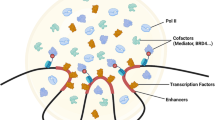
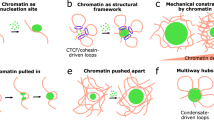
Abstract
Transcription factors relay information from the external environment to gene regulatory networks that control cell physiology. To confer signalling specificity, robustness and coordination, these signalling networks use temporal communication codes, such as the amplitude, duration or frequency of signals. Although much is known about how temporal information is encoded, a mechanistic understanding of how gene regulatory networks decode signalling dynamics is lacking. Recent advances in our understanding of phase separation of transcriptional condensates provide new biophysical frameworks for both temporal encoding and decoding mechanisms. In this Perspective, we summarize the mechanisms by which transcriptional condensates could enable temporal decoding through signal adaptation, memory and persistence. We further outline methods to probe and manipulate dynamic communication codes of transcription factors and condensates to rationally control gene activation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pearson, S., Sroczynska, P., Lacaud, G. & Kouskoff, V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development 135, 1525–1535 (2008).
Kang, M., Garg, V. & Hadjantonakis, A.-K. Lineage establishment and progression within the inner cell mass of the mouse blastocyst requires FGFR1 and FGFR2. Dev. Cell 41, 496–510.e5 (2017).
Molotkov, A., Mazot, P., Brewer, J. R., Cinalli, R. M. & Soriano, P. Distinct requirements for FGFR1 and FGFR2 in primitive endoderm development and exit from pluripotency. Dev. Cell 41, 511–526.e4 (2017).
Yamanaka, Y., Lanner, F. & Rossant, J. FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 137, 715–724 (2010).
Lee, R. E. C., Walker, S. R., Savery, K., Frank, D. A. & Gaudet, S. Fold change of nuclear NF-κB determines TNF-induced transcription in single cells. Mol. Cell 53, 867–879 (2014).
Murphy, L. O., Smith, S., Chen, R.-H., Fingar, D. C. & Blenis, J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 4, 556–564 (2002).
Murphy, L. O., MacKeigan, J. P. & Blenis, J. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol. Cell. Biol. 24, 144–153 (2004).
Lahav, G. et al. Dynamics of the p53–Mdm2 feedback loop in individual cells. Nat. Genet. 36, 147–150 (2004).
Nelson, D. E. Oscillations in NF-kB signaling control the dynamics of gene expression. Science 306, 704–708 (2004).
Goentoro, L. & Kirschner, M. W. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol. Cell 36, 872–884 (2009). This study showed that cells read out relative changes (instead of absolute levels) of β-catenin concentrations.
Barkai, N. & Leibler, S. Robustness in simple biochemical networks. Nature 387, 913–917 (1997).
Muzzey, D., Gómez-Uribe, C. A., Mettetal, J. T. & van Oudenaarden, A. A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell 138, 160–171 (2009).
Ryu, H. et al. Frequency modulation of ERK activation dynamics rewires cell fate. Mol. Syst. Biol. 12, 866 (2016).
Batchelor, E., Loewer, A., Mock, C. & Lahav, G. Stimulus-dependent dynamics of p53 in single cells. Mol. Syst. Biol. 7, 488 (2011).
Purvis, J. E. et al. p53 Dynamics control cell fate. Science 336, 1440–1444 (2012). This paper reported that cells leverage p53 dynamics to differentially control downstream programs involved in DNA repair and cellular senescence.
Heasley, L. E. & Johnson, G. L. The β-PDGF receptor induces neuronal differentiation of PC12 cells. Mol. Biol. Cell 3, 545–553 (1992).
Toettcher, J. E., Weiner, O. D. & Lim, W. A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434 (2013).
Traverse, S. et al. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr. Biol. 4, 694–701 (1994).
Traverse, S., Gomez, N., Paterson, H., Marshall, C. & Cohen, P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 288, 351–355 (1992).
