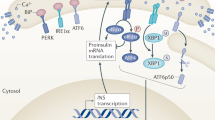
Abstract
Insufficient insulin secretion by pancreatic β cells is central to the pathogenesis of diabetes mellitus. As insulin is synthesized in the endoplasmic reticulum (ER), perturbations in ER homeostasis lead to ER stress and activate the ER stress response. Over the past two decades, considerable data have accumulated on the role of β cell ER stress in diabetes mellitus. Several monogenic forms of diabetes mellitus are caused by excessive ER stress, perturbed ER stress response signalling or impaired ER–Golgi protein trafficking. These pathways are now recognized to contribute to β cell failure in both type 1 and type 2 diabetes mellitus. This Review considers the role of β cell ER stress in common forms of diabetes mellitus and examines whether it is a cause or a consequence of these diseases. The strong genetic evidence for a causal role of ER stress in 15 monogenic forms of diabetes mellitus is summarized, and the effects of ER stress on human β cell differentiation, function and survival are described. Although definitive proof is lacking that ER stress responses can be therapeutically targeted to improve β cell function in diabetes mellitus, existing and novel treatments that aim to restore ER homeostasis are also outlined.
Key points
-
The unfolded protein response (UPR) is an essential adaptive mechanism in β cells that is activated by perturbed functioning of the endoplasmic reticulum (ER) and/or Golgi organelles.
-
The effects of ER stress on β cell proliferation, differentiation, function, apoptosis and immune cell crosstalk depend on the β cell developmental stage, duration of ER stress and genetic background.
-
Of ~70 known monogenic forms of diabetes mellitus, 15 provide indisputable genetic evidence for the critical roles of ER stress, dysregulated UPR and impaired ER–Golgi trafficking in human β cells.
-
ER stress occurs during both early and late stages in the pathogenesis of polygenic type 1 and type 2 diabetes mellitus, but causality has yet to be firmly demonstrated.
-
ER stress modulators have been shown to protect β cells in preclinical studies, and a few early clinical studies are underway.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402, 203–234 (2023).
American Diabetes Association Professional Practice Committee. 2. Diagnosis and classification of diabetes: standards of care in diabetes – 2025. Diabetes Care 48, S27–S49 (2025).
Piron, A. et al. Identification of novel type 1 and type 2 diabetes genes by co-localization of human islet eQTL and GWAS variants with colocRedRibbon. Preprint at medRxiv https://doi.org/10.1101/2024.10.19.24315808 (2024).
Chiou, J. et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature 594, 398–402 (2021).
Robertson, C. C. et al. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. Nat. Genet. 53, 962–971 (2021).
Suzuki, K. et al. Genetic drivers of heterogeneity in type 2 diabetes pathophysiology. Nature 627, 347–357 (2024).
Smith, K. et al. Multi-ancestry polygenic mechanisms of type 2 diabetes. Nat. Med. 30, 1065–1074 (2024).
Redondo, M. J. & Morgan, N. G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 19, 542–554 (2023).
Ahlqvist, E. et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6, 361–369 (2018).
Nair, A. T. N. et al. Heterogeneity in phenotype, disease progression and drug response in type 2 diabetes. Nat. Med. 28, 982–988 (2022).
Mansour et al. Genome-wide association analyses highlight etiological differences underlying newly defined subtypes of diabetes. Nat. Genet. 53, 1534–1542 (2021).
Udler, M. S. et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: a soft clustering analysis. PLoS Med. 15, e1002654 (2018).
Marhfour, I. et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 55, 2417–2420 (2012).
Laybutt, D. R. et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50, 752–763 (2007).
Huang, C. J. et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated β-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56, 2016–2027 (2007).
Marchetti, P. et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50, 2486–2494 (2007).
Kang, S. W. et al. Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127, 999–1013 (2006).
Guo, H. et al. Inefficient translocation of preproinsulin contributes to pancreatic β cell failure and late-onset diabetes. J. Biol. Chem. 289, 16290–16302 (2014).
Melnyk, A., Lang, S., Sicking, M., Zimmermann, R. & Jung, M. Co-chaperones of the human endoplasmic reticulum: an update. Subcell. Biochem. 101, 247–291 (2023).
Boriushkin, E., Wang, J. J. & Zhang, S. X. Role of p58IPK in endoplasmic reticulum stress-associated apoptosis and inflammation. J. Ophthalmic Vis. Res. 9, 134–143 (2014).
Yan, Y., Rato, C., Rohland, L., Preissler, S. & Ron, D. MANF antagonizes nucleotide exchange by the endoplasmic reticulum chaperone BiP. Nat. Commun. 10, 541 (2019).
Preissler, S., Rato, C., Perera, L., Saudek, V. & Ron, D. FICD acts bifunctionally to AMPylate and de-AMPylate the endoplasmic reticulum chaperone BiP. Nat. Struct. Mol. Biol. 24, 23–29 (2017).
Siwecka, N. et al. The structure, activation and signaling of IRE1 and its role in determining cell fate. Biomedicines 9, 156 (2021).
Bone, R. N. et al. A computational approach for defining a signature of β-cell Golgi stress in diabetes. Diabetes 69, 2364–2376 (2020).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell. Biol. 8, 519–529 (2007).
Xu, X. et al. Proteasomal degradation of WT proinsulin in pancreatic beta cells. J. Biol. Chem. 298, 102406 (2022).
Kadowaki, H. et al. Pre-emptive quality control protects the ER from protein overload via the proximity of ERAD components and SRP. Cell Rep. 13, 944–956 (2015).
Braunstein, I., Zach, L., Allan, S., Kalies, K. U. & Stanhill, A. Proteasomal degradation of preemptive quality control (pQC) substrates is mediated by an AIRAPL–p97 complex. Mol. Biol. Cell 26, 3719–3727 (2015).
