Abstract
Adipose tissue, a pivotal player in whole-body energy homeostasis and insulin sensitivity, undergoes considerable remodelling throughout the ageing process, a facet that has garnered little attention until the past decade. This Review comprehensively summarizes the dynamic metabolic, cellular and functional changes that occur in white and thermogenic adipose tissue during distinct ageing stages, across different adipose tissue depots. We emphasize the influence of ageing on different cell types within adipose tissue, including adipocytes, adipocyte progenitors, immune cells and senescent cells, and their collective effect on adipose tissue function and systemic metabolism. We also decipher the correlation between adipose tissue ageing and prevalent age-related conditions such as metabolic dysfunction-associated fatty liver disease and cardiovascular diseases. Finally, the Review delves into the potential of current anti-ageing interventions to beneficially affect adipose tissue, encompassing caloric restriction, metformin, glucagon-like peptide 1 receptor agonists and senolytics. The discussion extends to the exploration of whether targeting adipose tissue through such interventions could emerge as a prominent therapeutic strategy for mitigating age-related diseases and enhancing the healthspan and lifespan of the ageing population.
Key points
-
Adipose tissue is among the first organs to undergo changes in metabolic responses and endocrine function during early ageing, considerably affecting other metabolically active organs and contributing to systemic ageing and declining metabolic health.
-
White adipose tissue ages differently across anatomical depots and these alterations do not follow a linear trajectory with advancing age.
-
Robust adipogenesis is triggered during the early stages of ageing, particularly in the visceral adipose tissue of male individuals. Age-specific remodelling of adipose progenitor cells unlocks their adipogenic potential.
-
Therapeutic strategies that enhance white adipose tissue function, without necessarily reducing its mass, have shown promising effects in improving metabolic health in older individuals.
-
Reduced thermogenic activity in brown and beige adipocytes contributes to age-related cold sensitivity, diminishing the ability of older individuals to maintain core body temperature in colder environments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vaupel, J. W. Biodemography of human ageing. Nature 464, 536–542 (2010).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Cypess, A. M. Reassessing human adipose tissue. N. Engl. J. Med. 386, 768–779 (2022).
Ou, M. Y., Zhang, H., Tan, P. C., Zhou, S. B. & Li, Q. F. Adipose tissue aging: mechanisms and therapeutic implications. Cell Death Dis. 13, 300 (2022).
Nguyen, T. T. & Corvera, S. Adipose tissue as a linchpin of organismal ageing. Nat. Metab. 6, 793–807 (2024).
Schaum, N. et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020).
Zhou, Q. et al. A landscape of murine long non-coding RNAs reveals the leading transcriptome alterations in adipose tissue during aging. Cell Rep. 31, 107694 (2020).
Scherer, P. E. The many secret lives of adipocytes: implications for diabetes. Diabetologia 62, 223–232 (2019).
Sakers, A., De Siqueira, M. K., Seale, P. & Villanueva, C. J. Adipose-tissue plasticity in health and disease. Cell 185, 419–446 (2022).
Maniyadath, B., Zhang, Q., Gupta, R. K. & Mandrup, S. Adipose tissue at single-cell resolution. Cell Metab. 35, 386–413 (2023).
Auger, C. & Kajimura, S. Adipose tissue remodeling in pathophysiology. Annu. Rev. Pathol. 18, 71–93 (2023).
Kuk, J. L., Saunders, T. J., Davidson, L. E. & Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 8, 339–348 (2009).
Cero, C. et al. beta3-adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. JCI Insight 6, e139160 (2021).
Darcy, J. & Tseng, Y. H. ComBATing aging-does increased brown adipose tissue activity confer longevity? Geroscience 41, 285–296 (2019).
Schwartz, R. S. et al. Body fat distribution in healthy young and older men. J. Gerontol. 45, M181–M185 (1990).
Baarts, R. B. et al. Age- and sex-specific changes in visceral fat mass throughout the life-span. Obesity 31, 1953–1961 (2023).
Yang, Y. C. et al. Life-course trajectories of body mass index from adolescence to old age: racial and educational disparities. Proc. Natl Acad. Sci. USA 118, e2020167118 (2021).
Mott, J. W. et al. Relation between body fat and age in 4 ethnic groups. Am. J. Clin. Nutr. 69, 1007–1013 (1999).
Ding, J. et al. Effects of birth cohort and age on body composition in a sample of community-based elderly. Am. J. Clin. Nutr. 85, 405–410 (2007).
Reinders, I., Visser, M. & Schaap, L. Body weight and body composition in old age and their relationship with frailty. Curr. Opin. Clin. Nutr. Metab. Care 20, 11–15 (2017).
