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 30, 279–292 (2023).
Winer, D. A. et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 17, 610–617 (2011).
Hagglof, T. et al. T-bet+ B cells accumulate in adipose tissue and exacerbate metabolic disorder during obesity. Cell Metab. 34, 1121–1136.e6 (2022).
Pestel, J., Blangero, F., Watson, J., Pirola, L. & Eljaafari, A. Adipokines in obesity and metabolic-related-diseases. Biochimie 212, 48–59 (2023).
Jacks, R. D. & Lumeng, C. N. Macrophage and T cell networks in adipose tissue. Nat. Rev. Endocrinol. 20, 50–61 (2024).
Saltiel, A. R. & Olefsky, J. M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127, 1–4 (2017).
Sharma, B. R. & Kanneganti, T. D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 22, 550–559 (2021).
Larsen, C. M. et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 (2007).
Sattar, L. et al. Efficacy and safety of colchicine in prevention of secondary cardiovascular outcomes among patients with coronary vessel disease: a meta-analysis. Cureus 14, e26680 (2022).
Cronstein, B. N. et al. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J. Clin. Invest. 96, 994–1002 (1995).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Demidowich, A. P. et al. Effects of colchicine in adults with metabolic syndrome: a pilot randomized controlled trial. Diabetes Obes. Metab. 21, 1642–1651 (2019).
Patel, T. P. et al. Immunomodulatory effects of colchicine on peripheral blood mononuclear cell subpopulations in human obesity: data from a randomized controlled trial. Obesity 31, 466–478 (2023). This study highlights the changes in circulating leukocyte populations in individuals with obesity after colchicine treatment.
Levine, J. A. et al. Effects of colchicine on lipolysis and adipose tissue inflammation in adults with obesity and metabolic syndrome. Obesity 30, 358–368 (2022).
Evangelatos, G., Bamias, G., Kitas, G. D., Kollias, G. & Sfikakis, P. P. The second decade of anti-TNF-a therapy in clinical practice: new lessons and future directions in the COVID-19 era. Rheumatol. Int. 42, 1493–1511 (2022).
Mantravadi, S. et al. Impact of tumor necrosis factor inhibitors and methotrexate on diabetes mellitus among patients with inflammatory arthritis. BMC Rheumatol. 4, 39 (2020).
Otsuka, Y. et al. Effects of tumor necrosis factor inhibitors and tocilizumab on the glycosylated hemoglobin levels in patients with rheumatoid arthritis; an observational study. PLoS ONE 13, e0196368 (2018).
Stanley, T. L. et al. TNF-α antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J. Clin. Endocrinol. Metab. 96, E146–E150 (2011).
Dominguez, H. et al. Metabolic and vascular effects of tumor necrosis factor-α blockade with etanercept in obese patients with type 2 diabetes. J. Vasc. Res. 42, 517–525 (2005).
Paquot, N., Castillo, M. J., Lefebvre, P. J. & Scheen, A. J. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J. Clin. Endocrinol. Metab. 85, 1316–1319 (2000).
Patsalos, O., Dalton, B., Leppanen, J., Ibrahim, M. A. A. & Himmerich, H. Impact of TNF-α inhibitors on body weight and BMI: a systematic review and meta-analysis. Front. Pharmacol. 11, 481 (2020).
Tiburca, L. et al. The treatment with interleukin 17 inhibitors and immune-mediated inflammatory diseases. Curr. Issues Mol. Biol. 44, 1851–1866 (2022).
Li, J. et al. Serum IL-17A concentration and a IL17RA single nucleotide polymorphism contribute to the risk of autoimmune type 1 diabetes. Diabetes Metab. Res. Rev. 38, e3547 (2022).
Honkanen, J. et al. IL-17 immunity in human type 1 diabetes. J. Immunol. 185, 1959–1967 (2010).
Catterall, T., Fynch, S., Kay, T. W. H., Thomas, H. E. & Sutherland, A. P. R. IL-17F induces inflammation, dysfunction and cell death in mouse islets. Sci. Rep. 10, 13077 (2020).
Salama-Frisbie, R. et al. Association of polymorphisms of the IL-17A and IL-17f genes with increased risk of hypertension and obesity in Mexican patients with COVID-19. J. Interferon Cytokine Res. 44, 60–67 (2024).
Qiao, Y. C. et al. Changes of regulatory T cells and of proinflammatory and immunosuppressive cytokines in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J. Diabetes Res. 2016, 3694957 (2016).