Legesse, A. et al. The role of RNF149 in the pre-emptive quality control substrate ubiquitination. Commun. Biol. 6, 385 (2023).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
Pirot, P. et al. Global profiling of genes modified by endoplasmic reticulum stress in pancreatic beta cells reveals the early degradation of insulin mRNAs. Diabetologia 50, 1006–1014 (2007).
Kovaleva, V. et al. MANF regulates neuronal survival and UPR through its ER-located receptor IRE1α. Cell Rep. 42, 112066 (2023).
Hu, P., Han, Z., Couvillon, A. D., Kaufman, R. J. & Exton, J. H. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 26, 3071–3084 (2006).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Piecyk, M., Ferraro-Peyret, C., Laville, D., Perros, F. & Chaveroux, C. Novel insights into the GCN2 pathway and its targeting. Therapeutic value in cancer and lessons from lung fibrosis development. FEBS J. 291, 4867–4889 (2024).
Girardin, S. E., Cuziol, C., Philpott, D. J. & Arnoult, D. The eIF2α kinase HRI in innate immunity, proteostasis, and mitochondrial stress. FEBS J. 288, 3094–3107 (2021).
Gal-Ben-Ari, S., Barrera, I., Ehrlich, M. & Rosenblum, K. PKR: a kinase to remember. Front. Mol. Neurosci. 11, 480 (2018).
Cnop, M., Toivonen, S., Igoillo Esteve, M. & Salpea, P. Endoplasmic reticulum stress and eIF2α phosphorylation: the achilles heel of pancreatic β cells. Mol. Metab. 6, 1024–1039 (2017).
Pappalardo, Z. et al. A whole-genome RNA interference screen reveals a role for Spry2 in insulin transcription and the unfolded protein response. Diabetes 66, 1703–1712 (2017).
Harding, H. P. et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099–1108 (2000).
Palam, L. R., Baird, T. D. & Wek, R. C. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem. 286, 10939–10949 (2011).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Novoa, I., Zeng, H., Harding, H. P. & Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 (2001).
Jousse, C. et al. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 163, 767–775 (2003).
van Huizen, R., Martindale, J. L., Gorospe, M. & Holbrook, N. J. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2α signaling. J. Biol. Chem. 278, 15558–15564 (2003).
Kim, I., Xu, W. & Reed, J. C. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug. Discov. 7, 1013–1030 (2008).
Gorasia, D. G. et al. A prominent role of PDIA6 in processing of misfolded proinsulin. Biochim. Biophys. Acta 1864, 715–723 (2016).
Eletto, D., Eletto, D., Boyle, S. & Argon, Y. PDIA6 regulates insulin secretion by selectively inhibiting the RIDD activity of IRE1. FASEB J. 30, 653–665 (2016).
Eletto, D., Eletto, D., Dersh, D., Gidalevitz, T. & Argon, Y. Protein disulfide isomerase A6 controls the decay of IRE1α signaling via disulfide-dependent association. Mol. Cell 53, 562–576 (2014).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999).
Brown, M. S., Ye, J., Rawson, R. B. & Goldstein, J. L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell 100, 391–398 (2000).
Schroder, M. & Kaufman, R. J. ER stress and the unfolded protein response. Mutat. Res. 569, 29–63 (2005).
Sharma, R. B., Darko, C. & Alonso, L. C. Intersection of the ATF6 and XBP1 ER stress pathways in mouse islet cells. J. Biol. Chem. 295, 14164–14177 (2020).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Yoshida, H. et al. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20, 6755–6767 (2000).
Fonseca, S. G. et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Invest. 120, 744–755 (2010).
Odisho, T., Zhang, L. & Volchuk, A. ATF6β regulates the Wfs1 gene and has a cell survival role in the ER stress response in pancreatic β-cells. Exp. Cell Res. 330, 111–122 (2015).
Bailey, D. & O’Hare, P. Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid. Redox Signal. 9, 2305–2321 (2007).
Murakami, T. et al. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat. Cell Biol. 11, 1205–1211 (2009).
Barbosa, S. et al. An orchestrated program regulating secretory pathway genes and cargos by the transmembrane transcription factor CREB-H. Traffic 14, 382–398 (2013).
Oh-Hashi, K., Hasegawa, T., Mizutani, Y., Takahashi, K. & Hirata, Y. Elucidation of brefeldin A-induced ER and Golgi stress responses in Neuro2a cells. Mol. Cell. Biochem. 476, 3869–3877 (2021).
Sampieri, L., Di Giusto, P. & Alvarez, C. CREB3 transcription factors: ER-Golgi stress transducers as hubs for cellular homeostasis. Front. Cell. Dev. Biol. 7, 123 (2019).
Knupp, J., Pletan, M. L., Arvan, P. & Tsai, B. Autophagy of the ER: the secretome finds the lysosome. FEBS J. 290, 5656–5673 (2023).
Fang, J. et al. COPII-dependent ER export: a critical component of insulin biogenesis and β-cell ER homeostasis. Mol. Endocrinol. 29, 1156–1169 (2015).
Staufner, C. et al. Defining clinical subgroups and genotype–phenotype correlations in NBAS-associated disease across 110 patients. Genet. Med. 22, 610–621 (2020).
Weigel, A. V. et al. ER-to-Golgi protein delivery through an interwoven, tubular network extending from ER. Cell 184, 2412–2429.e16 (2021).
Shomron, O. et al. COPII collar defines the boundary between ER and ER exit site and does not coat cargo containers. J. Cell. Biol. 220, e201907224 (2021).
Tojima, T., Suda, Y., Jin, N., Kurokawa, K. & Nakano, A. Spatiotemporal dissection of the Golgi apparatus and the ER–Golgi intermediate compartment in budding yeast. eLife 13, e92900 (2024).
Kim, W. K., Choi, W., Deshar, B., Kang, S. & Kim, J. Golgi stress response: new insights into the pathogenesis and therapeutic targets of human diseases. Mol. Cell 46, 191–199 (2023).
