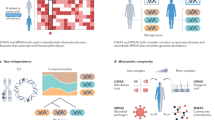
Abstract
The human microbiome is a complex ecosystem of microorganisms that inhabit the human body and have a crucial role in human health. Microbiome composition is shaped by its interaction with many factors, including human genetics. Advances in genomic technologies are improving the ability to quantify the effect of human genetics on the microbiome through improved heritability studies and microbiome genome-wide association studies (GWAS). Complementary studies using transcriptomic analyses are providing a more comprehensive view of the bidirectional relationship between host gene expression and the microbiome. The resulting insights into the genetic mechanisms driving host–microbiome interactions will ultimately contribute to the development of personalized medicine and targeted therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Valdes, A. M., Walter, J., Segal, E. & Spector, T. D. Role of the gut microbiota in nutrition and health. BMJ 361, k2179 (2018).
Zheng, D., Liwinski, T. & Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506 (2020).
Tarracchini, C. et al. Exploring the vitamin biosynthesis landscape of the human gut microbiota. mSystems 9, e0092924 (2024).
de Vos, W. M., Tilg, H., Van Hul, M. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022).
Geng, J., Ni, Q., Sun, W., Li, L. & Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 147, 112678 (2022).
Van Hul, M. & Cani, P. D. The gut microbiota in obesity and weight management: microbes as friends or foe? Nat. Rev. Endocrinol. 19, 258–271 (2023).
Kandalai, S., Li, H., Zhang, N., Peng, H. & Zheng, Q. The human microbiome and cancer: a diagnostic and therapeutic perspective. Cancer Biol. Ther. 24, 2240084 (2023).
Vivarelli, S. et al. Gut microbiota and cancer: from pathogenesis to therapy. Cancers 11, 38 (2019).
Vich Vila, A. et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 11, 362 (2020).
Amato, K. R. et al. The human gut microbiome and health inequities. Proc. Natl Acad. Sci. USA 118, e2017947118 (2021).
Leeming, E. R., Johnson, A. J., Spector, T. D. & Le Roy, C. I. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients 11, 2862 (2019).
Schmidt, T. S. B., Raes, J. & Bork, P. The human gut microbiome: from association to modulation. Cell 172, 1198–1215 (2018).
Turnbaugh, P. J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009).
Benson, A. K. et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl Acad. Sci. USA 107, 18933–18938 (2010).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014). This important early study uses data from twins to estimate gut microbiome heritability.
Visscher, P. M., Hill, W. G. & Wray, N. R. Heritability in the genomics era—concepts and misconceptions. Nat. Rev. Genet. 9, 255–266 (2008).
Goodrich, J. K. et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe 19, 731–743 (2016).
Davenport, E. R. et al. Genome-wide association studies of the human gut microbiota. PLoS One 10, e0140301 (2015).
O’Callaghan, A. & van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 7, 925 (2016).
Samuel, B. S. et al. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc. Natl Acad. Sci. USA 104, 10643–10648 (2007).
Ignatyeva, O. et al. Christensenella minuta, a new candidate next-generation probiotic: current evidence and future trajectories. Front. Microbiol. 14, 1241259 (2023).
Rothschild D et al. Environment dominates over host genetics in shaping human gut microbiota. Yearb. Pediatr. Endocrinol. https://doi.org/10.1530/ey.15.14.5 (2018). This key study argues that microbiome heritability is low and that environment is stronger than genetics in shaping the gut microbiome.
Hughes, D. A. et al. Genome-wide associations of human gut microbiome variation and implications for causal inference analyses. Nat. Microbiol. 5, 1079–1087 (2020).
Wang, J. et al. Meta-analysis of human genome-microbiome association studies: the MiBioGen consortium initiative. Microbiome 6, 101 (2018).
Kurilshikov, A. et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 53, 156–165 (2021).
Boulund, U. et al. Gut microbiome associations with host genotype vary across ethnicities and potentially influence cardiometabolic traits. Cell. Host. Microbe 30, 1464–1480.e6 (2022).
Gacesa, R. et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 604, 732–739 (2022).
Zhernakova, D. V. et al. Host genetic regulation of human gut microbial structural variation. Nature 625, 813–821 (2024). This study investigates the heritability of structural variation in the gut microbiome.
Beaumont, M. et al. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 17, 189 (2016).
Blekhman, R. et al. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 16, 191 (2015). This important early study demonstrates the feasibility of testing for genome-wide host genetic associations with gut microbiome measurements as quantitative traits.
Grieneisen, L. et al. Gut microbiome heritability is nearly universal but environmentally contingent. Science 373, 181–186 (2021). This article describes a large study of microbiome heritability in wild primates.
Zhernakova, A. et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352, 565–569 (2016).
Hejazi, J., Amiri, R., Nozarian, S., Tavasolian, R. & Rahimlou, M. Genetic determinants of food preferences: a systematic review of observational studies. BMC Nutr. 10, 24 (2024).
Vandeputte, D. et al. Temporal variability in quantitative human gut microbiome profiles and implications for clinical research. Nat. Commun. 12, 6740 (2021).