Timis, T. L., Beni, L., Florian, I. A., Orasan, M. & Orasan, R. I. Prevalence of metabolic syndrome and chronic inflammation in psoriasis before and after biologic therapy: a prospective study. Med. Pharm. Rep. 96, 368–383 (2023). This prospective study finds that the prevalence of metabolic syndrome in patients with psoriasis was reduced by biologic therapies (TNF inhibitors and IL-12–IL-23 and IL-17 antagonists) that were administered to treat their psoriasis.
Adamstein, N. H. et al. Association of interleukin 6 inhibition with ziltivekimab and the neutrophil-lymphocyte ratio: a secondary analysis of the RESCUE clinical trial. JAMA Cardiol. 8, 177–181 (2023).
Broch, K. et al. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 77, 1845–1855 (2021).
Ridker, P. M. et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397, 2060–2069 (2021).
Wada, Y., Jensen, C., Meyer, A. S. P., Zonoozi, A. A. M. & Honda, H. Efficacy and safety of interleukin-6 inhibition with ziltivekimab in patients at high risk of atherosclerotic events in Japan (RESCUE-2): a randomized, double-blind, placebo-controlled, phase 2 trial. J. Cardiol. 82, 279–285 (2023).
Wedell-Neergaard, A. S. et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 29, 844–855.e3 (2019).
Garbers, C., Heink, S., Korn, T. & Rose-John, S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 17, 395–412 (2018).
Wueest, S. & Konrad, D. The controversial role of IL-6 in adipose tissue on obesity-induced dysregulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 319, E607–E613 (2020).
Hasan, I., Rainsford, K. D. & Ross, J. S. Salsalate: a pleotropic anti-inflammatory drug in the treatment of diabetes, obesity, and metabolic diseases. Inflammopharmacology 31, 2781–2797 (2023).
Fleischman, A., Shoelson, S. E., Bernier, R. & Goldfine, A. B. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care 31, 289–294 (2008).
Goldfine, A. B. et al. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin. Transl. Sci. 1, 36–43 (2008).
Hawley, S. A. et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336, 918–922 (2012).
Goldfine, A. B. et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 152, 346–357 (2010).
Goldfine, A. B. et al. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 159, 1–12 (2013).
Mashayekhi, M. et al. Comparative effects of weight loss and incretin-based therapies on vascular endothelial function, fibrinolysis and inflammation in individuals with obesity and prediabetes: a randomized controlled trial. Diabetes Obes. Metab. 25, 570–580 (2023).
Sandsdal, R. M. et al. Combination of exercise and GLP-1 receptor agonist treatment reduces severity of metabolic syndrome, abdominal obesity, and inflammation: a randomized controlled trial. Cardiovasc. Diabetol. 22, 41 (2023).
Hammoud, R. & Drucker, D. J. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat. Rev. Endocrinol. 19, 201–216 (2023).
Lee, Y. S. et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 55, 2456–2468 (2012).
Wong, C. K. et al. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metab. 36, 130–143.e5 (2024).
Claria, J., Dalli, J., Yacoubian, S., Gao, F. & Serhan, C. N. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 189, 2597–2605 (2012).
Neuhofer, A. et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 62, 1945–1956 (2013).
White, P. J., Arita, M., Taguchi, R., Kang, J. X. & Marette, A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes 59, 3066–3073 (2010).
Barden, A. et al. Adiposity associates with lower plasma resolvin E1 (Rve1): a population study. Int. J. Obes. 48, 725–732 (2024). This population study has examined plasma levels of SPMs in relation to sex differences, lifestyle and a broad range of cardiovascular disease risk factors and has found that decreased plasma levels of resolvin E1 could be a consequence of obesity.
Fisk, H. L. et al. Modification of subcutaneous white adipose tissue inflammation by omega-3 fatty acids is limited in human obesity-a double blind, randomised clinical trial. EBioMedicine 77, 103909 (2022).
Lopez-Vicario, C. et al. Leukocytes from obese individuals exhibit an impaired SPM signature. FASEB J. 33, 7072–7083 (2019).
Titos, E. et al. Signaling and immunoresolving actions of resolvin D1 in inflamed human visceral adipose tissue. J. Immunol. 197, 3360–3370 (2016).