Cohen-Saidon, C., Cohen, A. A., Sigal, A., Liron, Y. & Alon, U. Dynamics and variability of ERK2 response to EGF in individual living cells. Mol. Cell 36, 885–893 (2009).
Santos, S. D. M., Verveer, P. J. & Bastiaens, P. I. H. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol. 9, 324–330 (2007).
Sasagawa, S., Ozaki, Y.-I., Fujita, K. & Kuroda, S. Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nat. Cell Biol. 7, 365–373 (2005).
Marshall, C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80, 179–185 (1995).
Cai, L., Dalal, C. K. & Elowitz, M. B. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature 455, 485–490 (2008).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Henninger, J. E. et al. RNA-mediated feedback control of transcriptional condensates. Cell 184, 207–225.e24 (2021). This paper demonstrated evidence for RNA-mediated feeback for the control of Mediator condensate formation and dissolution.
Meyer, K. et al. YAP charge patterning mediates signal integration through transcriptional co-condensates. Preprint at bioRxiv https://doi.org/10.1101/2024.08.10.607443 (2024).
Huang, W. Y. C. et al. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 363, 1098–1103 (2019). This study demonstrated that a phase transition is involved in kinetic proofreading in receptor-mediated activation of Ras.
Sun, S., GrandPre, T., Limmer, D. T. & Groves, J. T. Kinetic frustration by limited bond availability controls the LAT protein condensation phase transition on membranes. Sci. Adv. 8, eabo5295 (2022).
Sweeney, K. & McClean, M. N. Transcription factor localization dynamics and DNA binding drive distinct promoter interpretations. Cell Rep. 42, 112426 (2023).
Adler, M. & Alon, U. Fold-change detection in biological systems. Curr. Opin. Syst. Biol. 8, 81–89 (2018).
Ferrell, J. E. Jr. Perfect and near-perfect adaptation in cell signaling. Cell Syst. 2, 62–67 (2016).
Zhang, C., Tsoi, R., Wu, F. & You, L. Processing oscillatory signals by incoherent feedforward loops. PLoS Comput. Biol. 12, e1005101 (2016).
Goentoro, L., Shoval, O., Kirschner, M. W. & Alon, U. The incoherent feedforward loop can provide fold-change detection in gene regulation. Mol. Cell 36, 894–899 (2009).
Shen-Orr, S. S., Milo, R., Mangan, S. & Alon, U. Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 31, 64–68 (2002).
Milo, R. et al. Network motifs: simple building blocks of complex networks. Science 298, 824–827 (2002).
Lee, T. I. et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298, 799–804 (2002).
Milo, R. et al. Superfamilies of evolved and designed networks. Science 303, 1538–1542 (2004).
Boyer, L. A. et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956 (2005).
Ashall, L. et al. Pulsatile stimulation determines timing and specificity of NF-kB-dependent transcription. Science 324, 242–246 (2009).
Hoffmann, A., Levchenko, A., Scott, M. L. & Baltimore, D. The IkB–NF-kB signaling module: temporal control and selective gene activation. Science 298, 1241–1245 (2002).
Hansen, A. S. & O’Shea, E. K. Promoter decoding of transcription factor dynamics involves a trade-off between noise and control of gene expression. Mol. Syst. Biol. 9, 704 (2013).
Mittag, T. & Pappu, R. V. A conceptual framework for understanding phase separation and addressing open questions and challenges. Mol. Cell 82, 2201–2214 (2022).
Riback, J. A. et al. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168, 1028–1040.e19 (2017).
Cinar, H. et al. Temperature, hydrostatic pressure, and osmolyte effects on liquid–liquid phase separation in protein condensates: physical chemistry and biological implications. Chemistry 25, 13049–13069 (2019).
Mittag, T. & Parker, R. Multiple modes of protein-protein interactions promote RNP granule assembly. J. Mol. Biol. 430, 4636–4649 (2018).
