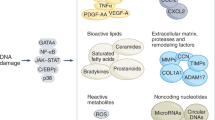
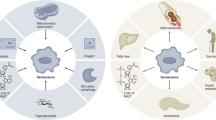
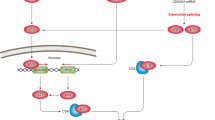
Abstract
Translational research on cellular senescence has led to numerous early-phase clinical trials targeting senescent cells to treat, prevent or alleviate multiple disorders and diseases, including metabolic diseases and their comorbidities. Cellular senescence is a cell fate that occurs in response to stressors, including metabolic disruptions, and is one of the hallmarks (or pillars) of ageing. In their senescent state, cells cease proliferation and can develop a senescence-associated secretory and metabolic phenotype that contributes to the pathogenesis of metabolic dysfunction associated with obesity and ageing. Metabolic stress, which is central to the development of metabolic diseases, can trigger cellular senescence, thereby enabling a vicious cycle that exacerbates metabolic dysfunction. Therapies targeting senescent cells (senotherapeutics), either alone or in combination with other gerotherapies or lifestyle interventions, hold great promise for addressing the ongoing obesity epidemic and the need for improved therapies to prevent and treat metabolic diseases and their complications and comorbidities. In this Review, we discuss novel senotherapeutics, including challenges related to the translation of these therapies and the need to establish gerodiagnostic biomarkers to track the elimination of senescent cells, define eligibility and measure efficacy, as well as considerations for clinical trial design and execution.
Key points
-
Cellular senescence is a fundamental ageing process that seems to contribute to the pathogenesis of many chronic diseases, including metabolic diseases.
-
Lifestyle and pharmacological interventions that affect metabolic disorders can prevent senescent cell accumulation or modulate their secretory phenotype.
-
Elimination of senescent cells with senolytic drugs or inhibition of the senescence-associated secretory phenotype have shown promise for preventing, alleviating or delaying metabolic diseases and associated comorbidities in preclinical models.
-
Senotherapies are a potentially viable intervention for treatment of metabolic diseases.
-
Early-phase clinical trials are evaluating the safety and tolerability of senolytic drugs, along with monitoring target engagement (the clearance of senescent cells) across multiple age-related diseases.
-
The development of gerodiagnostic biomarkers that target fundamental ageing processes will be critical for identifying individuals who will benefit the most from senolytic therapies and facilitate individualized approaches for treatment of metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Tchkonia, T., Palmer, A. K. & Kirkland, J. L. New horizons: novel approaches to enhance healthspan through targeting cellular senescence and related aging mechanisms. J. Clin. Endocrinol. Metab. 106, e1481–e1487 (2021).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Cellular senescence and senolytics: the path to the clinic. Nat. Med. 28, 1556–1568 (2022).
Wiley, C. D. & Campisi, J. The metabolic roots of senescence: mechanisms and opportunities for intervention. Nat. Metab. 3, 1290–1301 (2021).
Tchkonia, T. et al. Fat tissue, aging, and cellular senescence. Aging Cell 9, 667–684 (2010).
Palmer, A. K. et al. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64, 2289–2298 (2015).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl Acad. Sci. USA 112, E6301–E6310 (2015).
Gasek, N. S. et al. Clearance of p21 highly expressing senescent cells accelerates cutaneous wound healing. Nat. Aging 5, 21–27 (2025).
Tchkonia, T. et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 17, 644–656 (2013).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Munoz-Espin, D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013).
Storer, M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013).
Menon, R. et al. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging 8, 216–230 (2016).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 15, 978–990 (2013).
Zhu, Y. et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 9, 955–963 (2017).
Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Prata, L., Ovsyannikova, I. G., Tchkonia, T. & Kirkland, J. L. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin. Immunol. 40, 101275 (2018).
Ovadya, Y. et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 9, 5435 (2018).
Chaib, S. et al. The efficacy of chemotherapy is limited by intratumoral senescent cells expressing PD-L2. Nat. Cancer 5, 448–462 (2024).
Pereira, B. I. et al. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat. Commun. 10, 2387 (2019).