Guo, Y. P., Jiang, H. K., Jiang, H., Tian, H. Y. & Li, L. Lipoxin A4 may attenuate the progression of obesity-related glomerulopathy by inhibiting NF-κB and ERK/p38 MAPK-dependent inflammation. Life Sci. 198, 112–118 (2018).
Sotak, M. et al. Lipoxins reduce obesity-induced adipose tissue inflammation in 3D-cultured human adipocytes and explant cultures. iScience 25, 104602 (2022). Using 3D adipocyte spheroids and patient-specific explant cultures, this study provides proof-of-concept evidence supporting the therapeutic potential of lipoxins in reducing obesity-related human adipose tissue inflammation.
Levy, B. D., Clish, C. B., Schmidt, B., Gronert, K. & Serhan, C. N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 (2001).
Ioannidou, A. et al. Hypertrophied human adipocyte spheroids as in vitro model of weight gain and adipose tissue dysfunction. J. Physiol. 600, 869–883 (2022).
Lauschke, V. M. & Hagberg, C. E. Next-generation human adipose tissue culture methods. Curr. Opin. Genet. Dev. 80, 102057 (2023).
Beydag-Tasoz, B. S., Yennek, S. & Grapin-Botton, A. Towards a better understanding of diabetes mellitus using organoid models. Nat. Rev. Endocrinol. 19, 232–248 (2023).
Serhan, C. N., Fiore, S., Brezinski, D. A. & Lynch, S. Lipoxin A4 metabolism by differentiated HL-60 cells and human monocytes: conversion to novel 15-oxo and dihydro products. Biochemistry 32, 6313–6319 (1993).
Godson, C., Guiry, P. & Brennan, E. Lipoxin mimetics and the resolution of inflammation. Annu. Rev. Pharmacol. Toxicol. 63, 429–448 (2023).
Ozdemir, A. T., Nalbantsoy, A., Ozgul Ozdemir, R. B. & Berdeli, A. Effects of 15-lipoxygenase overexpressing adipose tissue mesenchymal stem cells on the Th17 / Treg plasticity. Prostaglandins Other Lipid Mediat. 159, 106610 (2022).
Fredman, G. et al. Resolvin D1 limits 5-lipoxygenase nuclear localization and leukotriene B4 synthesis by inhibiting a calcium-activated kinase pathway. Proc. Natl Acad. Sci. USA 111, 14530–14535 (2014).
Qin, C. X. et al. Formylpeptide receptor 2: nomenclature, structure, signalling and translational perspectives: IUPHAR review 35. Br. J. Pharmacol. 179, 4617–4639 (2022).
Tourki, B. et al. Lack of resolution sensor drives age-related cardiometabolic and cardiorenal defects and impedes inflammation-resolution in heart failure. Mol. Metab. 31, 138–149 (2020).
Takano, T. et al. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J. Exp. Med. 185, 1693–1704 (1997).
Dyall, S. C. et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 86, 101165 (2022).
Amiri Khosroshahi, R. et al. The effects of omega-3 fatty acids supplementation on inflammatory factors in cancer patients: a systematic review and dose-response meta-analysis of randomized clinical trials. Nutr. Cancer 76, 1–16 (2024).
Delpino, F. M. et al. Omega-3 supplementation and diabetes: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 62, 4435–4448 (2022). This meta-analysis evaluates the effects of ω-3 supplementation on parameters of T2DM in humans and demonstrates that the supplementation had a protective effect in approximately 70% of the studies.
Nomali, M. et al. Omega-3 supplementation and outcomes of heart failure: a systematic review of clinical trials. Medicine 103, e36804 (2024).
Kiecolt-Glaser, J. K. et al. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav. Immun. 26, 988–995 (2012).
Kratz, M. et al. n3 PUFAs do not affect adipose tissue inflammation in overweight to moderately obese men and women. J. Nutr. 143, 1340–1347 (2013).
Sherratt, S. C. R., Mason, R. P., Libby, P., Steg, P. G. & Bhatt, D. L. Do patients benefit from omega-3 fatty acids. Cardiovasc. Res. 119, 2884–2901 (2024).
Yokoyama, M. et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098 (2007).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2019).
Miyauchi, K. et al. Randomized trial for evaluation in secondary prevention efficacy of combination therapy-statin and eicosapentaenoic acid (RESPECT-EPA). Circulation 150, 425–434 (2024).
