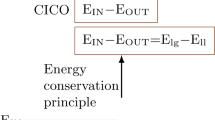
Abstract
Maintaining a ‘healthy’ body weight is crucial for survival and involves a partially understood regulatory system that adjusts energy intake and energy output (expenditure and losses) for that purpose. Several models of body weight regulation exist, but experiments testing their validity are lacking. This Review elaborates on how to test the validity of body weight regulation models in humans. We begin by highlighting the interaction between the obesogenic environment and the individual’s biological sensitivity to such environment, which triggers obesity in many, but not all, individuals. We discuss the identity of the regulated parameter(s), often considered to be body weight or body adiposity. We then focus on two models: set point and dual-intervention point. Under the set point model, obesity results from a malfunction of the system (leptin resistance) for preventing weight increases above the defended value. Under the dual-intervention point model, obesity occurs because the system tolerates a wide range of weights in some individuals. This key difference predicts different compensatory responses to energy balance perturbations in individuals according to their weight status, thus becoming instrumental in testing the validity of the models. Finally, we discuss the design of proof-of-concept experiments to advance the understanding of body weight regulation in humans.
Key points
-
The biological mechanisms that regulate body weight in humans are incompletely understood, yet their interaction with the environment determines body weight.
-
The nature of the regulated parameter, often considered to be body weight, is indeed unknown. Adiposity, lean mass and glycogen stores, among other parameters, are also potential candidates.
-
Different body weight regulation models have been proposed, which exert their regulation by triggering compensatory responses in energy intake and energy output (energy expenditure and energy losses).
-
The set point model proposes that body weight is defended at a fixed level, whereas the dual-intervention point model proposes that body weight is maintained between two boundaries, that is, the lower and upper intervention points.
-
We discuss key aspects for the design and interpretation of experiments aimed at testing the validity of body weight regulation models in humans, with a focus on comparing the set point and the dual-intervention point models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Busetto, L. et al. A new framework for the diagnosis, staging and management of obesity in adults. Nat. Med. 30, 2395–2399 (2024).
Rubino, F. et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 13, 221–262 (2025).
Farooqi, S. Obesity and thinness: insights from genetics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 378, 20220205 (2023).
Collet, T. H. et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 6, 1321–1329 (2017).
Farooqi, I. S. et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 110, 1093–1103 (2002).
Funcke, J. B. et al. Rare antagonistic leptin variants and severe, early-onset obesity. N. Engl. J. Med. 388, 2253–2261 (2023).
Licinio, J. et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc. Natl Acad. Sci. USA 101, 4531–4536 (2004).
Magkos, F. et al. On the pathogenesis of obesity: causal models and missing pieces of the puzzle. Nat. Metab. 6, 1856–1865 (2024).
Speakman, J. R., Sorensen, T. I. A., Hall, K. D. & Allison, D. B. Unanswered questions about the causes of obesity. Science 381, 944–946 (2023).
World Obesity Federation. World Obesity Atlas 2023. https://data.worldobesity.org/publications/?cat=19 (2023).
NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387, 1377–1396 (2016).
Boutari, C. & Mantzoros, C. S. A. 2022 update on the epidemiology of obesity and a call to action: as its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 133, 155217 (2022).
NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403, 1027–1050 (2024).
Pedersen, M. M., Ekstrom, C. T. & Sorensen, T. I. A. Emergence of the obesity epidemic preceding the presumed obesogenic transformation of the society. Sci. Adv. 9, eadg6237 (2023).
Fernandez-Verdejo, R., Sanchez-Delgado, G. & Ravussin, E. Energy expenditure in humans: principles, methods, and changes throughout the life course. Annu. Rev. Nutr. 44, 51–76 (2024).
Frankl, J., Piaggi, P., Foley, J. E., Krakoff, J. & Votruba, S. B. In vitro lipolysis is associated with whole-body lipid oxidation and weight gain in humans. Obesity 25, 207–214 (2017).
Ravussin, E. et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N. Engl. J. Med. 318, 467–472 (1988).
Swinburn, B. A. et al. Insulin resistance associated with lower rates of weight gain in Pima Indians. J. Clin. Invest. 88, 168–173 (1991).
Levitsky, D. A. et al. The rise and fall of physiological theories of the control of human eating behavior. Front. Nutr. 9, 826334 (2022).