Egashira, M. et al. F4/80+ macrophages contribute to clearance of senescent cells in the mouse postpartum uterus. Endocrinology 158, 2344–2353 (2017).
Wang, T. W. et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 611, 358–364 (2022).
Palmer, A. K. et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18, e12950 (2019).
Hickson, L. J. et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456 (2019).
Jeyapalan, J. C. & Sedivy, J. M. Cellular senescence and organismal aging. Mech. Ageing Dev. 129, 467–474 (2008).
Tchkonia, T. & Kirkland, J. L. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA 320, 1319–1320 (2018).
Yousefzadeh, M. J. et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19, e13094 (2020).
Niemann, B. et al. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J. Am. Coll. Cardiol. 57, 577–585 (2011).
Horvath, S. et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl Acad. Sci. USA 111, 15538–15543 (2014).
Field, A. E. et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch. Intern. Med. 161, 1581–1586 (2001).
Zhu, S. et al. Aging- and obesity-related peri-muscular adipose tissue accelerates muscle atrophy. PLoS ONE 14, e0221366 (2019).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Obesity, senescence, and senolytics. Handb. Exp. Pharmacol. 274, 165–180 (2022).
Firoz, A. & Haris, M. Metabolic syndrome in childhood cancer survivors. EXCLI J. 21, 380–386 (2022).
Suvakov, S. et al. Women with a history of preeclampsia exhibit accelerated aging and unfavorable profiles of senescence markers. Hypertension 81, 1550–1560 (2024).
Smith, W. A. et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort study. Cancer 120, 2742–2750 (2014).
Friedman, D. N., Tonorezos, E. S. & Cohen, P. Diabetes and metabolic syndrome in survivors of childhood cancer. Horm. Res. Paediatr. 91, 118–127 (2019).
Rosen, G. P., Nguyen, H. T. & Shaibi, G. Q. Metabolic syndrome in pediatric cancer survivors: a mechanistic review. Pediatr. Blood Cancer 60, 1922–1928 (2013).
Suvakov, S. et al. Epigenetic and senescence markers indicate an accelerated ageing-like state in women with preeclamptic pregnancies. EBioMedicine 70, 103536 (2021).
Kirkland, J. L. & Tchkonia, T. Cellular senescence: a translational perspective. EBioMedicine 21, 21–28 (2017).
Kirkland, J. L. & Tchkonia, T. Senolytic drugs: from discovery to translation. J. Intern. Med. 288, 518–536 (2020).
Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563 (2019).
Palmer, A. K., Gustafson, B., Kirkland, J. L. & Smith, U. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia 62, 1835–1841 (2019).
Fohr, T. et al. Metabolic syndrome and epigenetic aging: a twin study. Int. J. Obes. 48, 778–787 (2024).
Nannini, D. R. et al. Epigenetic age acceleration and metabolic syndrome in the coronary artery risk development in young adults study. Clin. Epigenetics 11, 160 (2019).
Revesz, D., Milaneschi, Y., Verhoeven, J. E., Lin, J. & Penninx, B. W. Longitudinal associations between metabolic syndrome components and telomere shortening. J. Clin. Endocrinol. Metab. 100, 3050–3059 (2015).
Morley, J. E. Diabetes, sarcopenia, and frailty. Clin. Geriatr. Med. 24, 455–469 (2008).
Aronson, D. & Edelman, E. R. Coronary artery disease and diabetes mellitus. Cardiol. Clin. 32, 439–455 (2014).
Eckel, R. H., Alberti, K. G., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet 375, 181–183 (2010).
Lusis, A. J., Attie, A. D. & Reue, K. Metabolic syndrome: from epidemiology to systems biology. Nat. Rev. Genet. 9, 819–830 (2008).
Moon, J. H. et al. Increased risk of metabolic disorders in healthy young adults with family history of diabetes: from the Korea National Health and Nutrition Survey. Diabetol. Metab. Syndr. 9, 16 (2017).
Spinelli, R. et al. Increased cell senescence in human metabolic disorders. J. Clin. Invest. 133, e169922 (2023).