Sue, N. et al. Independent activation of CREB3L2 by glucose fills a regulatory gap in mouse β-cells by co-ordinating insulin biosynthesis with secretory granule formation. Mol. Metab. 79, 101845 (2024).
Fox, R. M., Hanlon, C. D. & Andrew, D. J. The CrebA/Creb3-like transcription factors are major and direct regulators of secretory capacity. J. Cell Biol. 191, 479–492 (2010).
Sbodio, J. I., Snyder, S. H. & Paul, B. D. Golgi stress response reprograms cysteine metabolism to confer cytoprotection in Huntington’s disease. Proc. Natl Acad. Sci. USA 115, 780–785 (2018).
Wang, L. et al. WFS1 functions in ER export of vesicular cargo proteins in pancreatic β-cells. Nat. Commun. 12, 6996 (2021).
Taguchi, Y. et al. Yip1A, a novel host factor for the activation of the IRE1 pathway of the unfolded protein response during Brucella infection. PLoS Pathog. 11, e1004747 (2015).
Tang, B. L. et al. A membrane protein enriched in endoplasmic reticulum exit sites interacts with COPII. J. Biol. Chem. 276, 40008–40017 (2001).
Yoshida, Y. et al. YIPF5 and YIF1A recycle between the ER and the Golgi apparatus and are involved in the maintenance of the Golgi structure. Exp. Cell. Res. 314, 3427–3443 (2008).
Yang, J. et al. IER3IP1 is critical for maintaining glucose homeostasis through regulating the endoplasmic reticulum function and survival of β cells. Proc. Natl Acad. Sci. USA 119, e2204443119 (2022).
Esk, C. et al. A human tissue screen identifies a regulator of ER secretion as a brain-size determinant. Science 370, 935–941 (2020).
Zhong, X. et al. Essential requirement for IER3IP1 in B cell development. Proc. Natl Acad. Sci. USA 120, e2312810120 (2023).
Raote, I., Saxena, S., Campelo, F. & Malhotra, V. TANGO1 marshals the early secretory pathway for cargo export. Biochim. Biophys. Acta Biomembr. 1863, 183700 (2021).
Arnolds, O. & Stoll, R. Characterization of a fold in TANGO1 evolved from SH3 domains for the export of bulky cargos. Nat. Commun. 14, 2273 (2023).
Lekszas, C. et al. Biallelic TANGO1 mutations cause a novel syndromal disease due to hampered cellular collagen secretion. eLife 9, e51319 (2020).
Fan, J. et al. cTAGE5 deletion in pancreatic β cells impairs proinsulin trafficking and insulin biogenesis in mice. J. Cell Biol. 216, 4153–4164 (2017).
Raote, I. et al. TANGO1 builds a machine for collagen export by recruiting and spatially organizing COPII, tethers and membranes. eLife 7, e32723 (2018).
Aoki, T. et al. Identification of the neuroblastoma-amplified gene product as a component of the syntaxin 18 complex implicated in Golgi-to-endoplasmic reticulum retrograde transport. Mol. Biol. Cell 20, 2639–2649 (2009).
Wicksteed, B., Alarcon, C., Briaud, I., Lingohr, M. K. & Rhodes, C. J. Glucose-induced translational control of proinsulin biosynthesis is proportional to preproinsulin mRNA levels in islet β-cells but not regulated via a positive feedback of secreted insulin. J. Biol. Chem. 278, 42080–42090 (2003).
Vasiljevic, J., Torkko, J. M., Knoch, K. P. & Solimena, M. The making of insulin in health and disease. Diabetologia 63, 1981–1989 (2020).
Guest, P. C. 2D gel electrophoresis of insulin secretory granule proteins from biosynthetically labelled pancreatic islets. Adv. Exp. Med. Biol. 974, 167–174 (2017).
Itoh, N. & Okamoto, H. Translational control of proinsulin synthesis by glucose. Nature 283, 100–102 (1980).
Evans-Molina, C. et al. Glucose regulation of insulin gene transcription and pre-mRNA processing in human islets. Diabetes 56, 827–835 (2007).
Liu, M. et al. Normal and defective pathways in biogenesis and maintenance of the insulin storage pool. J. Clin. Invest. 131, e142240 (2021).
Sahin, G. S., Lee, H. & Engin, F. An accomplice more than a mere victim: the impact of β-cell ER stress on type 1 diabetes pathogenesis. Mol. Metab. 54, 101365 (2021).
Gao, Y. et al. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Mol. Cell. Biol. 32, 5129–5139 (2012).
Zhang, P. et al. The PERK eukaryotic initiation factor 2α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 (2002).
Zhang, W. et al. PERK EIF2AK3 control of pancreatic β cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell. Metab. 4, 491–497 (2006).
Harding, H. P. et al. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 (2001).
Xu, T. et al. The IRE1α–XBP1 pathway regulates metabolic stress-induced compensatory proliferation of pancreatic β-cells. Cell Res. 24, 1137–1140 (2014).
Tsuchiya, Y. et al. IRE1–XBP1 pathway regulates oxidative proinsulin folding in pancreatic β cells. J. Cell. Biol. 217, 1287–1301 (2018).
Hassler, J. R. et al. The IRE1α/XBP1s pathway is essential for the glucose response and protection of β cells. PLoS Biol. 13, e1002277 (2015).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Usui, M. et al. Atf6α-null mice are glucose intolerant due to pancreatic β-cell failure on a high-fat diet but partially resistant to diet-induced insulin resistance. Metabolism 61, 1118–1128 (2012).
Engin, F. et al. Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci. Transl. Med. 5, 211ra156 (2013).
Lytrivi, M., Castell, A. L., Poitout, V. & Cnop, M. Recent insights into mechanisms of β-cell lipo- and glucolipotoxicity in type 2 diabetes. J. Mol. Biol. 432, 1514–1534 (2020).