Zhang, Z. et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J. Clin. Invest. 129, 5327–5342 (2019).
Ezure, T., Amano, S. & Matsuzaki, K. Infiltration of subcutaneous adipose layer into the dermal layer with aging. Skin Res. Technol. 28, 311–316 (2022).
Ofenheimer, A. et al. Reference values of body composition parameters and visceral adipose tissue (VAT) by DXA in adults aged 18-81 years-results from the LEAD cohort. Eur. J. Clin. Nutr. 74, 1181–1191 (2020).
Justesen, J. et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2, 165–171 (2001).
Zamboni, M. et al. Body composition changes in stable-weight elderly subjects: the effect of sex. Aging Clin. Exp. Res. 15, 321–327 (2003).
Kuhn, J. P. et al. Pancreatic steatosis demonstrated at MR imaging in the general population: clinical relevance. Radiology 276, 129–136 (2015).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 (2011).
Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Ye, R. et al. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes beta-cell regeneration. eLife 3, e03851 (2014).
Ryu, J. et al. Adiponectin alleviates diet-induced inflammation in the liver by suppressing MCP-1 expression and macrophage infiltration. Diabetes 70, 1303–1316 (2021).
Coimbra, S., Brandao Proenca, J., Santos-Silva, A. & Neuparth, M. J. Adiponectin, leptin, and chemerin in elderly patients with type 2 diabetes mellitus: a close linkage with obesity and length of the disease. Biomed. Res. Int. 2014, 701915 (2014).
Atzmon, G. et al. Adiponectin levels and genotype: a potential regulator of life span in humans. J. Gerontol. A Biol. Sci. Med. Sci. 63, 447–453 (2008).
Hotta, K. et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50, 1126–1133 (2001).
Li, N. et al. Adiponectin preserves metabolic fitness during aging. eLife 10, e65108 (2021).
Holland, W. L. et al. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol. Metab. 6, 267–275 (2017).
Frederich, R. C. et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J. Clin. Invest. 96, 1658–1663 (1995).
Friedman, J. M. Leptin and the regulation of body weight. Keio J. Med. 60, 1–9 (2011).
Ma, X. H. et al. Aging is associated with resistance to effects of leptin on fat distribution and insulin action. J. Gerontol. A Biol. Sci. Med. Sci. 57, B225–B231 (2002).
Zhao, S. et al. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 30, 706–719.e6 (2019).
Gencer, B. et al. Association between resistin levels and cardiovascular disease events in older adults: the health, aging and body composition study. Atherosclerosis 245, 181–186 (2016).
Steppan, C. M. et al. The hormone resistin links obesity to diabetes. Nature 409, 307–312 (2001).
Acquarone, E., Monacelli, F., Borghi, R., Nencioni, A. & Odetti, P. Resistin: a reappraisal. Mech. Ageing Dev. 178, 46–63 (2019).
Bozaoglu, K. et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148, 4687–4694 (2007).
Lin, Y. et al. The chemerin-CMKLR1 axis limits thermogenesis by controlling a beige adipocyte/IL-33/type 2 innate immunity circuit. Sci. Immunol. 6, eabg9698 (2021).
Hart, R. & Greaves, D. R. Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J. Immunol. 185, 3728–3739 (2010).
Parolini, S. et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 109, 3625–3632 (2007).
Grabner, G. F., Xie, H., Schweiger, M. & Zechner, R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 3, 1445–1465 (2021).
Jaworski, K., Sarkadi-Nagy, E., Duncan, R. E., Ahmadian, M. & Sul, H. S. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G1–G4 (2007).
Li, E. et al. Control of lipolysis by a population of oxytocinergic sympathetic neurons. Nature 625, 175–180 (2024).
Palmer, A. K. & Jensen, M. D. Metabolic changes in aging humans: current evidence and therapeutic strategies. J. Clin. Invest. 132, e158451 (2022).
Gao, H. et al. Age-induced reduction in human lipolysis: a potential role for adipocyte noradrenaline degradation. Cell Metab. 32, 1–3 (2020).
Lonnqvist, F., Nyberg, B., Wahrenberg, H. & Arner, P. Catecholamine-induced lipolysis in adipose tissue of the elderly. J. Clin. Invest. 85, 1614–1621 (1990).
Markussen, L. K. et al. Lipolysis regulates major transcriptional programs in brown adipocytes. Nat. Commun. 13, 3956 (2022).
Camell, C. D. et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017).
Zhang, Z. et al. Dermal adipocytes contribute to the metabolic regulation of dermal fibroblasts. Exp. Dermatol. 30, 102–111 (2021).