Chow, C. C. & Hall, K. D. Short and long-term energy intake patterns and their implications for human body weight regulation. Physiol. Behav. 134, 60–65 (2014).
Dulloo, A. G. Physiology of weight regain: lessons from the classic Minnesota starvation experiment on human body composition regulation. Obes. Rev. 22, e13189 (2021).
Roberts, S. B. et al. Energy expenditure and subsequent nutrient intakes in overfed young men. Am. J. Physiol. 259, R461–R469 (1990).
Roberts, S. B. et al. Control of food intake in older men. JAMA 272, 1601–1606 (1994).
Qi, Q. et al. Sugar-sweetened beverages and genetic risk of obesity. N. Engl. J. Med. 367, 1387–1396 (2012).
Ravussin, E., Valencia, M. E., Esparza, J., Bennett, P. H. & Schulz, L. O. Effects of a traditional lifestyle on obesity in Pima Indians. Diabetes Care 17, 1067–1074 (1994).
Speakman, J. R. & Hall, K. D. Models of body weight and fatness regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 378, 20220231 (2023).
Keesey, R. E. & Powley, T. L. The regulation of body weight. Annu. Rev. Psychol. 37, 109–133 (1986).
Payne, P. R. & Dugdale, A. E. Mechanisms for the control of body-weight. Lancet 309, 583–586 (1977).
Wirtshafter, D. & Davis, J. D. Set points, settling points, and the control of body weight. Physiol. Behav. 19, 75–78 (1977).
Hall, K. D. & Guo, J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology 152, 1718–1727.e3 (2017).
Bar, A., Karin, O., Mayo, A., Ben-Zvi, D. & Alon, U. Rules for body fat interventions based on an operating point mechanism. iScience 26, 106047 (2023).
Speakman, J. R. & Elmquist, J. K. Obesity: an evolutionary context. Life Metab. 1, 10–24 (2022).
Levitsky, D. A. Putting behavior back into feeding behavior: a tribute to George Collier. Appetite 38, 143–148 (2002).
Adams, C. S., Korytko, A. I. & Blank, J. L. A novel mechanism of body mass regulation. J. Exp. Biol. 204, 1729–1734 (2001).
Jansson, J. O. et al. Body weight homeostat that regulates fat mass independently of leptin in rats and mice. Proc. Natl Acad. Sci. USA 115, 427–432 (2018).
Ohlsson, C. & Jansson, J. O. The gravitostat theory: more data needed. eClinicalMedicine 27, 100530 (2020).
Wiedmer, P., Boschmann, M. & Klaus, S. Gender dimorphism of body mass perception and regulation in mice. J. Exp. Biol. 207, 2859–2866 (2004).
Jansson, J. O. et al. The dual hypothesis of homeostatic body weight regulation, including gravity-dependent and leptin-dependent actions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 378, 20220219 (2023).
Kennedy, G. C. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B Biol. Sci. 140, 578–596 (1953).
Cnop, M. et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes 51, 1005–1015 (2002).
Tan, H. L. et al. Leptin-activated hypothalamic BNC2 neurons acutely suppress food intake. Nature 636, 198–205 (2024).
Halaas, J. L. et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995).
Murgatroyd, P. R. et al. Leptin does not respond to 48 h fat deposition or mobilization in women. Int. J. Obes. Relat. Metab. Disord. 27, 457–462 (2003).
Dulloo, A. G. Collateral fattening: when a deficit in lean body mass drives overeating. Obesity 25, 277–279 (2017).
Schwartz, M. W. et al. Is the energy homeostasis system inherently biased toward weight gain? Diabetes 52, 232–238 (2003).
Flatt, J. P. The difference in the storage capacities for carbohydrate and for fat, and its implications in the regulation of body weight. Ann. N. Y. Acad. Sci. 499, 104–123 (1987).
Sonko, B. J. et al. Non-invasive techniques for assessing carbohydrate flux: II. Measurement of deposition using 13C-glucose. Acta Physiol. Scand. 147, 99–108 (1993).
Pannacciulli, N. et al. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am. J. Clin. Nutr. 86, 625–632 (2007).