Arunagiri, A. et al. Proinsulin misfolding is an early event in the progression to type 2 diabetes. eLife 8, e44532 (2019).
Chan, J. Y., Luzuriaga, J., Bensellam, M., Biden, T. J. & Laybutt, D. R. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in β-cell gene expression and progression to diabetes. Diabetes 62, 1557–1568 (2013).
Cunha, D. A. et al. Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J. Cell Sci. 121, 2308–2318 (2008).
Cunha, D. A. et al. Death protein 5 and p53-upregulated modulator of apoptosis mediate the endoplasmic reticulum stress-mitochondrial dialog triggering lipotoxic rodent and human β-cell apoptosis. Diabetes 61, 2763–2775 (2012).
Eizirik, D. L., Pasquali, L. & Cnop, M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat. Rev. Endocrinol. 16, 349–362 (2020).
Oshima, M. et al. Stearoyl CoA desaturase is a gatekeeper that protects human beta cells against lipotoxicity and maintains their identity. Diabetologia 63, 395–409 (2020).
Kahn, S. E., Andrikopoulos, S. & Verchere, C. B. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 48, 241–253 (1999).
Wirth, F. et al. A human antibody against pathologic IAPP aggregates protects beta cells in type 2 diabetes models. Nat. Commun. 14, 6294 (2023).
Shrestha, S. et al. Aging compromises human islet beta cell function and identity by decreasing transcription factor activity and inducing ER stress. Sci. Adv. 8, eabo3932 (2022).
Green, A. et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia 64, 2741–2750 (2021).
Khan, M. A. B. et al. Epidemiology of type 2 diabetes — global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 10, 107–111 (2020).
Snyder, J. T., Darko, C., Sharma, R. B. & Alonso, L. C. Endoplasmic reticulum stress induced proliferation remains intact in aging mouse β-cells. Front. Endocrinol. 12, 734079 (2021).
Jaskulska, A., Janecka, A. E. & Gach-Janczak, K. Thapsigargin — from traditional medicine to anticancer drug. Int. J. Mol. Sci. 22, 4 (2020).
Song, J. et al. Aging impairs adaptive unfolded protein response and drives beta cell dedifferentiation in humans. J. Clin. Endocrinol. Metab. 107, 3231–3241 (2022).
Brozzi, F. et al. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 58, 2307–2316 (2015).
Brozzi, F. & Eizirik, D. L. ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes. Ups. J. Med. Sci. 121, 133–139 (2016).
van Kuppeveld, F. J., de Jong, A. S., Melchers, W. J. & Willems, P. H. Enterovirus protein 2B po(u)res out the calcium: a viral strategy to survive? Trends Microbiol. 13, 41–44 (2005).
Eizirik, D. L., Cardozo, A. K. & Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 (2008).
Schuit, F. C., In’t Veld, P. A. & Pipeleers, D. G. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc. Natl Acad. Sci. USA 85, 3865–3869 (1988).
Eizirik, D. L. & Cnop, M. ER stress in pancreatic β cells: the thin red line between adaptation and failure. Sci. Signal. 3, pe7 (2010).
Marroqui, L. et al. Pancreatic α cells are resistant to metabolic stress-induced apoptosis in type 2 diabetes. eBioMedicine 2, 378–385 (2015).
Eizirik, D. L., Szymczak, F. & Mallone, R. Why does the immune system destroy pancreatic β-cells but not α-cells in type 1 diabetes? Nat. Rev. Endocrinol. 19, 425–434 (2023).
Yong, J., Johnson, J. D., Arvan, P., Han, J. & Kaufman, R. J. Therapeutic opportunities for pancreatic β-cell ER stress in diabetes mellitus. Nat. Rev. Endocrinol. 17, 455–467 (2021).
Sharma, R. B. et al. Insulin demand regulates β cell number via the unfolded protein response. J. Clin. Invest. 125, 3831–3846 (2015).
Perl, S. et al. Significant human β-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J. Clin. Endocrinol. Metab. 95, E234–E239 (2010).
Cnop, M. et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 53, 321–330 (2010).
Mezza, T. & Kulkarni, R. N. The regulation of pre- and post-maturational plasticity of mammalian islet cell mass. Diabetologia 57, 1291–1303 (2014).
Dai, C. et al. Stress-impaired transcription factor expression and insulin secretion in transplanted human islets. J. Clin. Invest. 126, 1857–1870 (2016).
Riahi, Y. et al. Inhibition of mTORC1 by ER stress impairs neonatal β-cell expansion and predisposes to diabetes in the Akita mouse. eLife 7, e38472 (2018).
Balboa, D. et al. Insulin mutations impair beta-cell development in a patient-derived iPSC model of neonatal diabetes. eLife 7, e38519 (2018).
Zhang, Y. et al. Permanent neonatal diabetes-causing insulin mutations have dominant negative effects on beta cell identity. Mol. Metab. 80, 101879 (2024).
Szabat, M. et al. Reduced insulin production relieves endoplasmic reticulum stress and induces β cell proliferation. Cell. Metab. 23, 179–193 (2016).
Chen, C. W. et al. Adaptation to chronic ER stress enforces pancreatic β-cell plasticity. Nat. Commun. 13, 4621 (2022).
Mezza, T. et al. β-cell fate in human insulin resistance and type 2 diabetes: a perspective on islet plasticity. Diabetes 68, 1121–1129 (2019).
Lee, H. et al. Beta cell dedifferentiation induced by IRE1α deletion prevents type 1 diabetes. Cell Metab. 31, 822–836.e5 (2020).
Lee, H. et al. Stress-induced β cell early senescence confers protection against type 1 diabetes. Cell Metab. 35, 2200–2215.e9 (2023).
Bensellam, M., Jonas, J. C. & Laybutt, D. R. Mechanisms of β-cell dedifferentiation in diabetes: recent findings and future research directions. J. Endocrinol. 236, R109–R143 (2018).