Hariri, M. et al. Does omega-3 fatty acids supplementation affect circulating leptin levels? A systematic review and meta-analysis on randomized controlled clinical trials. Clin. Endocrinol. 82, 221–228 (2015).
Zhang, Y. Y., Liu, W., Zhao, T. Y. & Tian, H. M. Efficacy of omega-3 polyunsaturated fatty acids supplementation in managing overweight and obesity: a meta-analysis of randomized clinical trials. J. Nutr. Health Aging 21, 187–192 (2017).
Delpino, F. M., Figueiredo, L. M. & da Silva, B. G. C. Effects of omega-3 supplementation on body weight and body fat mass: a systematic review. Clin. Nutr. ESPEN 44, 122–129 (2021).
Barden, A. et al. Effect of weight loss on neutrophil resolvins in the metabolic syndrome. Prostaglandins Leukot. Essent. Fat. Acids 148, 25–29 (2019).
Bakker, N. et al. Oral omega-3 PUFA supplementation modulates inflammation in adipose tissue depots in morbidly obese women: a randomized trial. Nutrition 111, 112055 (2023).
Titos, E. et al. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 187, 5408–5418 (2011).
Gemperle, C. et al. Resolvin D1 reduces inflammation in co-cultures of primary human macrophages and adipocytes by triggering macrophages. Prostaglandins Leukot. Essent. Fat. Acids 174, 102363 (2021).
Hellmann, J., Tang, Y., Kosuri, M., Bhatnagar, A. & Spite, M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 25, 2399–2407 (2011).
Pal, A. et al. Resolvin E1 derived from eicosapentaenoic acid prevents hyperinsulinemia and hyperglycemia in a host genetic manner. FASEB J. 34, 10640–10656 (2020).
Shimizu, T. et al. Resolvin E3 ameliorates high-fat diet-induced insulin resistance via the phosphatidylinositol-3-kinase/Akt signaling pathway in adipocytes. FASEB J. 36, e22188 (2022).
Sima, C. et al. ERV1 overexpression in myeloid cells protects against high fat diet induced obesity and glucose intolerance. Sci. Rep. 7, 12848 (2017).
Trilleaud, C. et al. Agonist anti-ChemR23 mAb reduces tissue neutrophil accumulation and triggers chronic inflammation resolution. Sci. Adv. 7, eabd1453 (2021).
Laguna-Fernandez, A. et al. ERV1/ChemR23 signaling protects against atherosclerosis by modifying oxidized low-density lipoprotein uptake and phagocytosis in macrophages. Circulation 138, 1693–1705 (2018).
Jung, T. W., Chung, Y. H., Kim, H. C., Abd El-Aty, A. M. & Jeong, J. H. Protectin DX attenuates LPS-induced inflammation and insulin resistance in adipocytes via AMPK-mediated suppression of the NF-κB pathway. Am. J. Physiol. Endocrinol. Metab. 315, E543–E551 (2018).
Martinez-Fernandez, L. et al. Maresin 1 improves insulin sensitivity and attenuates adipose tissue inflammation in ob/ob and diet-induced obese mice. FASEB J. 31, 2135–2145 (2017).
Martinez-Fernandez, L. et al. Maresin 1 regulates insulin signaling in human adipocytes as well as in adipose tissue and muscle of lean and obese mice. J. Physiol. Biochem. 77, 167–173 (2021).
Laiglesia, L. M. et al. Maresin 1 inhibits TNF-alpha-induced lipolysis and autophagy in 3T3-L1 adipocytes. J. Cell Physiol. 233, 2238–2246 (2018).
Martinez-Fernandez, L. et al. Maresin 1 exerts a tissue-specific regulation of adipo-hepato-myokines in diet-induced obese mice and modulates adipokine expression in cultured human adipocytes in basal and inflammatory conditions. Biomolecules 13, 919 (2023).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Sugimoto, S. et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat. Metab. 4, 775–790 (2022). This study highlights that the synthesis of maresins in brown adipose tissue can be induced in response to cold exposure, resulting in the subsequent stimulation of inflammatory resolution pathways.
Felix-Soriano, E. et al. Changes in brown adipose tissue lipid mediator signatures with aging, obesity, and DHA supplementation in female mice. FASEB J. 35, e21592 (2021).
Laiglesia, L. M. et al. Maresin 1 activates brown adipose tissue and promotes browning of white adipose tissue in mice. Mol. Metab. 74, 101749 (2023).