Stubbs, R. J., Harbron, C. G., Murgatroyd, P. R. & Prentice, A. M. Covert manipulation of dietary fat and energy density: effect on substrate flux and food intake in men eating ad libitum. Am. J. Clin. Nutr. 62, 316–329 (1995).
Shetty, P. S. et al. Alterations in fuel selection and voluntary food intake in response to isoenergetic manipulation of glycogen stores in humans. Am. J. Clin. Nutr. 60, 534–543 (1994).
Stubbs, R. J., Murgatroyd, P. R., Goldberg, G. R. & Prentice, A. M. Carbohydrate balance and the regulation of day-to-day food intake in humans. Am. J. Clin. Nutr. 57, 897–903 (1993).
Snitker, S., Larson, D. E., Tataranni, P. A. & Ravussin, E. Ad libitum food intake in humans after manipulation of glycogen stores. Am. J. Clin. Nutr. 65, 941–946 (1997).
Galgani, J. E., de Jonge, L., Most, M. M., Bray, G. A. & Smith, S. R. Effect of a 3-day high-fat feeding period on carbohydrate balance and ad libitum energy intake in humans. Int. J. Obes. 34, 886–891 (2010).
Eckel, R. H. et al. Carbohydrate balance predicts weight and fat gain in adults. Am. J. Clin. Nutr. 83, 803–808 (2006).
Hervey, G. R. Regulation of energy balance. Nature 222, 629–631 (1969).
Cabanac, M. & Richard, D. The nature of the ponderostat: Hervey’s hypothesis revived. Appetite 26, 45–54 (1996).
Rivest, S., Deshaies, Y. & Richard, D. Effects of corticotropin-releasing factor on energy balance in rats are sex dependent. Am. J. Physiol. 257, R1417–R1422 (1989).
Perry, R. J. et al. Leptin’s hunger-suppressing effects are mediated by the hypothalamic–pituitary–adrenocortical axis in rodents. Proc. Natl Acad. Sci. USA 116, 13670–13679 (2019).
Izzi-Engbeaya, C. et al. Effects of corticosterone within the hypothalamic arcuate nucleus on food intake and body weight in male rats. Mol. Metab. 36, 100972 (2020).
Rasmussen, M., Almdal, T., Bratholm, P. & Christensen, N. J. Elevated β2-adrenoceptor protein concentration in adipose tissue from obese subjects is closely related to the body mass index and waist/hip ratio. Clin. Sci. 104, 93–102 (2003).
Asnicar, M. A. et al. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology 142, 4394–4400 (2001).
Haluzik, M., Matoulek, M., Svacina, S., Hilgertova, J. & Haas, T. The influence of short-term fasting on serum leptin levels, and selected hormonal and metabolic parameters in morbidly obese and lean females. Endocr. Res. 27, 251–260 (2001).
Rojdmark, S. & Rossner, S. Decreased dopaminergic control of prolactin secretion in male obesity: normalization by fasting. Metabolism 40, 191–195 (1991).
Wolfe, R. R. et al. Effect of short-term fasting on lipolytic responsiveness in normal and obese human subjects. Am. J. Physiol. 252, E189–E196 (1987).
Colling, C. et al. Changes in serum cortisol levels after 10 days of overfeeding and fasting. Am. J. Physiol. Endocrinol. Metab. 324, E506–E513 (2023).
Sorensen, T. I. A. An adiposity force induces obesity in humans independently of a normal energy balance system — a thought experiment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 378, 20220203 (2023).
Kim, J. Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Medina-Gomez, G. et al. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 3, e64 (2007).
Chitraju, C. et al. Mice lacking triglyceride synthesis enzymes in adipose tissue are resistant to diet-induced obesity. Elife 12, RP88049 (2023).
Arner, P., Andersson, D. P., Backdahl, J., Dahlman, I. & Ryden, M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metab. 28, 45–54.e3 (2018).
Blundell, J. E. et al. The drive to eat in homo sapiens: energy expenditure drives energy intake. Physiol. Behav. 219, 112846 (2020).
Hopkins, M. et al. Modelling the associations between fat-free mass, resting metabolic rate and energy intake in the context of total energy balance. Int. J. Obes. 40, 312–318 (2016).
Casanova, N. et al. Associations between high-metabolic rate organ masses and fasting hunger: a study using whole-body magnetic resonance imaging in healthy males. Physiol. Behav. 250, 113796 (2022).