Yu, L. et al. IgG is an aging factor that drives adipose tissue fibrosis and metabolic decline. Cell Metab. 36, 793–807.e5 (2024).
Cushman, S. W. & Wardzala, L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J. Biol. Chem. 255, 4758–4762 (1980).
Scherer, P. E. et al. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J. Cell Biol. 127, 1233–1243 (1994).
Fazakerley, D. J., Krycer, J. R., Kearney, A. L., Hocking, S. L. & James, D. E. Muscle and adipose tissue insulin resistance: malady without mechanism? J. Lipid Res. 60, 1720–1732 (2019).
Halberg, N. et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 29, 4467–4483 (2009).
Zhang, L. et al. Aging is associated with hypoxia and oxidative stress in adipose tissue: implications for adipose function. Am. J. Physiol. Endocrinol. Metab. 301, E599–E607 (2011).
Crewe, C., An, Y. A. & Scherer, P. E. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J. Clin. Invest. 127, 74–82 (2017).
Sun, K., Halberg, N., Khan, M., Magalang, U. J. & Scherer, P. E. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Mol. Cell Biol. 33, 904–917 (2013).
Divoux, A. et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 (2010).
Miller, K. N. et al. Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell 16, 497–507 (2017).
Laforest, S., Labrecque, J., Michaud, A., Cianflone, K. & Tchernof, A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit. Rev. Clin. Lab. Sci. 52, 301–313 (2015).
Weyer, C., Foley, J. E., Bogardus, C., Tataranni, P. A. & Pratley, R. E. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 43, 1498–1506 (2000).
Bluher, M. et al. Role of insulin action and cell size on protein expression patterns in adipocytes. J. Biol. Chem. 279, 31902–31909 (2004).
Skurk, T., Alberti-Huber, C., Herder, C. & Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 92, 1023–1033 (2007).
Acosta, J. R. et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia 59, 560–570 (2016).
Rajbhandari, P. et al. Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife 8, e49501 (2019).
Sarvari, A. K. et al. Plasticity of epididymal adipose tissue in response to diet-induced obesity at single-nucleus resolution. Cell Metab. 33, 437–453.e5 (2021).
Emont, M. P. et al. A single-cell atlas of human and mouse white adipose tissue. Nature 603, 926–933 (2022).
Backdahl, J. et al. Spatial mapping reveals human adipocyte subpopulations with distinct sensitivities to insulin. Cell Metab. 33, 1869–1882.e6 (2021).
Xie, L. et al. Single-nucleus RNA sequencing reveals heterogeneity among multiple white adipose tissue depots. Life Metab. 2, load045 (2023).
Holman, C. D. et al. Aging impairs cold-induced beige adipogenesis and adipocyte metabolic reprogramming. eLife 12, RP87756 (2024).
Hepler, C., Vishvanath, L. & Gupta, R. K. Sorting out adipocyte precursors and their role in physiology and disease. Genes Dev. 31, 127–140 (2017).
Ghaben, A. L. & Scherer, P. E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 20, 242–258 (2019).
Burl, R. B. et al. Deconstructing adipogenesis induced by beta3-adrenergic receptor activation with single-cell expression profiling. Cell Metab. 28, 300–309 (2018).
Merrick, D. et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, eaav2501 (2019).
Ferrero, R., Rainer, P. & Deplancke, B. Toward a consensus view of mammalian adipocyte stem and progenitor cell heterogeneity. Trends Cell Biol. 30, 937–950 (2020).
Loft, A. et al. Towards a consensus atlas of human and mouse adipose tissue at single-cell resolution. Nat. Metab. 7, 875–894 (2025).
Hepler, C. et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7, e39636 (2018).
Schwalie, P. C. et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108 (2018).
Nguyen, H. P. et al. Aging-dependent regulatory cells emerge in subcutaneous fat to inhibit adipogenesis. Dev. Cell 56, 1437–1451.e3 (2021).
Kotani, K. et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int. J. Obes. Relat. Metab. Disord. 18, 207–202 (1994).
Kirkland, J. L. & Dobson, D. E. Preadipocyte function and aging: links between age-related changes in cell dynamics and altered fat tissue function. J. Am. Geriatr. Soc. 45, 959–967 (1997).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28, 1556–1568 (2022).
Kokai, L. E. et al. Adipose stem cell function maintained with age: an intra-subject study of long-term cryopreserved cells. Aesthet. Surg. J. 37, 454–463 (2017).
Wang, G. et al. Distinct adipose progenitor cells emerging with age drive active adipogenesis. Science 388, eadj0430 (2025).
Jacks, R. D. & Lumeng, C. N. Macrophage and T cell networks in adipose tissue. Nat. Rev. Endocrinol. 20, 50–61 (2024).