Org, E. et al. Genetic and environmental control of host–gut microbiota interactions. Genome Res. 25, 1558–1569 (2015).
Chen, C. et al. Contribution of host genetics to the variation of microbial composition of cecum lumen and feces in pigs. Front. Microbiol. 9, 2626 (2018).
Doms, S. et al. Key features of the genetic architecture and evolution of host–microbe interactions revealed by high-resolution genetic mapping of the mucosa-associated gut microbiome in hybrid mice. eLife 11, e75419 (2022).
McKnite, A. M. et al. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS One 7, e39191 (2012).
Leamy, L. J. et al. Host genetics and diet, but not immunoglobulin A expression, converge to shape compositional features of the gut microbiome in an advanced intercross population of mice. Genome Biol. 15, 552 (2014).
Turpin, W. et al. Association of host genome with intestinal microbial composition in a large healthy cohort. Nat. Genet. 48, 1413–1417 (2016).
Bonder, M. J. et al. The effect of host genetics on the gut microbiome. Nat. Genet. 48, 1407–1412 (2016). This paper describes an early study on the effects of host genetics on the gut microbiome.
Wang, J. et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 48, 1396–1406 (2016). This early microbiome GWAS finds associations with the gene encoding for the vitamin D receptor.
Ishida, S. et al. Genome-wide association studies and heritability analysis reveal the involvement of host genetics in the Japanese gut microbiota. Commun. Biol. 3, 686 (2020).
Rühlemann, M. C. et al. Genome-wide association study in 8956 German individuals identifies influence of ABO histo-blood groups on gut microbiome. Nat. Genet. 53, 147–155 (2021). This large GWAS identifies effects of the ABO blood group on the human gut microbiome.
Liu, X. et al. A genome-wide association study for gut metagenome in Chinese adults illuminates complex diseases. Cell Discov. 7, 9 (2021).
Liu, X. et al. Mendelian randomization analyses support causal relationships between blood metabolites and the gut microbiome. Nat. Genet. 54, 52–61 (2022).
Lopera-Maya, E. A. et al. Effect of host genetics on the gut microbiome in 7738 participants of the Dutch Microbiome Project. Nat. Genet. 54, 143–151 (2022). This large GWAS of the human gut microbiome validates the association of variants at the LCT locus and finds that the association is modulated by lactose intake.
Qin, Y. et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 54, 134–142 (2022).
Kolde, R. et al. Host genetic variation and its microbiome interactions within the human microbiome project. Genome Med. 10, 6 (2018).
Scepanovic, P. et al. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome 7, 130 (2019).
Tomofuji, Y. et al. Analysis of gut microbiome, host genetics, and plasma metabolites reveals gut microbiome–host interactions in the Japanese population. Cell Rep. 42, 113324 (2023).
Moitinho-Silva, L. et al. Host genetic factors related to innate immunity, environmental sensing and cellular functions are associated with human skin microbiota. Nat. Commun. 13, 6204 (2022).
Liu, X. et al. A genome-wide association study reveals the relationship between human genetic variation and the nasal microbiome. Commun. Biol. 7, 139 (2024).
Fang, Z. Y. et al. Networks of human milk microbiota are associated with host genomics, childhood asthma, and allergic sensitization. Cell Host Microbe 32, 1838–1852.e5 (2024).
Enattah, N. S. et al. Identification of a variant associated with adult-type hypolactasia. Nat. Genet. 30, 233–237 (2002).
Rasinperä, H. et al. Transcriptional downregulation of the lactase (LCT) gene during childhood. Gut 54, 1660–1661 (2005).
Garrido, D., Barile, D. & Mills, D. A. A molecular basis for bifidobacterial enrichment in the infant gastrointestinal tract. Adv. Nutr. 3, 415S–421S (2012).
Itan, Y., Jones, B. L., Ingram, C. J. E., Swallow, D. M. & Thomas, M. G. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol. Biol. 10, 36 (2010).
Jajosky, R. P. et al. ABO blood group antigens and differential glycan expression: perspective on the evolution of common human enzyme deficiencies. iScience 26, 105798 (2023).
Mäkivuokko, H. et al. Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiol. 12, 94 (2012).
Rausch, P. et al. Multigenerational influences of the Fut2 gene on the dynamics of the gut microbiota in mice. Front. Microbiol. 8, 991 (2017).
Folseraas, T. et al. Extended analysis of a genome-wide association study in primary sclerosing cholangitis detects multiple novel risk loci. J. Hepatol. 57, 366–375 (2012).
Rausch, P. et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc. Natl Acad. Sci. USA 108, 19030–19035 (2011).
Wacklin, P. et al. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One 6, e20113 (2011).
Tong, M. et al. Reprograming of gut microbiome energy metabolism by the FUT2 Crohn’s disease risk polymorphism. ISME J. 8, 2193–2206 (2014).
Yang, H. et al. ABO genotype alters the gut microbiota by regulating GalNAc levels in pigs. Nature 606, 358–367 (2022).