Spiering, M. J. The mystery of metformin. J. Biol. Chem. 294, 6689–6691 (2019).
Coll, A. P. et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 578, 444–448 (2020).
Gerstein, H. C. et al. Growth differentiation factor 15 as a novel biomarker for metformin. Diabetes Care 40, 280–283 (2017).
Tsai, V. W. W., Husaini, Y., Sainsbury, A., Brown, D. A. & Breit, S. N. The MIC–1/GDF15–GFRAL pathway in energy homeostasis: implications for obesity, cachexia, and other associated diseases. Cell Metab. 28, 353–368 (2018).
Lund, J. & Clemmensen, C. Physiological protection against weight gain: evidence from overfeeding studies and future directions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 378, 20220229 (2023).
Ravussin, Y., Leibel, R. L. & Ferrante, A. W. Jr A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab. 20, 565–572 (2014).
Galgani, J. E. & Fernandez-Verdejo, R. Pathophysiological role of metabolic flexibility on metabolic health. Obes. Rev. 22, e13131 (2021).
Maclean, P. S., Bergouignan, A., Cornier, M. A. & Jackman, M. R. Biology’s response to dieting: the impetus for weight regain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R581–R600 (2011).
Moran, T. H. & Ladenheim, E. E. Adiposity signaling and meal size control. Physiol. Behav. 103, 21–24 (2011).
Leibel, R. L., Rosenbaum, M. & Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621–628 (1995).
Goldsmith, R. et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R79–R88 (2010).
Diaz, E. O., Prentice, A. M., Goldberg, G. R., Murgatroyd, P. R. & Coward, W. A. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am. J. Clin. Nutr. 56, 641–655 (1992).
Johannsen, D. L., Marlatt, K. L., Conley, K. E., Smith, S. R. & Ravussin, E. Metabolic adaptation is not observed after 8 weeks of overfeeding but energy expenditure variability is associated with weight recovery. Am. J. Clin. Nutr. 110, 805–813 (2019).
Lundgren, J. R. et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N. Engl. J. Med. 384, 1719–1730 (2021).
Martin, C. K. et al. Effect of different doses of supervised exercise on food intake, metabolism, and non-exercise physical activity: the E-MECHANIC randomized controlled trial. Am. J. Clin. Nutr. 110, 583–592 (2019).
Popkin, B. M., Duffey, K. & Gordon-Larsen, P. Environmental influences on food choice, physical activity and energy balance. Physiol. Behav. 86, 603–613 (2005).
Ferrannini, G. et al. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 38, 1730–1735 (2015).
Allison, K. C., Berkowitz, R. I., Brownell, K. D., Foster, G. D. & Wadden, T. A. Albert J. (“Mickey”) Stunkard, M.D.Obesity 22, 1937–1938 (2014).
Ranea-Robles, P. et al. Time-resolved effects of short-term overfeeding on energy balance in mice. Diabetes 74, 502–513 (2025).
Ravussin, Y. et al. Evidence for a non-leptin system that defends against weight gain in overfeeding. Cell Metab. 28, 289–299.e5 (2018).
Muller, M. J., Heymsfield, S. B. & Bosy-Westphal, A. Are metabolic adaptations to weight changes an artefact? Am. J. Clin. Nutr. 114, 1386–1395 (2021).
Basolo, A. et al. Effects of underfeeding and oral vancomycin on gut microbiome and nutrient absorption in humans. Nat. Med. 26, 589–598 (2020).
Yoshimura, E. et al. Effects of energy loads on energy and nutrient absorption rates and gut microbiome in humans: a randomized crossover trial. Obesity 32, 262–272 (2024).
Speakman, J. R. et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis. Model. Mech. 4, 733–745 (2011).
Franz, M. J. et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 107, 1755–1767 (2007).
Fothergill, E. et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity 24, 1612–1619 (2016).
Sumithran, P. et al. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 365, 1597–1604 (2011).
Bouchard, C. et al. Overfeeding in identical twins: 5-year postoverfeeding results. Metabolism 45, 1042–1050 (1996).
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Heymsfield, S. B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282, 1568–1575 (1999).
Flier, J. S. & Maratos-Flier, E. Leptin’s physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 26, 24–26 (2017).
Magkos, F. et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 23, 591–601 (2016).