Cabral, S. E., Otis, J. P. & Mowry, K. L. Multivalent interactions with RNA drive recruitment and dynamics in biomolecular condensates in Xenopus oocytes. iScience 25, 104811 (2022).
Kahn, J. D., Lemke, E. A. & Pappu, R. V. Faces, facets, and functions of biomolecular condensates driven by multivalent proteins and nucleic acids. Biophys. J. 120, E1–E4 (2021).
Brangwynne, C. P., Tompa, P. & Pappu, R. V. Polymer physics of intracellular phase transitions. Nat. Phys. 11, 899–904 (2015).
Pak, C. W. et al. Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein. Mol. Cell 63, 72–85 (2016).
Jiang, H. et al. Phase transition of spindle-associated protein regulate spindle apparatus assembly. Cell 163, 108–122 (2015).
Choi, J.-M., Holehouse, A. S. & Pappu, R. V. Physical principles underlying the complex biology of intracellular phase transitions. Annu. Rev. Biophys. 49, 107–133 (2020).
Zhang, Y. et al. The exchange dynamics of biomolecular condensates. eLife 12, RP91680 (2024).
Taylor, N. O., Wei, M.-T., Stone, H. A. & Brangwynne, C. P. Quantifying dynamics in phase-separated condensates using fluorescence recovery after photobleaching. Biophys. J. 117, 1285–1300 (2019).
Shen, Y. et al. The liquid-to-solid transition of FUS is promoted by the condensate surface. Proc. Natl Acad. Sci. USA 120, e2301366120 (2023).
Patel, A. et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077 (2015).
Lin, Y., Protter, D. S. W., Rosen, M. K. & Parker, R. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 (2015).
Phair, R. D. & Misteli, T. High mobility of proteins in the mammalian cell nucleus. Nature 404, 604–609 (2000).
Kar, S. et al. The Balbiani body is formed by microtubule-controlled molecular condensation of Buc in early oogenesis. Curr. Biol. 35, 315–332.e7 (2025).
Boke, E. et al. Amyloid-like self-assembly of a cellular compartment. Cell 166, 637–650 (2016).
Linsenmeier, M. et al. Dynamic arrest and aging of biomolecular condensates are modulated by low-complexity domains, RNA and biochemical activity. Nat. Commun. 13, 3030 (2022).
Folkmann, A. W., Putnam, A., Lee, C. F. & Seydoux, G. Regulation of biomolecular condensates by interfacial protein clusters. Science 373, 1218–1224 (2021). This study suggested that Pickering agents tune condensate–cytoplasmic exchange of biomolecular condensates.
Pullara, P., Alshareedah, I. & Banerjee, P. R. Temperature-dependent reentrant phase transition of RNA–polycation mixtures. Soft Matter 18, 1342–1349 (2022).
Krainer, G. et al. Reentrant liquid condensate phase of proteins is stabilized by hydrophobic and non-ionic interactions. Nat. Commun. 12, 1085 (2021).
Zhang, H. et al. RNA controls PolyQ protein phase transitions. Mol. Cell 60, 220–230 (2015).
Maharana, S. et al. RNA buffers the phase separation behavior of prion-like RNA binding proteins. Science 360, 918–921 (2018).
Lee, C.-Y. S. et al. Recruitment of mRNAs to P granules by condensation with intrinsically-disordered proteins. eLife 9, e52896 (2020).
Milin, A. N. & Deniz, A. A. Reentrant phase transitions and non-equilibrium dynamics in membraneless organelles. Biochemistry 57, 2470–2477 (2018).
Banerjee, P. R., Milin, A. N., Moosa, M. M., Onuchic, P. L. & Deniz, A. A. Reentrant phase transition drives dynamic substructure formation in ribonucleoprotein droplets. Angew. Chem. Int. Ed. Engl. 129, 11512–11517 (2017).