Zhao, Z. et al. CMKLR1 senses chemerin/resolvin E1 to control adipose thermogenesis and modulate metabolic homeostasis. Fundam. Res. 4, 575–588 (2022).
Kristof, E. et al. Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp. Cell Res. 377, 47–55 (2019).
Wang, Q., Jin, F., Zhang, J., Li, Z. & Yu, D. Lipoxin A4 promotes adipogenic differentiation and browning of mouse embryonic fibroblasts. Vitr. Cell Dev. Biol. Anim. 57, 953–961 (2021).
Goetz, L. H. & Schork, N. J. Personalized medicine: motivation, challenges, and progress. Fertil. Steril. 109, 952–963 (2018).
Morgan, P. et al. Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat. Rev. Drug Discov. 17, 167–181 (2018).
Miner, J. J. & Fitzgerald, K. A. A path towards personalized medicine for autoinflammatory and related diseases. Nat. Rev. Rheumatol. 19, 182–189 (2023).
Toro-Dominguez, D. & Alarcon-Riquelme, M. E. Precision medicine in autoimmune diseases: fact or fiction. Rheumatology 60, 3977–3985 (2021).
Passaro, A. et al. Cancer biomarkers: emerging trends and clinical implications for personalized treatment. Cell 187, 1617–1635 (2024).
Sethi, Y. et al. Precision medicine and the future of cardiovascular diseases: a clinically oriented comprehensive review. J. Clin. Med. 12, 1799 (2023).
Buckler, A. J. et al. Virtual transcriptomics: noninvasive phenotyping of atherosclerosis by decoding plaque biology from computed tomography angiography imaging. Arterioscler. Thromb. Vasc. Biol. 41, 1738–1750 (2021).
Buckler, A. J. et al. In silico model of atherosclerosis with individual patient calibration to enable precision medicine for cardiovascular disease. Comput. Biol. Med. 152, 106364 (2023).
DeGroat, W. et al. Discovering biomarkers associated and predicting cardiovascular disease with high accuracy using a novel nexus of machine learning techniques for precision medicine. Sci. Rep. 14, 1 (2024).
Ridker, P. M. et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 391, 319–328 (2018).
Al-Shaer, A. E. et al. Modeling human heterogeneity of obesity with diversity outbred mice reveals a fat mass-dependent therapeutic window for resolvin E1. FASEB J. 36, e22354 (2022).
Saraswathi, V., Heineman, R., Alnouti, Y., Shivaswamy, V. & Desouza, C. V. A combination of omega-3 PUFAs and COX inhibitors: a novel strategy to manage obesity-linked dyslipidemia and adipose tissue inflammation. J. Diabetes Complicat. 34, 107494 (2020).
Yin, S. et al. Effect of α-linolenic acid supplementation on cardiovascular disease risk profile in individuals with obesity or overweight: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 14, 1644–1655 (2023).
Moosavian, S. P., Arab, A., Mehrabani, S., Moradi, S. & Nasirian, M. The effect of omega-3 and vitamin E on oxidative stress and inflammation: systematic review and meta-analysis of randomized controlled trials. Int. J. Vitam. Nutr. Res. 90, 553–563 (2020).
Musazadeh, V. et al. Can omega-3 fatty acids and vitamin E co-supplementation affect obesity indices? Int. J. Vitam. Nutr. Res. 93, 471–480 (2023).
Al-Shaer, A. E., Buddenbaum, N. & Shaikh, S. R. Polyunsaturated fatty acids, specialized pro-resolving mediators, and targeting inflammation resolution in the age of precision nutrition. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1866, 158936 (2021).
Kraft, J. D. et al. Lipoxins modulate neutrophil oxidative burst, integrin expression and lymphatic transmigration differentially in human health and atherosclerosis. FASEB J. 36, e22173 (2022). This study shows that, in humans, lipoxins regulate neutrophil oxidative burst and the activation of the CD11b–CD18 integrin (which has a central role in clot activation and atherosclerosis), and it highlights the potential effects of lipoxins on thrombus formation in individuals with atherosclerosis.
Borgeson, E. et al. Lipoxin A4 inhibits Porphyromonas gingivalis-induced aggregation and reactive oxygen species production by modulating neutrophil-platelet interaction and CD11b expression. Infect. Immun. 79, 1489–1497 (2011). This manuscript demonstrates the mechanism underlying lipoxin-mediated attenuation of the aggregation of platelets and leukocytes, and resulting reactive oxygen species production, in human whole blood.
de Gaetano, G., Cerletti, C. & Evangelista, V. Recent advances in platelet-polymorphonuclear leukocyte interaction. Haemostasis 29, 41–49 (1999).