Galgani, J. E. et al. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J. Clin. Endocrinol. Metab. 95, 851–855 (2010).
Knuth, N. D. et al. Metabolic adaptation following massive weight loss is related to the degree of energy imbalance and changes in circulating leptin. Obesity 22, 2563–2569 (2014).
Redman, L. M. et al. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 27, 805–815.e4 (2018).
Kissileff, H. R. et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am. J. Clin. Nutr. 95, 309–317 (2012).
Ferrannini, E., Rosenbaum, M. & Leibel, R. L. The threshold shift paradigm of obesity: evidence from surgically induced weight loss. Am. J. Clin. Nutr. 100, 996–1002 (2014).
Zelissen, P. M. et al. Effect of three treatment schedules of recombinant methionyl human leptin on body weight in obese adults: a randomized, placebo-controlled trial. Diabetes Obes. Metab. 7, 755–761 (2005).
Zhao, S. et al. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 30, 706–719.e6 (2019).
Speakman, J. R. A nonadaptive scenario explaining the genetic predisposition to obesity: the “predation release” hypothesis. Cell Metab. 6, 5–12 (2007).
Speakman, J. R. The evolution of body fatness: trading off disease and predation risk. J. Exp. Biol. 221, jeb167254 (2018).
Yanovski, J. A. et al. A prospective study of holiday weight gain. N. Engl. J. Med. 342, 861–867 (2000).
Zheng, Y. et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 318, 255–269 (2017).
Murthy, V. L. et al. Metabolic liability for weight gain in early adulthood. Cell Rep. Med. 5, 101548 (2024).
Lund, C. et al. Protection against overfeeding-induced weight gain is preserved in obesity but does not require FGF21 or MC4R. Nat. Commun. 15, 1192 (2024).
Welt, C. K. et al. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 351, 987–997 (2004).
Pickering, T. R., Clarke, R. J. & Moggi-Cecchi, J. Role of carnivores in the accumulation of the Sterkfontein Member 4 hominid assemblage: a taphonomic reassessment of the complete hominid fossil sample (1936–1999). Am. J. Phys. Anthropol. 125, 1–15 (2004).
Shultz, S., Nelson, E. & Dunbar, R. I. Hominin cognitive evolution: identifying patterns and processes in the fossil and archaeological record. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2130–2140 (2012).
van Galen, K. A. et al. Brain responses to nutrients are severely impaired and not reversed by weight loss in humans with obesity: a randomized crossover study. Nat. Metab. 5, 1059–1072 (2023).
Levine, J. A. et al. Interindividual variation in posture allocation: possible role in human obesity. Science 307, 584–586 (2005).
Wardle, J. & Boniface, D. Changes in the distributions of body mass index and waist circumference in English adults, 1993/1994 to 2002/2003. Int. J. Obes. 32, 527–532 (2008).
Flegal, K. M. & Troiano, R. P. Changes in the distribution of body mass index of adults and children in the US population. Int. J. Obes. Relat. Metab. Disord. 24, 807–818 (2000).
Nymo, S. et al. Physiological predictors of weight regain at 1-year follow-up in weight-reduced adults with obesity. Obesity 27, 925–931 (2019).
Adam, T. C., Lejeune, M. P. & Westerterp-Plantenga, M. S. Nutrient-stimulated glucagon-like peptide 1 release after body-weight loss and weight maintenance in human subjects. Br. J. Nutr. 95, 160–167 (2006).
Buso, M. E. C. et al. Can a higher protein/low glycemic index vs. a conventional diet attenuate changes in appetite and gut hormones following weight loss? A 3-year PREVIEW sub-study. Front. Nutr. 8, 640538 (2021).
DeBenedictis, J. N. et al. Changes in the homeostatic appetite system after weight loss reflect a normalization toward a lower body weight. J. Clin. Endocrinol. Metab. 105, e2538–e2546 (2020).
Rosenbaum, M., Hirsch, J., Gallagher, D. A. & Leibel, R. L. Long-term persistence of adaptive thermogenesis in subjects who have maintained a reduced body weight. Am. J. Clin. Nutr. 88, 906–912 (2008).
Hall, K. D. et al. Effect of a plant-based, low-fat diet versus an animal-based, ketogenic diet on ad libitum energy intake. Nat. Med. 27, 344–353 (2021).
