Abstract
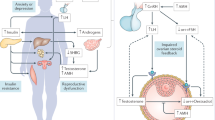
Polycystic ovary syndrome (PCOS) is a heterogeneous familial disorder affecting up to one in five women. The aetiology remains unclear, but available evidence suggests it is a polygenic disorder with epigenetic, developmental, and environmental components. The diagnostic criteria for PCOS are based on reproductive features, and the syndrome is categorized into several phenotypes that can vary by race and ethnicity. Insulin resistance and metabolic dysfunction have a crucial role in the pathogenesis of the syndrome and contribute to many adverse metabolic outcomes that place a substantial burden on the health of women with PCOS across their lifespan. Metabolic abnormalities like those identified in women with PCOS are also present in their female and male first-degree relatives. Overall, more emphasis is required on defining PCOS as a metabolic disorder in addition to a reproductive one. This approach could affect the management and future treatment options for the syndrome. The rationale of the current review is to identify and analyse existing evidence for PCOS as a metabolic, as well as a reproductive, disease.
Key points
-
Beyond reproductive features, polycystic ovary syndrome (PCOS) is associated with an increased prevalence of several metabolic abnormalities, some of which are also observed in the first-degree relatives of women with PCOS.
-
Insulin resistance and compensatory hyperinsulinaemia, intrinsic to PCOS and exacerbated by obesity, are major drivers of metabolic complications and strong determinants of reproductive dysfunction and hyperandrogenaemia in affected women.
-
The clinical presentation of PCOS is heterogeneous and can be categorized into several phenotypes that can vary by life stage, race and/or ethnicity and degree of adiposity.
-
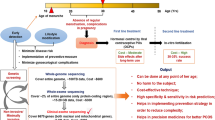
Cluster analysis suggests that there are reproducible reproductive and metabolic PCOS subtypes with distinct genetic architecture, which cannot be identified by the current diagnostic criteria.
-
With advances in omics-based studies and artificial intelligence-based methods, it might be possible to classify women with PCOS into subgroups that correlate with the clinical severity and identify those at high risk of long-term metabolic complications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lizneva, D. et al. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 106, 6–15 (2016).
Bozdag, G., Mumusoglu, S., Zengin, D., Karabulut, E. & Yildiz, B. O. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 31, 2841–2855 (2016).
Azziz, R. et al. Polycystic ovary syndrome. Nat. Rev. Dis. Prim. 2, 16057 (2016).
Riestenberg, C., Jagasia, A., Markovic, D., Buyalos, R. P. & Azziz, R. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J. Clin. Endocrinol. Metab. 107, 575–585 (2022).
Ruddenklau, A. & Campbell, R. E. Neuroendocrine impairments of polycystic ovary syndrome. Endocrinology 160, 2230–2242 (2019).
Moore, A. M. Impaired steroid hormone feedback in polycystic ovary syndrome: evidence from preclinical models for abnormalities within central circuits controlling fertility. Clin. Endocrinol. 97, 199–207 (2022).
Garg, A., Patel, B., Abbara, A. & Dhillo, W. S. Treatments targeting neuroendocrine dysfunction in polycystic ovary syndrome (PCOS). Clin. Endocrinol. 97, 156–164 (2022).
Cimino, I. et al. Novel role for anti-Müllerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 7, 10055 (2016).
Dewailly, D., Barbotin, A. L., Dumont, A., Catteau-Jonard, S. & Robin, G. Role of anti-Müllerian hormone in the pathogenesis of polycystic ovary syndrome. Front. Endocrinol. 11, 641 (2020).
Cassar, S. et al. Insulin resistance in polycystic ovary syndrome: a systematic review and meta-analysis of euglycaemic-hyperinsulinaemic clamp studies. Hum. Reprod. 31, 2619–2631 (2016).
Tosi, F., Bonora, E. & Moghetti, P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum. Reprod. 32, 2515–2521 (2017).
Diamanti-Kandarakis, E. & Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 33, 981–1030 (2012).
Zhao, H., Zhang, J., Cheng, X., Nie, X. & He, B. Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J. Ovarian Res. 16, 9 (2023).
Angelidi, A. M., Filippaios, A. & Mantzoros, C. S. Severe insulin resistance syndromes. J. Clin. Invest. 131, e142245 (2021).