Murphy, S. Understanding childhood and adolescent obesity. Clin. Integr. Care 13, 100114 (2022).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Nerstedt, A. & Smith, U. The impact of cellular senescence in human adipose tissue. J. Cell Commun. Signal. 17, 563–573 (2023).
Wang, L. et al. Targeting p21Cip1 highly expressing cells in adipose tissue alleviates insulin resistance in obesity. Cell Metab. 34, 75–89 (2022).
Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 8, 15691 (2017).
Bianchi, A. et al. Moderate exercise inhibits age-related inflammation, liver steatosis, senescence, and tumorigenesis. J. Immunol. 206, 904–916 (2021).
Meijnikman, A. S. et al. Hyperinsulinemia is highly associated with markers of hepatocytic senescence in two independent cohorts. Diabetes 71, 1929–1936 (2022).
Aguayo-Mazzucato, C. Functional changes in beta cells during ageing and senescence. Diabetologia 63, 2022–2029 (2020).
Rubin de Celis, M. F. et al. PAHSAs reduce cellular senescence and protect pancreatic beta cells from metabolic stress through regulation of Mdm2/p53. Proc. Natl Acad. Sci. USA 119, e2206923119 (2022).
Cha, J., Aguayo-Mazzucato, C. & Thompson, P. J. Pancreatic β-cell senescence in diabetes: mechanisms, markers and therapies. Front. Endocrinol. (Lausanne) 14, 1212716 (2023).
Palmer, A. K., Tchkonia, T. & Kirkland, J. L. Targeting cellular senescence in metabolic disease. Mol. Metab. 66, 101601 (2022).
Sousa-Victor, P. et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316–321 (2014).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016).
Wang, J. et al. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 132, 1909–1919 (2015).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Minamino, T. & Komuro, I. Vascular cell senescence: contribution to atherosclerosis. Circ. Res. 100, 15–26 (2007).
Katakami, N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J. Atheroscler. Thromb. 25, 27–39 (2018).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Gardner, S. E., Humphry, M., Bennett, M. R. & Clarke, M. C. Senescent Vascular smooth muscle cells drive inflammation through an interleukin-1α-dependent senescence-associated secretory phenotype. Arterioscler. Thromb. Vasc. Biol. 35, 1963–1974 (2015).
Kaistha, A. et al. Premature cell senescence promotes vascular smooth muscle cell phenotypic modulation and resistance to re-differentiation. Cardiovasc. Res. 121, 1448–1463 (2025).
Katsuumi, G., Shimizu, I., Yoshida, Y. & Minamino, T. Vascular senescence in cardiovascular and metabolic diseases. Front. Cardiovasc. Med. 5, 18 (2018).
Suda, M. et al. Senescent cells: a therapeutic target in cardiovascular diseases. Cells 12, 1296 (2023).
Bian, X. et al. Senescence marker activin A is increased in human diabetic kidney disease: association with kidney function and potential implications for therapy. BMJ Open. Diabetes Res. Care 7, e000720 (2019).
Ogrodnik, M. et al. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 29, 1061–1077 (2019).
Gonzales, M. M. et al. Senolytic therapy in mild Alzheimer’s disease: a phase 1 feasibility trial. Nat. Med. 29, 2481–2488 (2023).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal. Transduct. Target. Ther. 8, 200 (2023).
Zhang, K. et al. Metabolic diseases and healthy aging: identifying environmental and behavioral risk factors and promoting public health. Front. Public. Health 11, 1253506 (2023).
Sun, J. K. et al. Chronic alcohol metabolism results in DNA repair infidelity and cell cycle-induced senescence in neurons. Aging Cell 22, e13772 (2023).
Jin, H. et al. Oroxylin A inhibits ethanol-induced hepatocyte senescence via YAP pathway. Cell Prolif. 51, e12431 (2018).
Nyunoya, T. et al. Cigarette smoke induces cellular senescence. Am. J. Respir. Cell Mol. Biol. 35, 681–688 (2006).