Swinburn, B. A. et al. The global syndemic of obesity, undernutrition, and climate change: the Lancet Commission Report. Lancet 393, 791–846 (2019).
Finch, G. M., Day, J. E., Razak, Welch, D. A. & Rogers, P. J. Appetite changes under free-living conditions during Ramadan fasting. Appetite 31, 159–170 (1998).
Suarez-Reyes, M. & Fernandez-Verdejo, R. Work/household, transport, and leisure domains account for the sex gap in physical activity in Chile. Front. Public. Health 10, 1011790 (2022).
Murakami, H. et al. Accuracy of wearable devices for estimating total energy expenditure: comparison with metabolic chamber and doubly labeled water method. JAMA Intern. Med. 176, 702–703 (2016).
Fernandez-Verdejo, R., Aguirre, C. & Galgani, J. E. Issues in measuring and interpreting energy balance and its contribution to obesity. Curr. Obes. Rep. 8, 88–97 (2019).
Jeran, S., Steinbrecher, A. & Pischon, T. Prediction of activity-related energy expenditure using accelerometer-derived physical activity under free-living conditions: a systematic review. Int. J. Obes. 40, 1187–1197 (2016).
Speakman, J. R. & Pontzer, H. Quantifying physical activity energy expenditure based on doubly labelled water and basal metabolism calorimetry: what are we actually measuring? Curr. Opin. Clin. Nutr. Metab. Care 26, 401–408 (2023).
Basolo, A. et al. Procedures for measuring excreted and ingested calories to assess nutrient absorption using bomb calorimetry. Obesity 28, 2315–2322 (2020).
Berrington de Gonzalez, A. et al. Body-mass index and mortality among 1.46 million white adults. N. Engl. J. Med. 363, 2211–2219 (2010).
Bhaskaran, K., Dos-Santos-Silva, I., Leon, D. A., Douglas, I. J. & Smeeth, L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 6, 944–953 (2018).
Hall, K. D. et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 378, 826–837 (2011).
Bray, G. A. & Bouchard, C. The biology of human overfeeding: a systematic review. Obes. Rev. 21, e13040 (2020).
Unlu, Y. et al. Impaired metabolic flexibility to fasting is associated with increased ad libitum energy intake in healthy adults. Obesity 32, 949–958 (2024).
Goldberg, G. R., Murgatroyd, P. R., McKenna, A. P., Heavey, P. M. & Prentice, A. M. Dietary compensation in response to covert imposition of negative energy balance by removal of fat or carbohydrate. Br. J. Nutr. 80, 141–147 (1998).
Reinhardt, M. et al. A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes 64, 2859–2867 (2015).
Pontzer, H. The energetics of movement, from exercise to ecology and evolution. J. Exp. Biol. 228, JEB247988 (2025).
Bailly, M. et al. Definition and diagnosis of constitutional thinness: a systematic review. Br. J. Nutr. 124, 531–547 (2020).
Dulloo, A. G. & Jacquet, J. The control of partitioning between protein and fat during human starvation: its internal determinants and biological significance. Br. J. Nutr. 82, 339–356 (1999).
Clayton, D. J., Creese, M., Skidmore, N., Stensel, D. J. & James, L. J. No effect of 24 h severe energy restriction on appetite regulation and ad libitum energy intake in overweight and obese males. Int. J. Obes. 40, 1662–1670 (2016).
Jandacek, R. J. Review of the effects of dilution of dietary energy with olestra on energy intake. Physiol. Behav. 105, 1124–1131 (2012).
Porikos, K. P. & Pi-Sunyer, F. X. Regulation of food intake in human obesity: studies with caloric dilution and exercise. Clin. Endocrinol. Metab. 13, 547–561 (1984).
Karl, J. P. et al. Altered metabolic homeostasis is associated with appetite regulation during and following 48-h of severe energy deprivation in adults. Metabolism 65, 416–427 (2016).
O’Leary, T. J. et al. Sex differences in energy balance, body composition, and metabolic and endocrine markers during prolonged arduous military training. J. Appl. Physiol. 136, 938–948 (2024).
Colman, E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul. Toxicol. Pharmacol. 48, 115–117 (2007).
Lebon, V. et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. J. Clin. Invest. 108, 733–737 (2001).