Lungu, A. O., Zadeh, E. S., Goodling, A., Cochran, E. & Gorden, P. Insulin resistance is a sufficient basis for hyperandrogenism in lipodystrophic women with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 97, 563–567 (2012).
Malek, M. et al. Treatment of type B insulin resistance: a novel approach to reduce insulin receptor autoantibodies. J. Clin. Endocrinol. Metab. 95, 3641–3647 (2010).
Long, C. et al. Prevalence of polycystic ovary syndrome in patients with type 2 diabetes: a systematic review and meta-analysis. Front. Endocrinol. 13, 980405 (2022).
Bayona, A. et al. Prevalence of PCOS and related hyperandrogenic traits in premenopausal women with type 1 diabetes: a systematic review and meta-analysis. Hum. Reprod. Update 28, 501–517 (2022).
Kataoka, J. et al. Prevalence of polycystic ovary syndrome in women with severe obesity – effects of a structured weight loss programme. Clin. Endocrinol. 91, 750–758 (2019).
Teede, H. et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023. Monash.edu www.monash.edu/__data/assets/pdf_file/0003/3379521/Evidence-Based-Guidelines-2023.pdf (2023).
Brothers, K. J. et al. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 12, 295–305 (2010).
Liu, C. et al. High-fat and high-sucrose diet impairs female reproduction by altering ovarian transcriptomic and metabolic signatures. J. Transl. Med. 22, 145 (2024).
Wu, S. et al. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes 63, 1270–1282 (2014).
Franks, S., Stark, J. & Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Update 14, 367–378 (2008).
Barber, T. M. et al. Global adiposity rather than abnormal regional fat distribution characterizes women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93, 999–1004 (2008).
Mannerås-Holm, L. et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J. Clin. Endocrinol. Metab. 96, E304–E311 (2011).
Zhu, S. et al. Imaging-based body fat distribution in polycystic ovary syndrome: a systematic review and meta-analysis. Front. Endocrinol. 12, 697223 (2021).
Bril, F. et al. Adipose tissue dysfunction in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 109, 10–24 (2023).
Ezeh, U., Chen, I. Y. D., Chen, Y. H. & Azziz, R. Adipocyte expression of glucose transporter 1 and 4 in PCOS: relationship to insulin-mediated and non-insulin-mediated whole-body glucose uptake. Clin. Endocrinol. 90, 542–552 (2019).
Ezeh, U. et al. Alterations in plasma non-esterified fatty acid (NEFA) kinetics and relationship with insulin resistance in polycystic ovary syndrome. Hum. Reprod. 34, 335–344 (2019).
Ezeh, U., Chen, I. Y. D., Chen, Y. H. & Azziz, R. Adipocyte insulin resistance in PCOS: relationship with GLUT-4 expression and whole-body glucose disposal and β-cell function. J. Clin. Endocrinol. Metab. 105, e2408–e2420 (2020).
Dumesic, D. A. et al. Adipose insulin resistance in normal-weight women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 104, 2171–2183 (2019).
Echiburú, B. et al. Enlarged adipocytes in subcutaneous adipose tissue associated to hyperandrogenism and visceral adipose tissue volume in women with polycystic ovary syndrome. Steroids 130, 15–21 (2018).
Chazenbalk, G. et al. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 78, 920–926 (2013).
Blouin, K. et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin. Endocrinol. 72, 176–188 (2010).
Dumesic, D. A., Padmanabhan, V., Chazenbalk, G. D. & Abbott, D. H. Polycystic ovary syndrome as a plausible evolutionary outcome of metabolic adaptation. Reprod. Biol. Endocrinol. 20, 12 (2022).
O’Reilly, M. W. et al. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 102, 3327–3339 (2017).
Raeisi, T. et al. Circulating resistin and follistatin levels in obese and non-obese women with polycystic ovary syndrome: a systematic review and meta-analysis. PLoS ONE 16, e0246200 (2021).
Lin, K., Sun, X., Wang, X., Wang, H. & Chen, X. Circulating adipokine levels in nonobese women with polycystic ovary syndrome and in nonobese control women: a systematic review and meta-analysis. Front. Endocrinol. 11, 537809 (2021).
Livadas, S. et al. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine 47, 631–638 (2014).
Huang, A., Brennan, K. & Azziz, R. Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil. Steril. 93, 1938–1941 (2010).
Rosenfield, R. L. & Ehrmann, D. A. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 37, 467–520 (2016).