Cottage, C. T. et al. Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun. Biol. 2, 307 (2019).
Kaur, G., Muthumalage, T. & Rahman, I. Clearance of senescent cells reverts the cigarette smoke-induced lung senescence and airspace enlargement in p16-3MR mice. Aging Cell 22, e13850 (2023).
Hohensinner, P. J. et al. Reduction of premature aging markers after gastric bypass surgery in morbidly obese patients. Obes. Surg. 28, 2804–2810 (2018).
Madeo, F., Carmona-Gutierrez, D., Hofer, S. J. & Kroemer, G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 29, 592–610 (2019).
Fontana, L. & Klein, S. Aging, adiposity, and calorie restriction. JAMA 297, 986–994 (2007).
Green, C. L., Lamming, D. W. & Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 23, 56–73 (2022).
Krishnamurthy, J. et al. Ink4a/Arf expression is a biomarker of aging. J. Clin. Invest. 114, 1299–1307 (2004).
Longo, V. D. & Anderson, R. M. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell 185, 1455–1470 (2022).
Fontana, L. et al. The effects of graded caloric restriction: XII. Comparison of mouse to human impact on cellular senescence in the colon. Aging Cell 17, e12746 (2018).
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span — from yeast to humans. Science 328, 321–326 (2010).
Wang, C. et al. Adult-onset, short-term dietary restriction reduces cell senescence in mice. Aging 2, 555–566 (2010).
Justice, J. N. et al. Caloric restriction intervention alters specific circulating biomarkers of the senescence-associated secretome in middle-aged and older adults with obesity and prediabetes in an 18-week randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 79, glad214 (2024).
Aversa, Z. et al. Calorie restriction reduces biomarkers of cellular senescence in humans. Aging Cell 23, e14038 (2024).
Schafer, M. J. et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes 65, 1606–1615 (2016).
Englund, D. A. et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 20, e13415 (2021).
Demaria, M. et al. Long-term intensive endurance exercise training is associated to reduced markers of cellular senescence in the colon mucosa of older adults. NPJ Aging 9, 3 (2023).
Justice, J. N. et al. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci. 73, 939–945 (2018).
Moiseeva, O. et al. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 12, 489–498 (2013) .
Kulkarni, A. S., Gubbi, S. & Barzilai, N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 32, 15–30 (2020).
Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016).
Kim, M. N., Moon, J. H. & Cho, Y. M. Sodium-glucose cotransporter-2 inhibition reduces cellular senescence in the diabetic kidney by promoting ketone body-induced NRF2 activation. Diabetes Obes. Metab. 23, 2561–2571 (2021).
Madonna, R. et al. Empagliflozin reduces the senescence of cardiac stromal cells and improves cardiac function in a murine model of diabetes. J. Cell Mol. Med. 24, 12331–12340 (2020).
Katsuumi, G. et al. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat. Aging 4, 926–938 (2024).
Shah, M. & Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 15, 181–187 (2014).
Oeseburg, H. et al. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler. Thromb. Vasc. Biol. 30, 1407–1414 (2010).
Meloni, A. R., DeYoung, M. B., Lowe, C. & Parkes, D. G. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes. Metab. 15, 15–27 (2013).
Baboota, R. K. et al. Chronic hyperinsulinemia promotes human hepatocyte senescence. Mol. Metab. 64, 101558 (2022).
Westhoff, J. H. et al. Hypertension induces somatic cellular senescence in rats and humans by induction of cell cycle inhibitor p16INK4a. Hypertension 52, 123–129 (2008).
Liu, S. et al. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci. Rep. 5, 17895 (2015).
Belakova, B. et al. Lipophilic statins eliminate senescent endothelial cells by inducing anoikis-related cell death. Cells 12, 2836 (2023).
Klein, S. et al. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab. Invest. 92, 1440–1450 (2012).
Sierra-Ramirez, A. et al. Transient metabolic improvement in obese mice treated with navitoclax or dasatinib/quercetin. Aging 12, 11337–11348 (2020).
Hense, J. D. et al. MASLD does not affect fertility and senolytics fail to prevent MASLD progression in male mice. Sci. Rep. 14, 17332 (2024).