Song, B., Scheuner, D., Ron, D., Pennathur, S. & Kaufman, R. J. Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118, 3378–3389 (2008).
Oyadomari, S. et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 (2002).
Yong, J. et al. Chop/Ddit3 depletion in β cells alleviates ER stress and corrects hepatic steatosis in mice. Sci. Transl. Med. 13, eaba9796 (2021).
Lee, J.-H. et al. Endoplasmic reticulum stress in pancreatic β cells induces incretin desensitization and β-cell dysfunction via ATF4-mediated PDE4D expression. Am. J. Physiol. Endocrinol. Metab. 325, E448–E465 (2023).
Zhang, I. X., Ren, J., Vadrevu, S., Raghavan, M. & Satin, L. S. ER stress increases store-operated Ca2+ entry (SOCE) and augments basal insulin secretion in pancreatic beta cells. J. Biol. Chem. 295, 5685–5700 (2020).
Zhang, I. X. et al. ER stress increases expression of intracellular calcium channel RyR1 to modify Ca2+ homeostasis in pancreatic beta cells. J. Biol. Chem. 299, 105065 (2023).
Marre, M. L. et al. Modifying enzymes are elicited by ER stress, generating epitopes that are selectively recognized by CD4+ T cells in patients with type 1 diabetes. Diabetes 67, 1356–1368 (2018).
Kracht, M. J. et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat. Med. 23, 501–507 (2017).
Nasteska, D. et al. PDX1LOW MAFALOW β-cells contribute to islet function and insulin release. Nat. Commun. 12, 674 (2021).
Li, X. et al. Requirement for translocon-associated protein (TRAP) α in insulin biogenesis. Sci. Adv. 5, eaax0292 (2019).
Fuchsberger, C. et al. The genetic architecture of type 2 diabetes. Nature 536, 41–47 (2016).
Sandhu, M. S. et al. Common variants in WFS1 confer risk of type 2 diabetes. Nat. Genet. 39, 951–953 (2007).
Costanzo, M. C. et al. The type 2 diabetes knowledge portal: an open access genetic resource dedicated to type 2 diabetes and related traits. Cell Metab. 35, 695–710.e6 (2023).
Hanson, R. L. et al. Association of CREBRF variants with obesity and diabetes in Pacific Islanders from Guam and Saipan. Diabetologia 62, 1647–1652 (2019).
Williamson, A. et al. Genome-wide association study and functional characterization identifies candidate genes for insulin-stimulated glucose uptake. Nat. Genet. 55, 973–983 (2023).
Ghosh, C. et al. Involvement of Cdkal1 in the etiology of type 2 diabetes mellitus and microvascular diabetic complications: a review. J. Diabetes Metab. Disord. 21, 991–1001 (2022).
Rehman, S. U. et al. Alternative exon splicing and differential expression in pancreatic islets reveals candidate genes and pathways implicated in early diabetes development. Mamm. Genome 32, 153–172 (2021).
Mahajan, A. et al. Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nat. Genet. 54, 560–572 (2022).
Loffler, D. et al. Functional and clinical relevance of novel and known PCSK1 variants for childhood obesity and glucose metabolism. Mol. Metab. 6, 295–305 (2017).
Robertson, C. C. et al. Untangling the genetics of beta cell dysfunction and death in type 1 diabetes. Mol. Metab. 86, 101973 (2024).
Greenwald, W. W. et al. Pancreatic islet chromatin accessibility and conformation reveals distal enhancer networks of type 2 diabetes risk. Nat. Commun. 10, 2078 (2019).
Wang, G. et al. Integrating genetics with single-cell multiomic measurements across disease states identifies mechanisms of beta cell dysfunction in type 2 diabetes. Nat. Genet. 55, 984–994 (2023).
Vinuela, A. et al. Genetic variant effects on gene expression in human pancreatic islets and their implications for T2D. Nat. Commun. 11, 4912 (2020).
Russell, M. A. et al. HLA class II antigen processing and presentation pathway components demonstrated by transcriptome and protein analyses of islet β-cells from donors with type 1 diabetes. Diabetes 68, 988–1001 (2019).
Alonso, L. et al. TIGER: the gene expression regulatory variation landscape of human pancreatic islets. Cell Rep. 37, 109807 (2021).
Fadista, J. et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc. Natl Acad. Sci. USA 111, 13924–13929 (2014).
Marselli, L. et al. Persistent or transient human β cell dysfunction induced by metabolic stress: specific signatures and shared gene expression with type 2 diabetes. Cell Rep. 33, 108466 (2020).
Yi, X. et al. Mining the transcriptome of target tissues of autoimmune and degenerative pancreatic β-cell and brain diseases to discover therapies. iScience 25, 105376 (2022).
Nyalwidhe, J. O. et al. Comparative quantitative proteomic analysis of disease stratified laser captured microdissected human islets identifies proteins and pathways potentially related to type 1 diabetes. PLoS ONE 12, e0183908 (2017).
Yi, X. & Eizirik, D. L. β-cell gene expression stress signatures in types 1 and 2 diabetes. J. Diabetes 16, e70026 (2024).
Hartman, M. G. et al. Role for activating transcription factor 3 in stress-induced β-cell apoptosis. Mol. Cell. Biol. 24, 5721–5732 (2004).
Engin, F., Nguyen, T., Yermalovich, A. & Hotamisligil, G. S. Aberrant islet unfolded protein response in type 2 diabetes. Sci. Rep. 4, 4054 (2014).
Tersey, S. A. et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61, 818–827 (2012).
Yang, C. et al. Pathological endoplasmic reticulum stress mediated by the IRE1 pathway contributes to pre-insulitic beta cell apoptosis in a virus-induced rat model of type 1 diabetes. Diabetologia 56, 2638–2646 (2013).