Molofsky, A. B. et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 210, 535–549 (2013).
Hildreth, A. D. et al. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat. Immunol. 22, 639–653 (2021).
Dahlquist, K. J. V. & Camell, C. D. Aging leukocytes and the inflammatory microenvironment of the adipose tissue. Diabetes 71, 23–30 (2022).
Camell, C. D. et al. Aging induces an Nlrp3 inflammasome-dependent expansion of adipose B cells that impairs metabolic homeostasis. Cell Metab. 30, 1024–1039 (2019).
Carey, A. et al. Age-associated accumulation of B cells promotes macrophage inflammation and inhibits lipolysis in adipose tissue during sepsis. Cell Rep. 43, 113967 (2024).
Zhang, Z. et al. A panoramic view of cell population dynamics in mammalian aging. Science 387, eadn3949 (2025).
Bruno, M. E. C. et al. Accumulation of gammadelta T cells in visceral fat with aging promotes chronic inflammation. Geroscience 44, 1761–1778 (2022).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Brigger, D. et al. Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age. Nat. Metab. 2, 688–702 (2020).
Goldberg, E. L. et al. IL-33 causes thermogenic failure in aging by expanding dysfunctional adipose ILC2. Cell Metab. 33, 2277–2287.e5 (2021).
Shan, B. et al. Cold-responsive adipocyte progenitors couple adrenergic signaling to immune cell activation to promote beige adipocyte accrual. Genes Dev. 35, 1333–1338 (2021).
Mahlakoiv, T. et al. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci. Immunol. 4, eaax0416 (2019).
Zeyda, M. et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int. J. Obes. 37, 658–665 (2013).
Spallanzani, R. G. et al. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci. Immunol. 4, eaaw3658 (2019).
Vasanthakumar, A. et al. Sex-specific adipose tissue imprinting of regulatory T cells. Nature 579, 581–585 (2020).
Cipolletta, D. et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486, 549–553 (2012).
Li, Y. et al. Insulin signaling establishes a developmental trajectory of adipose regulatory T cells. Nat. Immunol. 22, 1175–1185 (2021).
Bapat, S. P. et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528, 137–141 (2015).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Petrocelli, J. J. et al. Disuse-induced muscle fibrosis, cellular senescence, and senescence-associated secretory phenotype in older adults are alleviated during re-ambulation with metformin pre-treatment. Aging Cell 22, e13936 (2023).
Le Pelletier, L. et al. Metformin alleviates stress-induced cellular senescence of aging human adipose stromal cells and the ensuing adipocyte dysfunction. eLife 10, e62635 (2021).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Wang, B. et al. An inducible p21-Cre mouse model to monitor and manipulate p21-highly-expressing senescent cells in vivo. Nat. Aging 1, 962–973 (2021).
Palmer, A. K. et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18, e12950 (2019).
Xu, M. et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 4, e12997 (2015).
Zhou, Z. et al. Type 2 cytokine signaling in macrophages protects from cellular senescence and organismal aging. Immunity 57, 513–527 (2024).
Han, H. S. et al. Impaired BCAA catabolism in adipose tissues promotes age-associated metabolic derangement. Nat. Aging 3, 982–1000 (2023).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Gasek, N. S., Kuchel, G. A., Kirkland, J. L. & Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 1, 870–879 (2021).
Zhang, L. et al. Cellular senescence: a key therapeutic target in aging and diseases. J. Clin. Investig. 132, e158450 (2022).
Zhu, Y. et al. Orally-active, clinically-translatable senolytics restore α-Klotho in mice and humans. eBioMedicine 77, 103912 (2022).
He, Y. et al. PPARγ acetylation in adipocytes exacerbates BAT whitening and worsens age-associated metabolic dysfunction. Cells 12, 1424 (2023).
Hamaoka, T. et al. Near-infrared time-resolved spectroscopy for assessing brown adipose tissue density in humans: a review. Front. Endocrinol. 11, 261 (2020).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Ikeda, K., Maretich, P. & Kajimura, S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab. 29, 191–200 (2018).
Cypess, A. M. et al. Emerging debates and resolutions in brown adipose tissue research. Cell Metab. 37, 12–33 (2025).
Golozoubova, V. et al. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 15, 2048–2050 (2001).
Kazak, L. et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 26, 693 (2017).
Bertholet, A. M. et al. Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell Metab. 25, 811–822.e4 (2017).
Ikeda, K. et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 23, 1454–1465 (2017).
Kramarova, T. V. et al. Mitochondrial ATP synthase levels in brown adipose tissue are governed by the c-Fo subunit P1 isoform. FASEB J. 22, 55–63 (2008).