Raffaele, M. et al. Mild exacerbation of obesity- and age-dependent liver disease progression by senolytic cocktail dasatinib + quercetin. Cell Commun. Signal. 19, 44 (2021).
Hense, J. D. et al. Senolytic treatment reverses obesity-mediated senescent cell accumulation in the ovary. Geroscience 44, 1747–1759 (2022).
Avila, B. M. et al. Effect of senolytic drugs in young female mice chemically induced to estropause. Life Sci. 357, 123073 (2024).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Wilson, W. H. et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 11, 1149–1159 (2010).
Afreen, S. et al. BCL-XL expression is essential for human erythropoiesis and engraftment of hematopoietic stem cells. Cell Death Dis. 11, 8 (2020).
Josefsson, E. C., Vainchenker, W. & James, C. Regulation of platelet production and life span: role of Bcl-xL and potential implications for human platelet diseases. Int. J. Mol. Sci. 21, 7591 (2020).
Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 23, 1072–1079 (2017).
Naqvi, K. et al. Long-term follow-up of lower dose dasatinib (50 mg daily) as frontline therapy in newly diagnosed chronic-phase chronic myeloid leukemia. Cancer 126, 67–75 (2020).
Ottmann, O. et al. Long-term efficacy and safety of dasatinib in patients with chronic myeloid leukemia in accelerated phase who are resistant to or intolerant of imatinib. Blood Cancer J. 8, 88 (2018).
Christopher, L. J. et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug. Metab. Dispos. 36, 1357–1364 (2008).
Graefe, E. U. et al. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 41, 492–499 (2001).
Touil, Y. S. et al. Fisetin disposition and metabolism in mice: Identification of geraldol as an active metabolite. Biochem. Pharmacol. 82, 1731–1739 (2011).
Grosse, L. et al. Defined p16high senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99 (2020).
Born, E. et al. Eliminating senescent cells can promote pulmonary hypertension development and progression. Circulation 147, 650–666 (2023).
Mannick, J. B. et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 10, eaaq1564 (2018).
Bitto, A. et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife 5, e16351 (2016).
Fuhrmann-Stroissnigg, H. et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun. 8, 422 (2017).
Lazaro, I. et al. Targeting HSP90 ameliorates nephropathy and atherosclerosis through suppression of NF-κB and STAT signaling pathways in diabetic mice. Diabetes 64, 3600–3613 (2015).
Lee, J. H. et al. Heat shock protein 90 (HSP90) inhibitors activate the heat shock factor 1 (HSF1) stress response pathway and improve glucose regulation in diabetic mice. Biochem. Biophys. Res. Commun. 430, 1109–1113 (2013).
Samakkarnthai, P. et al. In vitro and in vivo effects of zoledronic acid on senescence and senescence-associated secretory phenotype markers. Aging 15, 3331–3355 (2023).
Xu, Q. et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 3, 1706–1726 (2021).
Liu, Y. et al. Senolytic and senomorphic agent procyanidin C1 alleviates structural and functional decline in the aged retina. Proc. Natl Acad. Sci. USA 121, e2311028121 (2024).
Shao, M. et al. Procyanidin C1 inhibits bleomycin-induced pulmonary fibrosis in mice by selective clearance of senescent myofibroblasts. FASEB J. 38, e23749 (2024).
Gonzalez-Gualda, E. et al. Galacto-conjugation of navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 19, e13142 (2020).
Munoz-Espin, D. et al. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med. 10, e9355 (2018).
Guerrero, A. et al. Galactose-modified duocarmycin prodrugs as senolytics. Aging Cell 19, e13133 (2020).
Chini, C. C. S. et al. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat. Metab. 2, 1284–1304 (2020).
Chini, C. et al. The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD+ decline. Biochem. Biophys. Res. Commun. 513, 486–493 (2019).
Ren, C. et al. Nicotinamide mononucleotide ameliorates cellular senescence and inflammation caused by sodium iodate in RPE. Oxid. Med. Cell Longev. 2022, 5961123 (2022).