Sanchez-Garrido, M. A. & Tena-Sempere, M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 35, 100937 (2020).
Escobar-Morreale, H. F. The role of androgen excess in metabolic dysfunction in women: androgen excess and female metabolic dysfunction. Adv. Exp. Med. Biol. 1043, 597–608 (2017).
Aboeldalyl, S. et al. The role of chronic inflammation in polycystic ovarian syndrome–a systematic review and meta-analysis. Int. J. Mol. Sci. 22, 2734 (2021).
Huang, Z. H. et al. PCOS is associated with increased CD11c expression and crown-like structures in adipose tissue and increased central abdominal fat depots independent of obesity. J. Clin. Endocrinol. Metab. 98, E17–E24 (2013).
Sengupta, P., Dutta, S. & Fallah Hassan, M. Polycystic ovary syndrome (PCOS) and oxidative stress. J. Integr. Sci. Technol. 12, 752–752 (2024).
Bhattacharya, K. et al. Polycystic ovary syndrome and its management: in view of oxidative stress. Biomol. Concepts 15 https://doi.org/10.1515/bmc-2022-0038 (2024).
Lewis, R. D., Narayanaswamy, A. K., Farewell, D. & Rees, D. A. Complement activation in polycystic ovary syndrome occurs in the postprandial and fasted state and is influenced by obesity and insulin sensitivity. Clin. Endocrinol. 94, 74–84 (2021).
Butler, A. E., Moin, A. S. M., Sathyapalan, T. & Atkin, S. L. Components of the complement cascade differ in polycystic ovary syndrome. Int. J. Mol. Sci. 23, 12232 (2022).
Butler, A. E., Moin, A. S. M., Sathyapalan, T. & Atkin, S. L. Complement dysregulation in obese versus nonobese polycystic ovary syndrome patients. Cells 12, 2002 (2023).
Zawadzki, J. K. & Dunaif, A. in Polycystic Ovary Syndrome (eds Dunaif, A., Givens, J. R. & Haseltine, F.) 377–384 (Blackwell, 1992).
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 81, 19–25 (2004).
Azziz, R. et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil. Steril. 91, 456–488 (2009).
Day, F. et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 14, e1007813 (2018). This large-scale meta-analysis of genome-wide association studies in women with PCOS shows a similar underlying genetic architecture of PCOS phenotypes regardless of the diagnostic criteria used.
Joo, Y. Y. et al. A polygenic and phenotypic risk prediction for polycystic ovary syndrome evaluated by phenome-wide association studies. J. Clin. Endocrinol. Metab. 105, 1918–1936 (2020).
Daan, N. M. P. et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil. Steril. 102, 1444–1451 (2014).
Guastella, E., Longo, R. A. & Carmina, E. Clinical and endocrine characteristics of the main polycystic ovary syndrome phenotypes. Fertil. Steril. 94, 2197–2201 (2010).
Moghetti, P. et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 98, E628–E637 (2013).
Panidis, D. et al. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum. Reprod. 27, 541–549 (2012).
Moran, L. & Teede, H. Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum. Reprod. Update 15, 477–488 (2009).
Stepto, N. K. et al. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 28, 777–784 (2013). This study demonstrates the increased prevalence of insulin resistance even in lean women with PCOS, supporting the view that intrinsic insulin resistance is associated with the syndrome.
Diamanti-Kandarakis, E. & Panidis, D. Unravelling the phenotypic map of polycystic ovary syndrome (PCOS): a prospective study of 634 women with PCOS. Clin. Endocrinol. 67, 735–742 (2007).
Wiltgen, D. & Spritzer, P. M. Variation in metabolic and cardiovascular risk in women with different polycystic ovary syndrome phenotypes. Fertil. Steril. 94, 2493–2496 (2010).
Carmina, E. & Lobo, R. A. Comparing lean and obese PCOS in different PCOS phenotypes: evidence that the body weight is more important than the Rotterdam phenotype in influencing the metabolic status. Diagnostics 12, 2313 (2022).
Rajska, A., Buszewska-Forajta, M., Rachoń, D. & Markuszewski, M. J. Metabolomic insight into polycystic ovary syndrome – an overview. Int. J. Mol. Sci. 21, 4853 (2020).
Insenser, M. & Escobar-Morreale, H. F. Androgen excess in women: proteomic and metabolomic approaches. Front. Horm. Res. 53, 162–176 (2019).