Hanssen, M. J. et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 21, 863–865 (2015).
Morrison, S. F., Madden, C. J. & Tupone, D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 19, 741–756 (2014).
Cypess, A. M. et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 21, 33–38 (2015).
Blondin, D. P. et al. Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab. 32, 287–300.e7 (2020).
Rahbani, J. F. et al. ADRA1A-Gα(q) signalling potentiates adipocyte thermogenesis through CKB and TNAP. Nat. Metab. 4, 1459–1473 (2022).
Chouchani, E. T., Kazak, L. & Spiegelman, B. M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab. 29, 27–37 (2019).
Sun, Y. et al. Mitochondrial TNAP controls thermogenesis by hydrolysis of phosphocreatine. Nature 593, 580–585 (2021).
Kazak, L. et al. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat. Metab. 1, 360–370 (2019).
Bunk, J. et al. The futile creatine cycle powers UCP1-independent thermogenesis in classical BAT. Nat. Commun. 16, 3221 (2025).
Fedorenko, A., Lishko, P. V. & Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413 (2012).
Lee, P. et al. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab. 23, 602–609 (2016).
Yoneshiro, T. et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019).
Weir, G. et al. Substantial metabolic activity of human brown adipose tissue during warm conditions and cold-induced lipolysis of local triglycerides. Cell Metab. 27, 1348–1355.e4 (2018).
Mills, E. L. et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 (2018).
Wang, Z. et al. Chronic cold exposure enhances glucose oxidation in brown adipose tissue. EMBO Rep. 21, e50085 (2020).
Wang, G. X. et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 20, 1436–1443 (2014).
Hondares, E. et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J. Biol. Chem. 286, 12983–12990 (2011).
Kong, X. et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 28, 631–643.e3 (2018).
Whittle, A. J. et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012).
Sugimoto, S. et al. Brown adipose tissue-derived MaR2 contributes to cold-induced resolution of inflammation. Nat. Metab. 4, 775–790 (2022).
Lynes, M. D. et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 23, 631–637 (2017).
Tajima, K. et al. Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat. Metab. 1, 886–898 (2019).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med. 27, 58–65 (2021).
Bahler, L. et al. Differences in sympathetic nervous stimulation of brown adipose tissue between the young and old, and the lean and obese. J. Nucl. Med. 57, 372–377 (2016).
Silva, G. D. N. & Amato, A. A. Thermogenic adipose tissue aging: mechanisms and implications. Front. Cell Dev. Biol. 10, 955612 (2022).
Yoneshiro, T. et al. Impact of UCP1 and beta3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int. J. Obes. 37, 993–998 (2013).
Feng, X. et al. Senescent immune cells accumulation promotes brown adipose tissue dysfunction during aging. Nat. Commun. 14, 3208 (2023).
Song, A. et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Invest. 130, 247–257 (2020).
Sun, W. et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102 (2020).
Sun, W., Modica, S., Dong, H. & Wolfrum, C. Plasticity and heterogeneity of thermogenic adipose tissue. Nat. Metab. 3, 751–761 (2021).
Lee, Y. H., Petkova, A. P., Konkar, A. A. & Granneman, J. G. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J. 29, 286–299 (2015).
Shamsi, F. et al. Vascular smooth muscle-derived Trpv1+ progenitors are a source of cold-induced thermogenic adipocytes. Nat. Metab. 3, 485–495 (2021).
Gabaldon, A. M., Florez-Duquet, M. L., Hamilton, J. S., McDonald, R. B. & Horwitz, B. A. Effects of age and gender on brown fat and skeletal muscle metabolic responses to cold in F344 rats. Am. J. Physiol. 268, R931–R941 (1995).
Huang, Z. et al. Brown adipose tissue involution associated with progressive restriction in progenitor competence. Cell Rep. 39, 110575 (2022).
Pan, X. X. et al. Senescent T cell induces brown adipose tissue “whitening” via secreting IFN-γ. Front. Cell Dev. Biol. 9, 637424 (2021).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Jiang, Y., Berry, D. C. & Graff, J. M. Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. eLife 6, e30329 (2017).
Rui, L. Brown and beige adipose tissues in health and disease. Compr. Physiol. 7, 1281–1306 (2017).
Ma, X., Xu, L., Gavrilova, O. & Mueller, E. Role of forkhead box protein A3 in age-associated metabolic decline. Proc. Natl Acad. Sci. USA 111, 14289–14294 (2014).
Wang, W. et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab. 30, 174–189.e5 (2019).
Wang, Q. et al. Post-translational control of beige fat biogenesis by PRDM16 stabilization. Nature 609, 151–158 (2022).