De Franco, E. et al. Dominant ER stress-inducing WFS1 mutations underlie a genetic syndrome of neonatal/infancy-onset diabetes, congenital sensorineural deafness, and congenital cataracts. Diabetes 66, 2044–2053 (2017).
Delepine, M. et al. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott–Rallison syndrome. Nat. Genet. 25, 406–409 (2000).
Collardeau-Frachon, S. et al. Microscopic and ultrastructural features in Wolcott–Rallison syndrome, a permanent neonatal diabetes mellitus: about two autopsy cases. Pediatr. Diabetes 16, 510–520 (2015).
Skopkova, M. et al. EIF2S3 mutations associated with severe X-linked intellectual disability syndrome MEHMO. Hum. Mutat. 38, 409–425 (2017).
De Franco, E. et al. De novo mutations in EIF2B1 affecting eIF2 signaling cause neonatal/early-onset diabetes and transient hepatic dysfunction. Diabetes 69, 477–483 (2020).
Lytrivi, M. et al. DNAJC3 deficiency induces β-cell mitochondrial apoptosis and causes syndromic young-onset diabetes. Eur. J. Endocrinol. 184, 455–468 (2021).
Abdulkarim, B. et al. A missense mutation in PPP1R15B causes a syndrome including diabetes, short stature, and microcephaly. Diabetes 64, 3951–3962 (2015).
Al-Fadhli, F. M. et al. Biallelic loss of function variant in the unfolded protein response gene PDIA6 is associated with asphyxiating thoracic dystrophy and neonatal-onset diabetes. Clin. Genet. 99, 694–703 (2021).
De Franco, E. et al. A biallelic loss-of-function PDIA6 variant in a second patient with polycystic kidney disease, infancy-onset diabetes, and microcephaly. Clin. Genet. 102, 457–458 (2022).
Ansar, M. et al. Mutation of ATF6 causes autosomal recessive achromatopsia. Hum. Genet. 134, 941–950 (2015).
Kohl, S. et al. Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat. Genet. 47, 757–765 (2015).
Lee, E. J. et al. Mutations in unfolded protein response regulator ATF6 cause hearing and vision loss syndrome. J. Clin. Invest. 135, e175562 (2025).
Reuschlé, Q. et al. Loss of function of XBP1 splicing activity of IRE1α favors B cell tolerance breakdown. J. Autoimmun. 142, 103152 (2024).
Perera, L. A. et al. Infancy-onset diabetes caused by de-regulated AMPylation of the human endoplasmic reticulum chaperone BiP. EMBO Mol. Med. 15, e16491 (2023).
Rigoli, L. & Di Bella, C. Wolfram syndrome 1 and Wolfram syndrome 2. Curr. Opin. Pediatr. 24, 512–517 (2012).
Gorgogietas, V. et al. GLP-1R agonists demonstrate potential to treat Wolfram syndrome in human preclinical models. Diabetologia 66, 1306–1321 (2023).
Shen, Z.-Q. et al. CISD2 maintains cellular homeostasis. Biochim. Biophys. Acta 1868, 118954 (2021).
Montaser, H. et al. Loss of MANF causes childhood-onset syndromic diabetes due to increased endoplasmic reticulum stress. Diabetes 70, 1006–1018 (2021).
Poulton, C. J. et al. Microcephaly with simplified gyration, epilepsy, and infantile diabetes linked to inappropriate apoptosis of neural progenitors. Am. J. Hum. Genet. 89, 265–276 (2011).
Anitei, M. et al. IER3IP1-mutations cause microcephaly by selective inhibition of ER-Golgi transport. Cell. Mol. Life Sci. 81, 334 (2024).
Montaser, H. et al. IER3IP1 mutations cause neonatal diabetes due to impaired proinsulin trafficking. Diabetes 74, 514–527 (2024).
De Franco, E. et al. YIPF5 mutations cause neonatal diabetes and microcephaly through endoplasmic reticulum stress. J. Clin. Invest. 130, 6338–6353 (2020).
Stoy, J. et al. In celebration of a century with insulin — update of insulin gene mutations in diabetes. Mol. Metab. 52, 101280 (2021).
Liu, M. et al. Proinsulin misfolding and diabetes: mutant INS gene-induced diabetes of youth. Trends Endocrinol. Metab. 21, 652–659 (2010).
Liu, M. et al. Impaired cleavage of preproinsulin signal peptide linked to autosomal-dominant diabetes. Diabetes 61, 828–837 (2012).
Haataja, L. et al. Distinct states of proinsulin misfolding in MIDY. Cell. Mol. Life Sci. 78, 6017–6031 (2021).
Stoy, J. et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl Acad. Sci. USA 104, 15040–15044 (2007).
Ribeiro, C. M. P. & Hull-Ryde, E. A. Functional role of the ER stress transducer IRE1α in CF airway epithelial inflammation. Curr. Opin. Pharmacol. 65, 102258 (2022).
Hull-Ryde, E. A. et al. IRE1α is a therapeutic target for cystic fibrosis airway inflammation. Int. J. Mol. Sci. 22, 3063 (2021).
Hetz, C. & Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 13, 477–491 (2017).
Lebeaupin, C. et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 69, 927–947 (2018).
Moreno, J. A. et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 5, 206ra138 (2013).
Kim, M. J. et al. Specific PERK inhibitors enhanced glucose-stimulated insulin secretion in a mouse model of type 2 diabetes. Metabolism 97, 87–91 (2019).
Kim, M. J. et al. Attenuation of PERK enhances glucose-stimulated insulin secretion in islets. J. Endocrinol. 236, 125–136 (2018).
Rai, S., Szaruga, M., Pitera, A. P. & Bertolotti, A. Integrated stress response activator halofuginone protects mice from diabetes-like phenotypes. J. Cell Biol. 223, e202405175 (2024).
Szaruga, M. et al. Activation of the integrated stress response by inhibitors of its kinases. Nat. Commun. 14, 5535 (2023).