Lu, Z. et al. Nicotinamide mononucleotide alleviates osteoblast senescence induction and promotes bone healing in osteoporotic mice. J. Gerontol. A Biol. Sci. Med. Sci. 78, 186–194 (2023).
Rajman, L., Chwalek, K. & Sinclair, D. A. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 27, 529–547 (2018).
Zhang, H. et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 352, 1436–1443 (2016).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Amor, C. et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging 4, 336–349 (2024).
Suda, M. et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 1, 1117–1126 (2021).
Marin, I. et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 13, 410–431 (2023).
Chen, H. A. et al. Senescence rewires microenvironment sensing to facilitate antitumor immunity. Cancer Discov. 13, 432–453 (2023).
Eskiocak, O. et al. Senolytic CAR T cells reverse aging-associated defects in intestinal regeneration and fitness. Preprint at bioRxiv https://doi.org/10.1101/2024.03.19.585779v1 (2024).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Wiley, C. D. & Campisi, J. From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab. 23, 1013–1021 (2016).
Onorati, A. et al. Upregulation of PD-L1 in senescence and aging. Mol. Cell Biol. 42, e0017122 (2022).
Quandt, Z., Young, A. & Anderson, M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin. Exp. Immunol. 200, 131–140 (2020).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019).
Hansen, E., Sahasrabudhe, D. & Sievert, L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol. Immunother. 65, 765–767 (2016).
Schwartz, C. et al. Innate PD-L1 limits T cell-mediated adipose tissue inflammation and ameliorates diet-induced obesity. Sci. Transl. Med. 14, eabj6879 (2022).
Nambiar, A. et al. Senolytics dasatinib and quercetin in idiopathic pulmonary fibrosis: results of a phase I, single-blind, single-center, randomized, placebo-controlled pilot trial on feasibility and tolerability. EBioMedicine 90, 104481 (2023).
Millar, C. L. et al. A pilot study of senolytics to improve cognition and mobility in older adults at risk for Alzheimer’s disease. EBioMedicine 113, 105612 (2025).
Farr, J. N. et al. Effects of intermittent senolytic therapy on bone metabolism in postmenopausal women: a phase 2 randomized controlled trial. Nat. Med. 30, 2605–2612 (2024).
Wyles, S. P. et al. Cellular senescence in human skin aging: leveraging senotherapeutics. Gerontology 70, 7–14 (2024).
Yu, G. T. et al. Mapping cellular senescence networks in human diabetic foot ulcers. Geroscience 46, 1071–1082 (2024).
Yu, G. T. et al. Mapping epidermal and dermal cellular senescence in human skin aging. Aging Cell 24, e14358 (2024).
Schafer, M. J. et al. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 8, 14532 (2017).
Xu, M. et al. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J. Gerontol. A Biol. Sci. Med. Sci. 72, 780–785 (2017).
Weigl, M. et al. Profiling microRNA expression during senescence and aging: mining for a diagnostic tool of senescent-cell burden. Preprint at bioRxiv https://doi.org/10.1101/2024.04.10.588794v2 (2024).
Lu, A. T. et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327 (2019).
Fohr, T. et al. Does the epigenetic clock GrimAge predict mortality independent of genetic influences: an 18 year follow-up study in older female twin pairs. Clin. Epigenetics 13, 128 (2021).
McCrory, C. et al. GrimAge outperforms other epigenetic clocks in the prediction of age-related clinical phenotypes and all-cause mortality. J. Gerontol. A Biol. Sci. Med. Sci. 76, 741–749 (2021).
Lu, A. T. et al. DNA methylation GrimAge version 2. Aging (Albany NY) 14, 9484–9549 (2022).
Vaughan, D. E., Rai, R., Khan, S. S., Eren, M. & Ghosh, A. K. Plasminogen activator inhibitor-1 is a marker and a mediator of senescence. Arterioscler. Thromb. Vasc. Biol. 37, 1446–1452 (2017).