Duteil, D. et al. Lsd1 prevents age-programed loss of beige adipocytes. Proc. Natl Acad. Sci. USA 114, 5265–5270 (2017).
Majeed, Y. et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 11, 8177 (2021).
Pardo, P. S. & Boriek, A. M. SIRT1 regulation in ageing and obesity. Mech. Ageing Dev. 188, 111249 (2020).
Altshuler-Keylin, S. et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 24, 402–419 (2016).
Wu, R. et al. Genetically prolonged beige fat in male mice confers long-lasting metabolic health. Nat. Commun. 14, 2731 (2023).
Park, J. et al. Estrogen counteracts age-related decline in beige adipogenesis through the NAMPT-regulated ER stress response. Nat. Aging 4, 839–853 (2024).
Berry, D. C. et al. Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Cell Metab. 25, 481 (2017).
Chen, H. J., Meng, T., Gao, P. J. & Ruan, C. C. The role of brown adipose tissue dysfunction in the development of cardiovascular disease. Front. Endocrinol. 12, 652246 (2021).
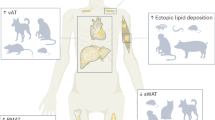
Gilani, A., Stoll, L., Homan, E. A. & Lo, J. C. Adipose signals regulating distal organ health and disease. Diabetes 73, 169–177 (2024).
Younossi, Z. M. Non-alcoholic fatty liver disease — a global public health perspective. J. Hepatol. 70, 531–544 (2019).
Castera, L., Friedrich-Rust, M. & Loomba, R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 156, 1264–1281.e4 (2019).
Mantovani, A. et al. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 111S, 154170 (2020).
Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184, 2537–2564 (2021).
Ipsen, D. H., Lykkesfeldt, J. & Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 75, 3313–3327 (2018).
Nassir, F. NAFLD: mechanisms, treatments, and biomarkers. Biomolecules 12, 824 (2022).
Saponaro, C. et al. Adipose tissue dysfunction and visceral fat are associated with hepatic insulin resistance and severity of NASH even in lean individuals. Liver Int. 42, 2418–2427 (2022).
Micu, E. S. et al. Systemic and adipose tissue inflammation in NASH: correlations with histopathological aspects. Rom. J. Morphol. Embryol. 62, 509–515 (2021).
Yamamuro, T. et al. Age-dependent loss of adipose Rubicon promotes metabolic disorders via excess autophagy. Nat. Commun. 11, 4150 (2020).
Azzu, V., Vacca, M., Virtue, S., Allison, M. & Vidal-Puig, A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology 158, 1899–1912 (2020).
Martinez-Sanchez, C. et al. Therapeutic targeting of adipose tissue macrophages ameliorates liver fibrosis in non-alcoholic fatty liver disease. JHEP Rep. 5, 100830 (2023).
Boesch, M. et al. Adipose tissue macrophage dysfunction is associated with a breach of vascular integrity in NASH. J. Hepatol. 80, 397–408 (2024).
Donia, T. & Khamis, A. Management of oxidative stress and inflammation in cardiovascular diseases: mechanisms and challenges. Env. Sci. Pollut. Res. Int. 28, 34121–34153 (2021).
Teixeira, T. M. et al. Activation of Nrf2-antioxidant signaling by 1,25-dihydroxycholecalciferol prevents leptin-induced oxidative stress and inflammation in human endothelial cells. J. Nutr. 147, 506–513 (2017).
Wang, Z. V. & Scherer, P. E. Adiponectin, the past two decades. J. Mol. Cell Biol. 8, 93–100 (2016).
Li, R. et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am. J. Physiol. Endocrinol. Metab. 293, E1703–E1708 (2007).
Dekker, J. M. et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J. Clin. Endocrinol. Metab. 93, 1489–1496 (2008).
Zhang, Y. et al. Adipocyte modulation of high-density lipoprotein cholesterol. Circulation 121, 1347–1355 (2010).
Chui, P. C., Guan, H. P., Lehrke, M. & Lazar, M. A. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Invest. 115, 2244–2256 (2005).
Schmidt, A. F. et al. Biomedical consequences of elevated cholesterol-containing lipoproteins and apolipoproteins on cardiovascular and non-cardiovascular outcomes. Commun. Med. 3, 9 (2023).
Mukama, T., Srour, B., Johnson, T., Katzke, V. & Kaaks, R. IGF-1 and risk of morbidity and mortality from cancer, cardiovascular diseases, and all causes in EPIC-Heidelberg. J. Clin. Endocrinol. Metab. 108, e1092–e1105 (2023).
Oikonomou, E. K. & Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 16, 83–99 (2019).
O’Mara, A. E. et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Invest. 130, 2209–2219 (2020).