Schede, H. H., Natarajan, P., Chakraborty, A. K. & Shrinivas, K. A model for organization and regulation of nuclear condensates by gene activity. Nat. Commun. 14, 4152 (2023).
Lin, A. Z. et al. Dynamical control enables the formation of demixed biomolecular condensates. Nat. Commun. 14, 7678 (2023). This paper reported that compositional identities of condensates can be achieved through dynamical control.
Meyer, K., Lammers, N. C., Bugaj, L. J., Garcia, H. G. & Weiner, O. D. Optogenetic control of YAP reveals a dynamic communication code for stem cell fate and proliferation. Nat. Commun. 14, 6929 (2023).
Shin, Y. et al. Liquid nuclear condensates mechanically sense and restructure the genome. Cell 176, 1518 (2019).
Quail, T. et al. Force generation by protein–DNA co-condensation. Nat. Phys. 17, 1007–1012 (2021). This study showed that the interaction between condensates and DNA generates forces.
Nguyen, T. et al. Chromatin sequesters pioneer transcription factor Sox2 from exerting force on DNA. Nat. Commun. 13, 3988 (2022).
Renger, R. et al. Co-condensation of proteins with single- and double-stranded DNA. Proc. Natl Acad. Sci. USA 119, e2107871119 (2022).
Gouveia, B. et al. Capillary forces generated by biomolecular condensates. Nature 609, 255–264 (2022).
Strom, A. R. et al. Condensate interfacial forces reposition DNA loci and probe chromatin viscoelasticity. Cell 187, 5282–5297.e20 (2024). This paper reported that biomolecular condensates generate capillary forces at targeted DNA loci, which were sufficient to reposition genomic loci.
Alam, S. G. et al. The mammalian LINC complex regulates genome transcriptional responses to substrate rigidity. Sci. Rep. 6, 38063 (2016).
Carley, E. et al. The LINC complex transmits integrin-dependent tension to the nuclear lamina and represses epidermal differentiation. eLife 10, e58541 (2021).
Morrison, S. J. et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 101, 499–510 (2000).
Gunne-Braden, A. et al. GATA3 mediates a fast, irreversible commitment to BMP4-driven differentiation in human embryonic stem cells. Cell Stem Cell 26, 693–706.e9 (2020).
Wang, J., Xu, L., Wang, E. & Huang, S. The potential landscape of genetic circuits imposes the arrow of time in stem cell differentiation. Biophys. J. 99, 29–39 (2010).
Iglesias, N. et al. Automethylation-induced conformational switch in Clr4 (Suv39h) maintains epigenetic stability. Nature 560, 504–508 (2018).
Ramaswami, M., Taylor, J. P. & Parker, R. Altered ribostasis: RNA–protein granules in degenerative disorders. Cell 154, 727–736 (2013).
Kim, H. J. et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473 (2013).
Du, M. et al. Direct observation of a condensate effect on super-enhancer controlled gene bursting. Cell 187, 2595–2598 (2024).
Christie, J. M., Salomon, M., Nozue, K., Wada, M. & Briggs, W. R. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc. Natl Acad. Sci. USA 96, 8779–8783 (1999).
Harper, S. M., Neil, L. C. & Gardner, K. H. Structural basis of a phototropin light switch. Science 301, 1541–1544 (2003).
Wu, Y. I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Strickland, D. et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384 (2012).
Liu, H. et al. Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322, 1535–1539 (2008).
Kennedy, M. J. et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods 7, 973–975 (2010).
Bugaj, L. J., Choksi, A. T., Mesuda, C. K., Kane, R. S. & Schaffer, D. V. Optogenetic protein clustering and signaling activation in mammalian cells. Nat. Methods 10, 249–252 (2013).
Ni, M., Tepperman, J. M. & Quail, P. H. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400, 781–784 (1999).
Shimizu-Sato, S., Huq, E., Tepperman, J. M. & Quail, P. H. A light-switchable gene promoter system. Nat. Biotechnol. 20, 1041–1044 (2002).