Blaho, V. A., Buczynski, M. W., Brown, C. R. & Dennis, E. A. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J. Biol. Chem. 284, 21599–21612 (2009).
Chan, M. M. & Moore, A. R. Resolution of inflammation in murine autoimmune arthritis is disrupted by cyclooxygenase-2 inhibition and restored by prostaglandin E2-mediated lipoxin A4 production. J. Immunol. 184, 6418–6426 (2010).
Loynes, C. A. et al. PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 4, eaar8320 (2018).
Schmid, T. & Brune, B. Prostanoids and resolution of inflammation — beyond the lipid-mediator class switch. Front. Immunol. 12, 714042 (2021).
Elajami, T. K. et al. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 30, 2792–2801 (2016).
Gomez, D. et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat. Med. 24, 1418–1429 (2018).
Hellmann, J. et al. Biosynthesis of D-series resolvins in skin provides insights into their role in tissue repair. J. Invest. Dermatol. 138, 2051–2060 (2018).
Higgins, G. et al. Activation of P2RY11 and ATP release by lipoxin A4 restores the airway surface liquid layer and epithelial repair in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 51, 178–190 (2014).
Borgeson, E. et al. Lipoxin A4 and benzo-lipoxin A4 attenuate experimental renal fibrosis. FASEB J. 25, 2967–2979 (2011).
Dormandy, J. A. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–1289 (2005).
Delgado-Lista, J. et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet 399, 1876–1885 (2022).
Jangsiripornpakorn, J. et al. The glucose-lowering effect of low-dose diacerein and its responsiveness metabolic markers in uncontrolled diabetes. BMC Res. Notes 15, 91 (2022).
Schebb, N. H. et al. Formation, signaling and occurrence of specialized pro-resolving lipid mediators — what is the evidence so far? Front. Pharmacol. 13, 838782 (2022).
Skarke, C. et al. Bioactive products formed in humans from fish oils. J. Lipid Res. 56, 1808–1820 (2015).
Witting, M. et al. Challenges and perspectives for naming lipids in the context of lipidomics. Metabolomics 20, 15 (2024).
Hasanzad, M. et al. Precision medicine journey through omics approach. J. Diabetes Metab. Disord. 21, 881–888 (2022).
Vande Walle, L. & Lamkanfi, M. Drugging the NLRP3 inflammasome: from signalling mechanisms to therapeutic targets. Nat. Rev. Drug Discov. 23, 43–66 (2024).
Acknowledgements
The work of E.B. is supported by the European Research Council (ERC-StG no. 804418), Independent Research Fund Denmark (DFF number 3165-00026B), Aarhus University Research Foundation (AUFF-E-2022-7-8), Novo Nordisk Foundation (NNF22OC0079363), the Swedish Research Council (VR 2023-02627), the Swedish state’s ALF-agreement (ALFGBG-978978), and Regionala FoU-medel Västra Götalandsregionen (OLG-2023-02-22). The authors thank S. Tavajoh and A. Harazin for their valuable input while assembling the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Mauro Perretti, who co-reviewed with Jianmin Chen, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soták, M., Clark, M., Suur, B.E. et al. Inflammation and resolution in obesity. Nat Rev Endocrinol 21, 45–61 (2025). https://doi.org/10.1038/s41574-024-01047-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41574-024-01047-y
This article is cited by
-
Pro-resolving lipid mediators in diseases: exploring the molecular basis and clinical implication
Molecular Biomedicine (2026)
-
Prognostic effect of triglyceride glucose-related parameters on all-cause and cardiovascular mortality in individuals with cardiovascular-kidney-metabolic syndrome: evidence from international multi-cohort studies
Cardiovascular Diabetology (2026)
-
Nimbolide ameliorates ARDS and ulcerative colitis by disrupting NLRP3 inflammasome activation
Communications Biology (2026)
-
Identification of modifiable plasma protein markers of cardiometabolic risk in children and adolescents with obesity
Nature Communications (2026)
-
Integrating multi-source data and machine learning to Decipher the psoriasis-COPD comorbidity
Clinical and Experimental Medicine (2026)