Finlin, B. S. et al. The beta3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Invest. 130, 2319–2331 (2020).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
Berbee, J. F. et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 6, 6356 (2015).
Cypess, A. M. et al. Emerging debates and resolutions in brown adipose tissue research. Cell Metab. https://doi.org/10.1016/j.cmet.2024.11.002 (2024).
Queiroz, M. & Sena, C. M. Perivascular adipose tissue in age-related vascular disease. Ageing Res. Rev. 59, 101040 (2020).
Gong, H. et al. Age-dependent tissue expression patterns of Sirt1 in senescence-accelerated mice. Mol. Med. Rep. 10, 3296–3302 (2014).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009).
Zoncu, R., Efeyan, A. & Sabatini, D. M. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 (2011).
Bluher, M., Kahn, B. B. & Kahn, C. R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299, 572–574 (2003).
Lee, D. J. W., Hodzic Kuerec, A. & Maier, A. B. Targeting ageing with rapamycin and its derivatives in humans: a systematic review. Lancet Healthy Longev. 5, e152–e162 (2024).
Houde, V. P. et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 59, 1338–1348 (2010).
Lamming, D. W. et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012).
Johnston, O., Rose, C. L., Webster, A. C. & Gill, J. S. Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J. Am. Soc. Nephrol. 19, 1411–1418 (2008).
Shan, T. et al. Adipocyte-specific deletion of mTOR inhibits adipose tissue development and causes insulin resistance in mice. Diabetologia 59, 1995–2004 (2016).
de Cabo, R., Carmona-Gutierrez, D., Bernier, M., Hall, M. N. & Madeo, F. The search for antiaging interventions: from elixirs to fasting regimens. Cell 157, 1515–1526 (2014).
Pontzer, H. et al. Daily energy expenditure through the human life course. Science 373, 808–812 (2021).
Speakman, J. R. & Westerterp, K. R. Associations between energy demands, physical activity, and body composition in adult humans between 18 and 96 y of age. Am. J. Clin. Nutr. 92, 826–834 (2010).
Corrales, P. et al. Long-term caloric restriction ameliorates deleterious effects of aging on white and brown adipose tissue plasticity. Aging Cell 18, e12948 (2019).
Corrales, P. et al. microRNAs-mediated regulation of insulin signaling in white adipose tissue during aging: role of caloric restriction. Aging Cell 22, e13919 (2023).
Yu, D. et al. Calorie-restriction-induced insulin sensitivity is mediated by adipose mTORC2 and not required for lifespan extension. Cell Rep. 29, 236–248.e3 (2019).
Chinnapaka, S., Malekzadeh, H., Tirmizi, Z. & Ejaz, A. Caloric restriction mitigates age-associated senescence characteristics in subcutaneous adipose tissue-derived stem cells. Aging 16, 7535–7552 (2024).
Suchacki, K. J. et al. The effects of caloric restriction on adipose tissue and metabolic health are sex- and age-dependent. eLife 12, e88080 (2023).
Panda, S., Maier, G. & Villareal, D. T. Targeting energy intake and circadian biology to engage mechanisms of aging in older adults with obesity: calorie restriction and time-restricted eating. J. Gerontol. Ser. A 78, 79–85 (2023).
Foretz, M., Guigas, B. & Viollet, B. Metformin: update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 19, 460–476 (2023).
Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016).
Bharath, L. P. et al. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab. 32, 44–55.e6 (2020).
Cabreiro, F. et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239 (2013).
Wang, C. P., Lorenzo, C. & Espinoza, S. E. Frailty attenuates the impact of metformin on reducing mortality in older adults with type 2 diabetes. J. Endocrinol. Diabetes Obes. 2, 1031 (2014).
Sundelin, E., Jensen, J. B., Jakobsen, S., Gormsen, L. C. & Jessen, N. Metformin biodistribution: a key to mechanisms of action? J. Clin. Endocrinol. Metab. 105, dgaa332 (2020).
Duca, F. A. et al. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 21, 506–511 (2015).
Grycel, S. et al. Metformin treatment affects adipocytokine secretion and lipid composition in adipose tissues of diet-induced insulin-resistant rats. Nutrition 63-64, 126–133 (2019).
Tokubuchi, I. et al. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE 12, e0171293 (2017).
Geerling, J. J. et al. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes 63, 880–891 (2014).
Holst, J. J. Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology 107, 1848–1855 (1994).
Kusminski, C. M. et al. Transforming obesity: the advancement of multi-receptor drugs. Cell 187, 3829–3853 (2024).
Day, J. W. et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 5, 749–757 (2009).