Chen, B., Xu, P., Wang, J. & Zhang, C. The role of miRNA in polycystic ovary syndrome (PCOS). Gene 706, 91–96 (2019).
Lizneva, D. et al. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: systematic review and meta-analysis. Fertil. Steril. 106, 1510–1520 (2016).
Sendur, S. N. & Yildiz, B. O. Influence of ethnicity on different aspects of polycystic ovary syndrome: a systematic review. Reprod. Biomed. Online 42, 799–818 (2021).
VanHise, K. et al. Racial and ethnic disparities in polycystic ovary syndrome. Fertil. Steril. 119, 348–354 (2023).
Kazemi, M. et al. Comprehensive evaluation of disparities in cardiometabolic and reproductive risk between Hispanic and White women with polycystic ovary syndrome in the United States: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 226, 187–204 (2022).
Lee, I., Vresilovic, J., Irfan, M., Gallop, R. & Dokras, A. Higher incidence of metabolic syndrome in black women with polycystic ovary syndrome: a longitudinal study. J. Clin. Endocrinol. Metab. 107, E1558–E1567 (2022).
Chan, J. L. et al. Racial and ethnic differences in the prevalence of metabolic syndrome and its components of metabolic syndrome in women with polycystic ovary syndrome: a regional cross-sectional study. Am. J. Obstet. Gynecol. 217, 189.e1–189.e8 (2017).
Ezeh, U., Ida Chen, Y. D. & Azziz, R. Racial and ethnic differences in the metabolic response of polycystic ovary syndrome. Clin. Endocrinol. 93, 163–172 (2020).
Lo, J. C. et al. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 91, 1357–1363 (2006).
Lofton, H., Ard, J. D., Hunt, R. R. & Knight, M. G. Obesity among African American people in the United States: a review. Obesity 31, 306–315 (2023).
Hyatt, T. C. et al. Insulin sensitivity in African-American and white women: association with inflammation. Obesity 17, 276–282 (2009).
Allister-Price, C., Craig, C. M., Spielman, D., Cushman, S. S. & McLaughlin, T. L. Metabolic markers, regional adiposity, and adipose cell size: relationship to insulin resistance in African-American as compared with Caucasian women. Int. J. Obes. 43, 1164–1173 (2019).
Armiyaw, L., Sarcone, C., Fosam, A. & Muniyappa, R. Increased β-cell responsivity independent of insulin sensitivity in healthy African American adults. J. Clin. Endocrinol. Metab. 105, e2429–e2438 (2020).
Chung, S. T. et al. Postprandial insulin response and clearance among black and white women: the Federal Women’s Study. J. Clin. Endocrinol. Metab. 104, 181–192 (2019).
Inaishi, J. & Saisho, Y. Ethnic similarities and differences in the relationship between beta cell mass and diabetes. J. Clin. Med. 6, 113 (2017).
Ibáñez, L. et al. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm. Res. Paediatr. 88, 371–395 (2017).
Cree-Green, M. et al. Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome. J. Endocr. Soc. 1, 931–944 (2017).
Winters, S. J., Talbott, E., Guzick, D. S., Zborowski, J. & McHugh, K. P. Serum testosterone levels decrease in middle age in women with the polycystic ovary syndrome. Fertil. Steril. 73, 724–729 (2000).
Alsamarai, S. et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J. Clin. Endocrinol. Metab. 94, 4961–4970 (2009).
Elting, M. W., Korsen, T. J. M., Rekers-Mombarg, L. T. M. & Schoemaker, J. Women with polycystic ovary syndrome gain regular menstrual cycles when ageing. Hum. Reprod. 15, 24–28 (2000).
Jacewicz-święcka, M., Wołczyński, S. & Kowalska, I. The effect of ageing on clinical, hormonal and sonographic features associated with PCOS – a long-term follow-up study. J. Clin. Med. 10, 2101 (2021).
van Keizerswaard, J., Dietz de Loos, A. L. P., Louwers, Y. V. & Laven, J. S. E. Changes in individual polycystic ovary syndrome phenotypical characteristics over time: a long-term follow-up study. Fertil. Steril. 117, 1059–1066 (2022). This long-term follow-up study provides evidence for the changes in the prevalence of PCOS phenotypes over time.