Cnop, M. et al. Selective inhibition of eukaryotic translation initiation factor 2α dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic β-cell dysfunction and apoptosis. J. Biol. Chem. 282, 3989–3997 (2007).
Ladrière, L. et al. Enhanced signaling downstream of ribonucleic acid-activated protein kinase-like endoplasmic reticulum kinase potentiates lipotoxic endoplasmic reticulum stress in human islets. J. Clin. Endocrinol. Metab. 95, 1442–1449 (2010).
Abdulkarim, B. et al. Guanabenz sensitizes pancreatic β cells to lipotoxic endoplasmic reticulum stress and apoptosis. Endocrinology 158, 1659–1670 (2017).
Muralidharan, C. et al. Inhibition of the eukaryotic initiation factor-2α kinase PERK decreases risk of autoimmune diabetes in mice. J. Clin. Invest. 134, e176136 (2024).
Zyryanova, A. F. et al. Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B. Science 359, 1533–1536 (2018).
Zyryanova, A. F. et al. ISRIB blunts the integrated stress response by allosterically antagonising the inhibitory effect of phosphorylated eIF2 on eIF2B. Mol. Cell 81, 88–103.e6 (2021).
Krukowski, K. et al. Small molecule cognitive enhancer reverses age-related memory decline in mice. eLife 9, e62048 (2020).
Chou, A. et al. Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc. Natl Acad. Sci. USA 114, E6420–E6426 (2017).
Young-Baird, S. K. et al. Suppression of MEHMO syndrome mutation in eIF2 by small molecule ISRIB. Mol. Cell 77, 875–886.e7 (2020).
Tang, X. et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 33, 1577–1591.e7 (2021).
Sato, H., Shiba, Y., Tsuchiya, Y., Saito, M. & Kohno, K. 4mu8C inhibits insulin secretion independent of IRE1α RNase activity. Cell Struct. Funct. 42, 61–70 (2017).
Ghosh, R. et al. Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158, 534–548 (2014).
Feldman, H. C. et al. ATP-competitive partial antagonists of the IRE1α RNase segregate outputs of the UPR. Nat. Chem. Biol. 17, 1148–1156 (2021).
Morita, S. et al. Targeting ABL-IRE1α signaling spares ER-stressed pancreatic β cells to reverse autoimmune diabetes. Cell Metab. 25, 883–897.e8 (2017).
Grandjean, J. M. D. et al. Pharmacologic IRE1/XBP1s activation confers targeted ER proteostasis reprogramming. Nat. Chem. Biol. 16, 1052–1061 (2020).
Madhavan, A. et al. Pharmacologic IRE1/XBP1s activation promotes systemic adaptive remodeling in obesity. Nat. Commun. 13, 608 (2022).
Gallagher, C. M. et al. Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. eLife 5, e11878 (2016).
Gallagher, C. M. & Walter, P. Ceapins inhibit ATF6α signaling by selectively preventing transport of ATF6α to the Golgi apparatus during ER stress. eLife 5, e11880 (2016).
Guan, M. et al. Nelfinavir induces liposarcoma apoptosis through inhibition of regulated intramembrane proteolysis of SREBP-1 and ATF6. Clin. Cancer Res. 17, 1796–1806 (2011).
Rosarda, J. D. et al. Imbalanced unfolded protein response signaling contributes to 1-deoxysphingolipid retinal toxicity. Nat. Commun. 14, 4119 (2023).
Sun, S. et al. Capturing the conversion of the pathogenic alpha-1-antitrypsin fold by ATF6 enhanced proteostasis. Cell Chem. Biol. 30, 22–42.e25 (2023).
Paxman, R. et al. Pharmacologic ATF6 activating compounds are metabolically activated to selectively modify endoplasmic reticulum proteins. eLife 7, e37168 (2018).
Wang, M. et al. Pharmacological activation of ATF6 remodels the proteostasis network to rescue pathogenic GABAA receptors. Cell Biosci. 12, 48 (2022).
Robinson, C. M., Duggan, A. & Forrester, A. ER exit in physiology and disease. Front. Mol. Biosci. 11, 1352970 (2024).
Yonemura, Y. et al. Inhibition of cargo export at ER exit sites and the trans-Golgi network by the secretion inhibitor FLI-06. J. Cell Sci. 129, 3868–3877 (2016).
Stechmann, B. et al. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell 141, 231–242 (2010).
Forrester, A. et al. Functional dissection of the retrograde Shiga toxin trafficking inhibitor Retro-2. Nat. Chem. Biol. 16, 327–336 (2020).
Batshaw, M. L., MacArthur, R. B. & Tuchman, M. Alternative pathway therapy for urea cycle disorders: twenty years later. J. Pediatr. 138, S46–S55 (2001).
Ma, W., Goldberg, E. & Goldberg, J. ER retention is imposed by COPII protein sorting and attenuated by 4-phenylbutyrate. eLife 6, e26624 (2017).
Gomez-Navarro, N. et al. Selective inhibition of protein secretion by abrogating receptor–coat interactions during ER export. Proc. Natl Acad. Sci. USA 119, e2202080119 (2022).
Yusta, B. et al. GLP-1 receptor activation improves β cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 4, 391–406 (2006).
Cunha, D. A. et al. Glucagon-like peptide-1 agonists protect pancreatic β-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes 58, 2851–2862 (2009).
Natalicchio, A. et al. Exendin-4 protects pancreatic beta cells from palmitate-induced apoptosis by interfering with GPR40 and the MKK4/7 stress kinase signalling pathway. Diabetologia 56, 2456–2466 (2013).
Shimoda, M. et al. The human glucagon-like peptide-1 analogue liraglutide preserves pancreatic beta cells via regulation of cell kinetics and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetologia 54, 1098–1108 (2011).
Kondo, M. et al. Activation of GLP-1 receptor signalling alleviates cellular stresses and improves beta cell function in a mouse model of Wolfram syndrome. Diabetologia 61, 2189–2201 (2018).