Bossart, M. et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP-1/GIP/GCG receptor triagonist. Cell Metab. 34, 59–74.e10 (2022).
Frias, J. P. et al. The sustained effects of a Dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 26, 343–352.e2 (2017).
Davis, E. M. & Sandoval, D. A. Glucagon-like peptide-1: actions and influence on pancreatic hormone function. Compr. Physiol. 10, 577–595 (2020).
Dossat, A. M., Lilly, N., Kay, K. & Williams, D. L. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J. Neurosci. 31, 14453–14457 (2011).
Nauck, M. A. et al. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 273, E981–E988 (1997).
Gerstein, H. C. et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 394, 121–130 (2019).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124 (2021).
Samms, R. J. et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J. Clin. Investig. 131, e146353 (2021).
Yu, X. et al. The GIP receptor activates futile calcium cycling in white adipose tissue to increase energy expenditure and drive weight loss in mice. Cell Metab. 37, 187–204.e7 (2025).
Beiroa, D. et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63, 3346–3358 (2014).
Gutierrez, A. D. et al. Anti-diabetic effects of GLP1 analogs are mediated by thermogenic interleukin-6 signaling in adipocytes. Cell Rep. Med. 3, 100813 (2022).
Samms, R. J. et al. Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue. Mol. Metab. 64, 101550 (2022).
Ida, S. et al. Effects of antidiabetic drugs on muscle mass in type 2 diabetes mellitus. Curr. Diabetes Rev. 17, 293–303 (2021).
Sodhi, M., Rezaeianzadeh, R., Kezouh, A. & Etminan, M. Risk of gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA 330, 1795–1797 (2023).
Chen, W., Cai, P., Zou, W. & Fu, Z. Psychiatric adverse events associated with GLP-1 receptor agonists: a real-world pharmacovigilance study based on the FDA adverse event reporting system database. Front. Endocrinol. 15, 1330936 (2024).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. eBioMedicine 36, 18–28 (2018).
Cohn, R. L., Gasek, N. S., Kuchel, G. A. & Xu, M. The heterogeneity of cellular senescence: insights at the single-cell level. Trends Cell Biol. 33, 9–17 (2023).
Saul, D. et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 13, 4827 (2022).
Tao, W., Yu, Z. & Han, J. J. Single-cell senescence identification reveals senescence heterogeneity, trajectory, and modulators. Cell Metab. 36, 1126–1143.e5 (2024).
Wang, Q. A., Tao, C., Gupta, R. K. & Scherer, P. E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 19, 1338–1344 (2013).
Vishvanath, L. et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 23, 350–359 (2016).
de Paula, F. J. A. & Rosen, C. J. Marrow adipocytes: origin, structure, and function. Annu. Rev. Physiol. 82, 461–484 (2020).
Horowitz, M. C. et al. Bone marrow adipocytes. Adipocyte 6, 193–204 (2017).
de Araujo, I. M. et al. Marrow adipose tissue spectrum in obesity and type 2 diabetes mellitus. Eur. J. Endocrinol. 176, 21–30 (2017).
Fazeli, P. K. et al. The dynamics of human bone marrow adipose tissue in response to feeding and fasting. JCI Insight 6, e138636 (2021).
Cawthorn, W. P. et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 20, 368–375 (2014).
Li, Z. et al. Lipolysis of bone marrow adipocytes is required to fuel bone and the marrow niche during energy deficits. eLife 11, e78496 (2022).
Tratwal, J., Rojas-Sutterlin, S., Bataclan, C., Blum, S. & Naveiras, O. Bone marrow adiposity and the hematopoietic niche: a historical perspective of reciprocity, heterogeneity, and lineage commitment. Best Pract. Res. Clin. Endocrinol. Metab. 35, 101564 (2021).
Acknowledgements
The authors are grateful to P. Scherer (University of Texas Southwestern Medical Center), T. McLaughlin (Stanford) and Z. Zhang (Wuhan University) for their insightful suggestions and comments. Q.A.W. is supported by National Institutes of Health grants R56AG063854, R01AG063854, R01HD096152, R01DK128907, California Institute for Regenerative Medicine grant DISC0-15689 and the American Diabetes Association Junior Faculty Development Award 1-19-JDF-023.
Author information
Authors and Affiliations
Contributions
Q.A.W. and G.W. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Li Qiang and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Song, A. & Wang, Q.A. Adipose tissue ageing: implications for metabolic health and lifespan. Nat Rev Endocrinol 21, 623–637 (2025). https://doi.org/10.1038/s41574-025-01142-8
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41574-025-01142-8
This article is cited by
-
Aging-Related Obesity: Unveiling Mitochondrial and Metabolic Dysfunction
Current Nutrition Reports (2025)