Evans, D. S. et al. Proteomic analysis of the senescence-associated secretory phenotype: GDF-15, IGFBP-2, and cystatin-C are associated with multiple aging traits. J. Gerontol. A Biol. Sci. Med. Sci. 79, glad265 (2024).
Islam, M. T. et al. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 22, e13767 (2023).
Aguayo-Mazzucato, C. et al. Acceleration of beta cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 30, 129–142 (2019).
Xu, M. et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4, e12997 (2015).
Kim, S. R. et al. Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J. Am. Soc. Nephrol. 32, 1987–2004 (2021).
Kim, S. R. et al. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl. Res. 213, 112–123 (2019).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147 (2017).
Mylonas, K. J. et al. Cellular senescence inhibits renal regeneration after injury in mice, with senolytic treatment promoting repair. Sci. Transl. Med. 13, eabb0203 (2021).
Moncsek, A. et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2−/−) mice. Hepatology 67, 247–259 (2018).
Yu, S. et al. Quercetin reverses cardiac systolic dysfunction in mice fed with a high-fat diet: role of angiogenesis. Oxid. Med. Cell Longev. 2021, 8875729 (2021).
Dookun, E. et al. Clearance of senescent cells during cardiac ischemia-reperfusion injury improves recovery. Aging Cell 19, e13249 (2020).
Walaszczyk, A. et al. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18, e12945 (2019).
Anderson, R. et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 38, e100492 (2019).
Lewis-McDougall, F. C. et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18, e12931 (2019).
Nath, K. A. et al. The murine dialysis fistula model exhibits a senescence phenotype: pathobiological mechanisms and therapeutic potential. Am. J. Physiol. Ren. Physiol. 315, F1493–F1499 (2018).
Sugihara, H. et al. Cellular senescence-mediated exacerbation of Duchenne muscular dystrophy. Sci. Rep. 10, 16385 (2020).
Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28 (2018).
Dungan, C. M. et al. Senolytic treatment rescues blunted muscle hypertrophy in old mice. Geroscience 44, 1925–1940 (2022).
Saccon, T. D. et al. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1895–1905 (2021).
Wang, B. et al. An inducible p21-Cre mouse model to monitor and manipulate p21-highly-expressing senescent cells in vivo. Nat. Aging 1, 962–973 (2021).
Klier, S. et al. Safety and efficacy of senolytic UBX1325 in diabetic macular edema. NEJM Evid. 4, EVIDoa2400009 (2025).
Tchkonia, T., Kritchevsky, S. B., Kuchel, G. A. & Kirkland, J. L. NIA Translational Geroscience Network: an infrastructure to facilitate geroscience-guided clinical trials. J. Am. Geriatr. Soc. 72, 1605–1607 (2024).
Acknowledgements
The authors acknowledge the support of the National Institutes of Health (grants R37AG13925, R33AG61456, R01AG72301, R01AG61414, R01AG69690, U54AG75941, R01AG89711 and R01AG75684), the Hevolution Foundation (HF-GRO-23-1199148-3), the Connor Fund, Robert J. and Theresa W. Ryan, and the Noaber Foundation. The authors are grateful to T. Evans, senior program coordinator of the Translational Geoscience Network, for contributing to clinical trials of senolytics.
Author information
Authors and Affiliations
Contributions
S.C. and A.K.P. contributed to all aspects of the article. S.P.W. and N.M. contributed to writing the article and to reviewing and/or editing the manuscript before submission. J.L.K. and T.T. contributed to discussion of the content, writing the article and to reviewing and/or editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.T., A.K.P. and J.L.K. have a financial interest related to this article, including patents and pending patents covering senolytic drugs and their uses. S.C. holds patents or pending patents on PDL2 at Mayo Clinic and Spanish National Cancer Research Center, some of which have been licensed to Rejuveron Senescence Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Cristina Aguayo-Mazzucato, Ippei Shimizu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaib, S., Palmer, A.K., Wyles, S.P. et al. Translating cellular senescence research into clinical practice for metabolic disease. Nat Rev Endocrinol (2025). https://doi.org/10.1038/s41574-025-01187-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41574-025-01187-9