Levskaya, A., Weiner, O. D., Lim, W. A. & Voigt, C. A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature 461, 997–1001 (2009).
Shaaya, M. et al. Light-regulated allosteric switch enables temporal and subcellular control of enzyme activity. eLife 9, e60647 (2020).
Krueger, D. et al. Principles and applications of optogenetics in developmental biology. Development 146, dev175067 (2019).
Tischer, D. & Weiner, O. D. Illuminating cell signalling with optogenetic tools. Nat. Rev. Mol. Cell Biol. 15, 551–558 (2014).
Lee, S. et al. Reversible protein inactivation by optogenetic trapping in cells. Nat. Methods 11, 633–636 (2014).
Niopek, D., Wehler, P., Roensch, J., Eils, R. & Di Ventura, B. Optogenetic control of nuclear protein export. Nat. Commun. 7, 10624 (2016).
Kögler, A. C. et al. Extremely rapid and reversible optogenetic perturbation of nuclear proteins in living embryos. Dev. Cell 56, 2348–2363.e8 (2021).
Niopek, D. et al. Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nat. Commun. 5, 4404 (2014).
Yumerefendi, H. et al. Control of protein activity and cell fate specification via light-mediated nuclear translocation. PLoS ONE 10, e0128443 (2015).
Chen, S. Y. et al. Optogenetic control reveals differential promoter interpretation of transcription factor nuclear translocation dynamics. Cell Syst. 11, 336–353.e24 (2020).
Zimmerman, S. P., Kuhlman, B. & Yumerefendi, H. Engineering and application of LOV2-based photoswitches. Methods Enzymol. 580, 169–190 (2016).
Wilson, M. Z., Ravindran, P. T., Lim, W. A. & Toettcher, J. E. Tracing information flow from Erk to target gene induction reveals mechanisms of dynamic and combinatorial control. Mol. Cell 67, 757–769.e5 (2017).
Shin, Y. et al. Spatiotemporal control of intracellular phase transitions using light-activated optoDroplets. Cell 168, 159–171.e14 (2017). This paper introduced an optogenetic platform (optoDroplets) that uses light to activate IDR-mediated phase transitions in living cells.
Bracha, D. et al. Mapping local and global liquid phase behavior in living cells using photo-oligomerizable seeds. Cell 176, 407 (2019).
Kim, Y. J. et al. Light-activated macromolecular phase separation modulates transcription by reconfiguring chromatin interactions. Sci. Adv. 9, eadg1123 (2023).
Dine, E., Gil, A. A., Uribe, G., Brangwynne, C. P. & Toettcher, J. E. Protein phase separation provides long-term memory of transient spatial stimuli. Cell Syst. 6, 655–663.e5 (2018). This study demonstrated spatial memory of phase-separated compartments.
Brumbaugh-Reed, E. H., Gao, Y., Aoki, K. & Toettcher, J. E. Rapid and reversible dissolution of biomolecular condensates using light-controlled recruitment of a solubility tag. Nat. Commun. 15, 6717 (2024).
Bertrand, E. et al. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2, 437–445 (1998).
Lee, M. et al. Optogenetic control of mRNA condensation reveals an intimate link between condensate material properties and functions. Nat. Commun. 15, 3216 (2024).
Taylor, P. Ostwald ripening in emulsions. Adv. Colloid Interface Sci. 75, 107–163 (1998).
Chung, C.-I., Yang, J. & Shu, X. Chemogenetic minitool for dissecting the roles of protein phase separation. ACS Cent. Sci. 9, 1466–1479 (2023).
Chung, C.-I. et al. Phase separation of YAP–MAML2 differentially regulates the transcriptome. Proc. Natl Acad. Sci. USA 121, e2310430121 (2024).
Yang, J. et al. MYC phase separation selectively modulates the transcriptome. Nat. Struct. Mol. Biol. 31, 1567–1579 (2024).