Millán-de-Meer, M., Luque-Ramírez, M., Nattero-Chávez, L. & Escobar-Morreale, H. F. PCOS during the menopausal transition and after menopause: a systematic review and meta-analysis. Hum. Reprod. Update 29, 741–772 (2023).
Behboudi-Gandevani, S. et al. Cardiometabolic risks in polycystic ovary syndrome: long-term population-based follow-up study. Fertil. Steril. 110, 1377–1386 (2018).
Kazemi Jaliseh, H. et al. Polycystic ovary syndrome is a risk factor for diabetes and prediabetes in middle-aged but not elderly women: a long-term population-based follow-up study. Fertil. Steril. 108, 1078–1084 (2017).
Polotsky, A. J. et al. Hyperandrogenic oligomenorrhea and metabolic risks across menopausal transition. J. Clin. Endocrinol. Metab. 99, 2120–2127 (2014).
Aksun, S. et al. Alterations of cardiometabolic risk profile in polycystic ovary syndrome: 13 years follow-up in an unselected population. J. Endocrinol. Invest. 47, 1129–1137 (2023).
Vink, J. M., Sadrzadeh, S., Lambalk, C. B. & Boomsma, D. I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J. Clin. Endocrinol. Metab. 91, 2100–2104 (2006).
Dapas, M. & Dunaif, A. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr. Rev. 43, 927–965 (2022).
Dapas, M. et al. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLoS Med. 17, e1003132 (2020). Using unsupervised cluster analysis in a genotyped cohort of women with PCOS, this study reveals reproducible reproductive and metabolic subtypes of PCOS with distinct genetic architecture.
Stener-Victorin, E. & Deng, Q. Epigenetic inheritance of polycystic ovary syndrome – challenges and opportunities for treatment. Nat. Rev. Endocrinol. 17, 521–533 (2021).
Risal, S. et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat. Med. 25, 1894–1904 (2019).
Lim, S. S., Davies, M. J., Norman, R. J. & Moran, L. J. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. Update 18, 618–637 (2012).
Yildiz, B. O. Polycystic ovary syndrome: is obesity a symptom? Women’s Health 9, 505–507 (2013).
Azziz, R. et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 89, 2745–2749 (2004).
Asunción, M. et al. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J. Clin. Endocrinol. Metab. 85, 2434–2438 (2000).
Kumarapeli, V., Seneviratne, R. D. A., Wijeyaratne, C. N., Yapa, R. M. S. C. & Dodampahala, S. H. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semi-urban population in Sri Lanka. Am. J. Epidemiol. 168, 321–328 (2008).
Yildiz, B. O., Knochenhauer, E. S. & Azziz, R. Impact of obesity on the risk for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93, 162–168 (2008).
Yildiz, B. O., Bozdag, G., Yapici, Z., Esinler, I. & Yarali, H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum. Reprod. 27, 3067–3073 (2012). This study highlights the risk of metabolic syndrome in different PCOS phenotypes in an unselected, medically unbiased population.
Escobar-Morreale, H. F., Santacruz, E., Luque-Ramírez, M. & Carretero, J. I. B. Prevalence of ‘obesity-associated gonadal dysfunction’ in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum. Reprod. Update 23, 390–408 (2017).
Koivuaho, E. et al. Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood – longitudinal analysis of BMI data from birth to age 46 in cases of PCOS. Int. J. Obes. 43, 1370–1379 (2019).
Ollila, M. M. E. et al. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. J. Clin. Endocrinol. Metab. 101, 739–747 (2016).
Brower, M. A. et al. Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum. Reprod. 34, 127–136 (2019).
Zhao, Y. et al. Body mass index and polycystic ovary syndrome: a 2-sample bidirectional mendelian randomization study. J. Clin. Endocrinol. Metab. 105, 1778–1784 (2020).
Liu, Q. et al. Genomic correlation, shared loci, and causal relationship between obesity and polycystic ovary syndrome: a large-scale genome-wide cross-trait analysis. BMC Med. 20, 66 (2022).
Lim, S. S., Norman, R. J., Davies, M. J. & Moran, L. J. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes. Rev. 14, 95–109 (2013).
Zhang, L. et al. Impact of body mass index on assisted reproductive technology outcomes in patients with polycystic ovary syndrome: a meta-analysis. Reprod. Biomed. Online 48, 103849 (2024).
Glueck, C. J. & Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism 92, 108–120 (2019).