Jo, S. & Alejandro, E. U. Imeglimin to the rescue: enhanced CHOP/GADD34/eIF2α signaling axis promotes β-cell survival. Diabetes 71, 376–378 (2022).
Li, J. et al. Imeglimin ameliorates β-cell apoptosis by modulating the endoplasmic reticulum homeostasis pathway. Diabetes 71, 424–439 (2022).
Hallakou-Bozec, S. et al. Mechanism of action of imeglimin: a novel therapeutic agent for type 2 diabetes. Diabetes Obes. Metab. 23, 664–673 (2021).
Yingyue, Q. et al. Stimulatory effect of imeglimin on incretin secretion. J. Diabetes Investig. 14, 746–755 (2023).
Sanada, J. et al. Imeglimin exerts favorable effects on pancreatic β-cells by improving morphology in mitochondria and increasing the number of insulin granules. Sci. Rep. 12, 13220 (2022).
Awazawa, M. et al. Imeglimin improves systemic metabolism by targeting brown adipose tissue and gut microbiota in obese model mice. Metabolism 153, 155796 (2024).
Akiyama, M. et al. Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia 52, 653–663 (2009).
Evans-Molina, C. et al. Peroxisome proliferator-activated receptor γ activation restores islet function in diabetic mice through reduction of endoplasmic reticulum stress and maintenance of euchromatin structure. Mol. Cell. Biol. 29, 2053–2067 (2009).
Kono, T. et al. PPAR-γ activation restores pancreatic islet SERCA2 levels and prevents β-cell dysfunction under conditions of hyperglycemic and cytokine stress. Mol. Endocrinol. 26, 257–271 (2012).
Louvet, C. et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 105, 18895–18900 (2008).
Wilson, C. S. et al. B lymphocytes protect islet β cells in diabetes prone NOD mice treated with imatinib. JCI Insight 5, e125317 (2019).
Hawley, J. A. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes Metab. Res. Rev. 20, 383–393 (2004).
Paula, F. M. M. et al. Exercise training protects human and rodent β cells against endoplasmic reticulum stress and apoptosis. FASEB J. 32, 1524–1536 (2018).
Coomans de Brachene, A. et al. Exercise as a non-pharmacological intervention to protect pancreatic beta cells in individuals with type 1 and type 2 diabetes. Diabetologia 66, 450–460 (2023).
Kars, M. et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59, 1899–1905 (2010).
Xiao, C., Giacca, A. & Lewis, G. F. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and β-cell dysfunction in humans. Diabetes 60, 918–924 (2011).
Gitelman, S. E. et al. Imatinib therapy for patients with recent-onset type 1 diabetes: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 9, 502–514 (2021).
Wilbon, S. S. & Kolonin, M. G. GLP1 receptor agonists — effects beyond obesity and diabetes. Cells 13, 65 (2024).
Bedi, B. et al. UPR modulation of host immunity by Pseudomonas aeruginosa in cystic fibrosis. Clin. Sci. 134, 1911–1934 (2020).
Lee, I. M. et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380, 219–229 (2012).
Coomans de Brachene, A. et al. Interferons are key cytokines acting on pancreatic islets in type 1 diabetes. Diabetologia 67, 908–927 (2024).
Sims, E. K., Geyer, S. M., Long, S. A. & Herold, K. C. High proinsulin:C-peptide ratio identifies individuals with stage 2 type 1 diabetes at high risk for progression to clinical diagnosis and responses to teplizumab treatment. Diabetologia 66, 2283–2291 (2023).
Sharma, R. B., Landa-Galván, H. V. & Alonso, L. C. Living dangerously: protective and harmful ER stress responses in pancreatic β-cells. Diabetes 70, 2431–2443 (2021).
Mirmira, R. G., Sims, E. K., Syed, F. & Evans-Molina, C. Biomarkers of β-cell stress and death in type 1 diabetes. Curr. Diabetes Rep. 16, 95 (2016).
Wagner, L. E., Melnyk, O., Duffett, B. E. & Linnemann, A. K. Mouse models and human islet transplantation sites for intravital imaging. Front. Endocrinol. 13, 992540 (2022).
Kracht, M. J. L., de Koning, E. J. P., Hoeben, R. C., Roep, B. O. & Zaldumbide, A. Bioluminescent reporter assay for monitoring ER stress in human beta cells. Sci. Rep. 8, 17738 (2018).
Acknowledgements
The laboratory of M.C. is supported by the European Union Horizon Health project NEMESIS, the European Foundation for the Study of Diabetes — Boehringer Ingelheim European Research Programme on Multi-System Challenges in Diabetes, The Leona M. and Harry B. Helmsley Charitable Trust, the Belgian Fonds National de la Recherche Scientifique (FNRS), Walloon Region strategic axis Fonds de la Recherche Scientifique (FRFS) — Walloon Excellence in Life Sciences and Biotechnology (WELBIO), and Research Foundation Flanders (FWO) & Fund for Scientific Research (FRS)-FNRS Excellence of Science (EOS) project Pandarome. Y.T. and E.V. are FNRS-Fund for Research Training in Industry and Agriculture (FRIA) fellows. M.L. is supported by FNRS and Erasmus Fund for Medical Research fellowships.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and wrote the manuscript. M.C., M.L., Y.T. and E.V. also contributed substantially to discussions of the article content. M.C. and M.L. also reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Laura C. Alonso and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
fGSEA: https://github.com/alserglab/fgsea
Human Pancreas Analysis Program: https://hpap.pmacs.upenn.edu
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lytrivi, M., Tong, Y., Virgilio, E. et al. Diabetes mellitus and the key role of endoplasmic reticulum stress in pancreatic β cells. Nat Rev Endocrinol 21, 546–563 (2025). https://doi.org/10.1038/s41574-025-01129-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41574-025-01129-5