O’Mara, A. E. et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Invest. 130, 2209–2219 (2020).
Sjostrom, L. et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet 352, 167–172 (1998).
Cameron, J. D. et al. Energy depletion by diet or aerobic exercise alone: impact of energy deficit modality on appetite parameters. Am. J. Clin. Nutr. 103, 1008–1016 (2016).
Thivel, D. et al. Energy depletion by 24-h fast leads to compensatory appetite responses compared with matched energy depletion by exercise in healthy young males. Br. J. Nutr. 120, 583–592 (2018).
Hagele, F. A. et al. Appetite control is improved by acute increases in energy turnover at different levels of energy balance. J. Clin. Endocrinol. Metab. 104, 4481–4491 (2019).
Elia, M., Stubbs, R. J. & Henry, C. J. Differences in fat, carbohydrate, and protein metabolism between lean and obese subjects undergoing total starvation. Obes. Res. 7, 597–604 (1999).
Bloom, W. L., Azar, G., Clark, J. & MacKay, J. H. Comparison of metabolic changes in fasting obese and lean patients. Ann. N. Y. Acad. Sci. 131, 623–631 (1965).
Rising, R. et al. Food intake measured by an automated food-selection system: relationship to energy expenditure. Am. J. Clin. Nutr. 55, 343–349 (1992).
Uribe-Cerda, S., Morselli, E. & Perez-Leighton, C. Updates on the neurobiology of food reward and their relation to the obesogenic environment. Curr. Opin. Endocrinol. Diabetes Obes. 25, 292–297 (2018).
Klein, S. et al. Leptin production during early starvation in lean and obese women. Am. J. Physiol. Endocrinol. Metab. 278, E280–E284 (2000).
Chin-Chance, C., Polonsky, K. S. & Schoeller, D. A. Twenty-four-hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J. Clin. Endocrinol. Metab. 85, 2685–2691 (2000).
Mars, M., de Graaf, C., de Groot, C. P., van Rossum, C. T. & Kok, F. J. Fasting leptin and appetite responses induced by a 4-day 65%-energy-restricted diet. Int. J. Obes. 30, 122–128 (2006).
Chan, J. L. et al. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: observational and interventional studies in humans. Diabetes 51, 2105–2112 (2002).
Bak, A. M. et al. Prolonged fasting-induced metabolic signatures in human skeletal muscle of lean and obese men. PLoS ONE 13, e0200817 (2018).
Bergman, B. C., Cornier, M. A., Horton, T. J. & Bessesen, D. H. Effects of fasting on insulin action and glucose kinetics in lean and obese men and women. Am. J. Physiol. Endocrinol. Metab. 293, E1103–E1111 (2007).
Obici, S. et al. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51, 271–275 (2002).
Ludwig, D. S. et al. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. Am. J. Clin. Nutr. 114, 1873–1885 (2021).
MacLean, P. S., Higgins, J. A., Giles, E. D., Sherk, V. D. & Jackman, M. R. The role for adipose tissue in weight regain after weight loss. Obes. Rev. 16, 45–54 (2015).
Stubbs, B. J. et al. A ketone ester drink lowers human ghrelin and appetite. Obesity 26, 269–273 (2018).
Fernandez-Verdejo, R., Mey, J. T. & Ravussin, E. Effects of ketone bodies on energy expenditure, substrate utilization, and energy intake in humans. J. Lipid Res. 64, 100442 (2023).
Fisler, J. S., Egawa, M. & Bray, G. A. Peripheral 3-hydroxybutyrate and food intake in a model of dietary-fat induced obesity: effect of vagotomy. Physiol. Behav. 58, 1–7 (1995).
Kimura, I. et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl Acad. Sci. USA 108, 8030–8035 (2011).
Hochsmann, C. et al. Effect of 8 weeks of supervised overfeeding on eating attitudes and behaviors, eating disorder symptoms, and body image: results from the PROOF and EAT studies. Eat. Behav. 43, 101570 (2021).
Forbes, G. B., Brown, M. R., Welle, S. L. & Lipinski, B. A. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br. J. Nutr. 56, 1–9 (1986).
Katzeff, H. L. et al. Metabolic studies in human obesity during overnutrition and undernutrition: thermogenic and hormonal responses to norepinephrine. Metabolism 35, 166–175 (1986).