Benham, J. L. et al. Impact of bariatric surgery on anthropometric, metabolic, and reproductive outcomes in polycystic ovary syndrome: a systematic review and meta-analysis. Obes. Rev. 25, e13737 (2024).
Bhandari, M. et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes. Surg. 32, 3305–3312 (2022).
Samarasinghe, S. N. S. et al. Bariatric surgery for spontaneous ovulation in women living with polycystic ovary syndrome: the BAMBINI multicentre, open-label, randomised controlled trial. Lancet 403, 2489–2503 (2024).
Benito, E. et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J. Clin. Endocrinol. Metab. 105, E3384–E3391 (2020).
Ehrmann, D. A. et al. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J. Clin. Invest. 96, 520–527 (1995). This study demonstrates β-cell dysfunction in women with PCOS in the absence of impaired glucose tolerance.
Kakoly, N. S. et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum. Reprod. Update 24, 455–467 (2018).
Slopien, R. et al. Menopause and diabetes: EMAS clinical guide. Maturitas 117, 6–10 (2018).
Anagnostis, P. et al. Risk of type 2 diabetes mellitus in polycystic ovary syndrome is associated with obesity: a meta-analysis of observational studies. Endocrine 74, 245–253 (2021).
Wekker, V. et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum. Reprod. Update 26, 942–960 (2020).
Ollila, M. M. E. et al. Overweight and obese but not normal weight women with PCOS are at increased risk of type 2 diabetes mellitus – a prospective, population-based cohort study. Hum. Reprod. 32, 423–431 (2017). This study provides long-term follow-up data on the risk of type 2 diabetes mellitus in women with PCOS.
Rubin, K. H., Glintborg, D., Nybo, M., Abrahamsen, B. & Andersen, M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 102, 3848–3857 (2017).
Ryu, K. J. et al. Risk of type 2 diabetes is increased in nonobese women with polycystic ovary syndrome: the National Health Insurance Service–National Sample Cohort Study. Fertil. Steril. 115, 1569–1575 (2021).
Zhu, T., Cui, J. & Goodarzi, M. O. Polycystic ovary syndrome and risk of type 2 diabetes, coronary heart disease, stroke. Diabetes 70, 627–637 (2021).
Sweeting, A., Wong, J., Murphy, H. R. & Ross, G. P. A clinical update on gestational diabetes mellitus. Endocr. Rev. 43, 763–793 (2022).
Palomba, S. et al. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update 21, 575–592 (2015).
Sha, T., Wang, X., Cheng, W. & Yan, Y. A meta-analysis of pregnancy-related outcomes and complications in women with polycystic ovary syndrome undergoing IVF. Reprod. Biomed. Online 39, 281–293 (2019).
Yan, Q. et al. The incidence of gestational diabetes mellitus among women with polycystic ovary syndrome: a meta-analysis of longitudinal studies. BMC Pregnancy Childbirth 22, 370 (2022).
Legro, R. S., Kunselman, A. R. & Dunaif, A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am. J. Med. 111, 607–613 (2001).
Wild, R. A., Rizzo, M., Clifton, S. & Carmina, E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil. Steril. 95, 1073–1079 (2011).
Roe, A. et al. Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J. Clin. Endocrinol. Metab. 99, E841–E847 (2014).
Alberti, K. G. M. M. et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International atherosclerosis society; and international association for the study of obesity. Circulation 120, 1640–1645 (2009).
Kakoly, N. S., Moran, L. J., Teede, H. J. & Joham, A. E. Cardiometabolic risks in PCOS: a review of the current state of knowledge. Expert. Rev. Endocrinol. Metab. 14, 23–33 (2019).
Lim, S. S. et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes. Rev. 20, 339–352 (2019).
Vernon, G., Baranova, A. & Younossi, Z. M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34, 274–285 (2011).
Tziomalos, K., G. Athyros, V. & Karagiannis, A. Non-alcoholic fatty liver disease in type 2 diabetes: pathogenesis and treatment options. Curr. Vasc. Pharmacol. 10, 162–172 (2012).
Kelley, C. E., Brown, A. J., Diehl, A. M. & Setji, T. L. Review of nonalcoholic fatty liver disease in women with polycystic ovary syndrome. World J. Gastroenterol. 20, 14172–14184 (2014).
Falzarano, C. et al. Nonalcoholic fatty liver disease in women and girls with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 107, 258–272 (2022).