Wagh, K., Stavreva, D. A., Upadhyaya, A. & Hager, G. L. Transcription factor dynamics: one molecule at a time. Annu. Rev. Cell Dev. Biol. 39, 277–305 (2023).
Hager, G. L., McNally, J. G. & Misteli, T. Transcription dynamics. Mol. Cell 35, 741–753 (2009).
Gebhardt, J. C. M. et al. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods 10, 421–426 (2013).
Izeddin, I. et al. Single-molecule tracking in live cells reveals distinct target-search strategies of transcription factors in the nucleus. eLife 3, e02230 (2014).
Loffreda, A. et al. Live-cell p53 single-molecule binding is modulated by C-terminal acetylation and correlates with transcriptional activity. Nat. Commun. 8, 313 (2017).
Hao, S. et al. YAP condensates are highly organized hubs. iScience 27, 109927 (2024).
Berrocal, A., Lammers, N. C., Garcia, H. G. & Eisen, M. B. Unified bursting strategies in ectopic and endogenous even-skipped expression patterns. eLife 12, RP88671 (2023).
Ha, T. et al. Probing the interaction between two single molecules: fluorescence resonance energy transfer between a single donor and a single acceptor. Proc. Natl Acad. Sci. USA 93, 6264–6268 (1996).
Joo, C. & Ha, T. Single-molecule FRET with total internal reflection microscopy. Cold Spring Harb. Protoc. 2012, db.top072058 (2012).
Graham, T. G. W., Ferrie, J. J., Dailey, G. M., Tjian, R. & Darzacq, X. Correction: detecting molecular interactions in live-cell single-molecule imaging with proximity-assisted photoactivation (PAPA). eLife 13, e97099 (2024).
Shoval, O. et al. Fold-change detection and scalar symmetry of sensory input fields. Proc. Natl Acad. Sci. USA 107, 15995–16000 (2010).
Riback, J. A. et al. Composition-dependent thermodynamics of intracellular phase separation. Nature 581, 209–214 (2020).
Nandi, S. K., Österle, D., Heidenreich, M., Levy, E. D. & Safran, S. A. Affinity and valence impact the extent and symmetry of phase separation of multivalent proteins. Phys. Rev. Lett. 129, 128102 (2022).
Farag, M., Borcherds, W. M., Bremer, A., Mittag, T. & Pappu, R. V. Phase separation of protein mixtures is driven by the interplay of homotypic and heterotypic interactions. Nat. Commun. 14, 5527 (2023).
Monahan, Z. et al. Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 36, 2951–2967 (2017).
Aumiller, W. M. Jr & Keating, C. D. Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat. Chem. 8, 129–137 (2016).
Kwon, I. et al. Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell 156, 374 (2014).
Sanders, D. W. et al. Competing protein–RNA interaction networks control multiphase intracellular organization. Cell 181, 306–324.e28 (2020).
Guillén-Boixet, J. et al. RNA-induced conformational switching and clustering of G3BP drive stress granule assembly by condensation. Cell 181, 346–361.e17 (2020).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Genetics thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Aggregate
-
State of proteins in which irreversible intermolecular interactions are established.
- Chemotaxis
-
The directed movement of a cell in response to a chemical stimulus.
- Demixing
-
The process of a mixture spontaneously separating into its constituent components owing to physical properties.
- Osmo-responses
-
The response of cells to changes in solute concentration of their environment. It is primarily driven by the flux of water across the cellular membrane to balance solute concentrations inside and outside the cell.
- Phase separation
-
The process by which a homogeneous mixture of molecules segregates into distinct phases with different properties.
- Surfactant
-
Amphiphilic molecules that decrease the surface tension or interfacial tension between two liquids.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meyer, K., Huang, B. & Weiner, O.D. Emerging roles of transcriptional condensates as temporal signal integrators. Nat Rev Genet 26, 559–570 (2025). https://doi.org/10.1038/s41576-025-00837-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41576-025-00837-y