Passmore, R., Strong, J. A., Swindells, Y. E. & Eldin, N. The effect of overfeeding on two fat young women. Br. J. Nutr. 17, 373–383 (1963).
Roser, M., Ritchie, H. & Rosado, P. Food supply. Our World in Data https://ourworldindata.org/food-supply (2023).
Hollstein, T. et al. Recharacterizing the metabolic state of energy balance in thrifty and spendthrift phenotypes. J. Clin. Endocrinol. Metab. 105, 1375–1392 (2020).
Lund, J., Gerhart-Hines, Z. & Clemmensen, C. Role of energy excretion in human body weight regulation. Trends Endocrinol. Metab. 31, 705–708 (2020).
Galgani, J. E. et al. Baseline body fat percentage is associated to weight and fat mass gain from high-fat overfeeding over 8 weeks. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgaf247 (2025).
Goossens, G. H., Jocken, J. W. E. & Blaak, E. E. Sexual dimorphism in cardiometabolic health: the role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 17, 47–66 (2021).
Mauvais-Jarvis, F. Sex differences in energy metabolism: natural selection, mechanisms and consequences. Nat. Rev. Nephrol. 20, 56–69 (2024).
Jastreboff, A. M. et al. Triple-hormone-receptor agonist retatrutide for obesity — a phase 2 trial. N. Engl. J. Med. 389, 514–526 (2023).
Wilding, J. P., Overgaard, R. V., Jacobsen, L. V., Jensen, C. B. & le Roux, C. W. Exposure-response analyses of liraglutide 3.0 mg for weight management. Diabetes Obes. Metab. 18, 491–499 (2016).
Swinburn, B., Egger, G. & Raza, F. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev. Med. 29, 563–570 (1999).
Handy, S. L., Boarnet, M. G., Ewing, R. & Killingsworth, R. E. How the built environment affects physical activity: views from urban planning. Am. J. Prev. Med. 23, 64–73 (2002).
Lake, A. & Townshend, T. Obesogenic environments: exploring the built and food environments. J. R. Soc. Promot. Health 126, 262–267 (2006).
Kerns, J. C. et al. Increased physical activity associated with less weight regain six years after “The biggest loser” competition. Obesity 25, 1838–1843 (2017).
Bejarano, C. M. et al. Neighborhood built environment associations with adolescents’ location-specific sedentary and screen time. Health Place. 56, 147–154 (2019).
Frank, L. D. et al. Objective assessment of obesogenic environments in youth: geographic information system methods and spatial findings from the Neighborhood Impact on Kids study. Am. J. Prev. Med. 42, e47–e55 (2012).
Sallis, J. F. et al. Evaluating a brief self-report measure of neighborhood environments for physical activity research and surveillance: Physical Activity Neighborhood Environment Scale (PANES). J. Phys. Act. Health 7, 533–540 (2010).
Gallagher, D. et al. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 72, 694–701 (2000).
Haynes, W. G., Morgan, D. A., Walsh, S. A., Mark, A. L. & Sivitz, W. I. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 100, 270–278 (1997).
Johnstone, A. M., Murison, S. D., Duncan, J. S., Rance, K. A. & Speakman, J. R. Factors influencing variation in basal metabolic rate include fat-free mass, fat mass, age, and circulating thyroxine but not sex, circulating leptin, or triiodothyronine. Am. J. Clin. Nutr. 82, 941–948 (2005).
Acknowledgements
The authors thank V. Cortés for critical reading and recommendations to improve the manuscript. The authors are grateful to M. Heaner for her assistance with proofreading the manuscript. The authors acknowledge support from ANID/CONICYT FONDECYT Regular 1220551 (J.E.G.) and a NORC Center Grant #P30DK072476 titled ‘Nutrition and Metabolic Health Through the Lifespan’ sponsored by NIDDK.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Mark Hopkins, David Levitsky, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fernández-Verdejo, R., Ravussin, E. & Galgani, J.E. Body weight regulation models in humans: insights for testing their validity. Nat Rev Endocrinol 21, 703–717 (2025). https://doi.org/10.1038/s41574-025-01149-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41574-025-01149-1
This article is cited by
-
Obesity: a disease of the ponderostat and the regulation of energy balance
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2025)