Gomez, J. M. D. et al. Subclinical atherosclerosis and polycystic ovary syndrome. Fertil. Steril. 117, 912–923 (2022).
Mousa, A. et al. Polycystic ovary syndrome: Technical report for the International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome, 2023. Monash.edu www.monash.edu/__data/assets/pdf_file/0010/3379591/TechnicalReport-2023.pdf (2023).
Yilmaz, B., Vellanki, P., Ata, B. & Yildiz, B. O. Metabolic syndrome, hypertension, and hyperlipidemia in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil. Steril. 109, 356–364 (2018). This systematic review and meta-analysis provides compelling evidence of increased prevalence of metabolic syndrome, hypertension and dyslipidaemia in first-degree relatives of women with PCOS compared with the general population.
Yilmaz, B., Vellanki, P., Ata, B. & Yildiz, B. O. Diabetes mellitus and insulin resistance in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil. Steril. 110, 523–533 (2018). This meta-analysis provides quantitative evidence of the clustering of insulin resistance and type 2 diabetes mellitus in the parents and siblings of women with PCOS.
Crisosto, N. et al. Reproductive and metabolic features during puberty in sons of women with polycystic ovary syndrome. Endocr. Connect. 6, 607–613 (2017).
Recabarren, S. E. et al. Metabolic profile in sons of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 93, 1820–1826 (2008).
Zhu, J. et al. Evidence from men for ovary-independent effects of genetic risk factors for polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 107, E1577–E1587 (2022).
Moolhuijsen, L. M. E. et al. Genomic and proteomic evidence for hormonal and metabolic foundations of polycystic ovary syndrome. Preprint at medRxiv https://doi.org/10.1101/2024.04.18.24306020 (2024).
Yildiz, B. O., Yarali, H., Oguz, H. & Bayraktar, M. Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 88, 2031–2036 (2003).
Shan, D. et al. Reproductive health in first-degree relatives of patients with polycystic ovary syndrome: a review and meta-analysis. J. Clin. Endocrinol. Metab. 107, 273–295 (2022).
Chang, S. & Dunaif, A. Diagnosis of polycystic ovary syndrome: which criteria to use and when? Endocrinol. Metab. Clin. North. Am. 50, 11–23 (2021).
Buchanan, T. A., Watanabe, R. M. & Xiang, A. H. Limitations in surrogate measures of insulin resistance. J. Clin. Endocrinol. Metab. 95, 4874–4876 (2010).
Silva, I. S. et al. Polycystic ovary syndrome: clinical and laboratory variables related to new phenotypes using machine-learning models. J. Endocrinol. Invest. 45, 497–505 (2022).
Suha, S. A. & Islam, M. N. An extended machine learning technique for polycystic ovary syndrome detection using ovary ultrasound image. Sci. Rep. 12, 17123 (2022).
Zhu, T. & Goodarzi, M. O. Causes and consequences of polycystic ovary syndrome: insights from mendelian randomization. J. Clin. Endocrinol. Metab. 107, E899–E911 (2022).
Bastarache, L., Denny, J. C. & Roden, D. M. Phenome-wide association studies. JAMA 327, 75–76 (2022).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Anna Benrick and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Helvaci, N., Yildiz, B.O. Polycystic ovary syndrome as a metabolic disease. Nat Rev Endocrinol 21, 230–244 (2025). https://doi.org/10.1038/s41574-024-01057-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41574-024-01057-w
This article is cited by
-
Exploring biomarkers of mitochondrial dynamics in polycystic ovary syndrome and the effect of mesenchymal stem cell transplantation on ovarian mitochondria
Journal of Ovarian Research (2026)
-
Plumbagin ameliorates ferroptosis of ovarian granulosa cells in polycystic ovary syndrome by down-regulating SLC7A5 m6A methylation modification through inhibition of YTHDF1
Journal of Ovarian Research (2025)
-
Lipid accumulation product and visceral adiposity index in polycystic ovary syndrome: a systematic review and meta-analysis of observational studies
Lipids in Health and Disease (2025)
-
Distribution of polycystic ovary syndrome (PCOS) phenotypes in Iranian women: a cross-sectional study
BMC Research Notes (2025)
-
Role and interaction of LncRNAs and insulin resistance in polycystic ovary syndrome: a narrative review
Journal of Ovarian Research (2025)