Ségurel, L. et al. The ABO blood group is a trans-species polymorphism in primates. Proc. Natl Acad. Sci. USA 109, 18493–18498 (2012).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open. Res. 4, 186 (2019).
Davey Smith, G. & Ebrahim, S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22 (2003).
Sanderson, E. et al. Mendelian randomization. Nat. Rev. Methods Prim. 2, 6 (2022).
Sanna, S. et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605 (2019). Together with Liu et al. (2022), this work exemplifies the use of Mendelian randomization to identify potential causal effects of the microbiome on human health.
Arora, T. & Tremaroli, V. Therapeutic potential of butyrate for treatment of type 2 diabetes. Front. Endocrinol. 12, 761834 (2021).
Stender, S., Gellert-Kristensen, H. & Smith, G. D. Reclaiming Mendelian randomization from the deluge of papers and misleading findings. Lipids Health Dis. 23, 286 (2024).
Wade, K. H. & Hall, L. J. Improving causality in microbiome research: can human genetic epidemiology help? Wellcome Open. Res. 4, 199 (2019).
Morgan, X. C. et al. Associations between host gene expression, the mucosal microbiome, and clinical outcome in the pelvic pouch of patients with inflammatory bowel disease. Genome Biol. 16, 67 (2015).
Häsler, R. et al. Uncoupling of mucosal gene regulation, mRNA splicing and adherent microbiota signatures in inflammatory bowel disease. Gut 66, 2087–2097 (2017).
Omar Al-Hassi, H., Ng, O. & Brookes, M. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 67, 395 (2018).
Bennet, S. M. P. et al. Altered intestinal antibacterial gene expression response profile in irritable bowel syndrome is linked to bacterial composition and immune activation: XXXX. Neurogastroenterol. Motil. 30, e13468 (2018).
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019). Together with Morgan et al. (2015), this key paper describes associations between the gut microbiome and host gene regulation in IBD.
Dayama, G., Priya, S., Niccum, D. E., Khoruts, A. & Blekhman, R. Interactions between the gut microbiome and host gene regulation in cystic fibrosis. Genome Med. 12, 12 (2020).
Mars, R. A. T. et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 182, 1460–1473.e17 (2020).
Chang, H. et al. Unveiling the links between microbial alteration and host gene disarray in Crohn’s disease via TAHMC. Adv. Biol. 8, e2400064 (2024).
Mirsepasi-Lauridsen, H. C., Vallance, B. A., Krogfelt, K. A. & Petersen, A. M. Escherichia coli pathobionts associated with inflammatory bowel disease. Clin. Microbiol. Rev. 32, e00060-18 (2019).
Xu, S. et al. Oxidative stress gene expression, DNA methylation, and gut microbiota interaction trigger Crohn’s disease: a multi-omics Mendelian randomization study. BMC Med. 21, 179 (2023).
Priya, S. et al. Identification of shared and disease-specific host gene–microbiome associations across human diseases using multi-omic integration. Nat. Microbiol. 7, 780–795 (2022). This important study elucidates patterns of host gene–microbiome interactions across diseases.
Fyhrquist, N. et al. Microbe–host interplay in atopic dermatitis and psoriasis. Nat. Commun. 10, 4703 (2019).
Edfeldt, G. et al. Distinct cervical tissue-adherent and luminal microbiome communities correlate with mucosal host gene expression and protein levels in Kenyan sex workers. Microbiome 11, 67 (2023).
Wang, Z. et al. Airway host–microbiome interactions in chronic obstructive pulmonary disease. Respir. Res. 20, 113 (2019).
Yan, Z. et al. Multi-omics analyses of airway host–microbe interactions in chronic obstructive pulmonary disease identify potential therapeutic interventions. Nat. Microbiol. 7, 1361–1375 (2022).
Johnson, K. E. et al. Human milk variation is shaped by maternal genetics and impacts the infant gut microbiome. Cell Genom. 4, 100638 (2024).
Yang, Y. et al. Exploratory multi-omics analysis reveals host–microbe interactions associated with disease severity in psoriatic skin. EBioMedicine 105, 105222 (2024).
Zhu, B. et al. Characteristics of vaginal microbes and classification of the vaginal microbiome. Preprint at bioRxiv https://doi.org/10.1101/2023.08.16.553525 (2023).
Galeano Niño, J. L. et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611, 810–817 (2022).
Cai, L. et al. Integrative analysis reveals associations between oral microbiota dysbiosis and host genetic and epigenetic aberrations in oral cavity squamous cell carcinoma. NPJ Biofilms Microbiomes 10, 39 (2024).
Camp, J. G. et al. Microbiota modulate transcription in the intestinal epithelium without remodeling the accessible chromatin landscape. Genome Res. 24, 1504–1516 (2014).
Sommer, F., Nookaew, I., Sommer, N., Fogelstrand, P. & Bäckhed, F. Site-specific programming of the host epithelial transcriptome by the gut microbiota. Genome Biol. 16, 62 (2015).
Pan, W.-H. et al. Exposure to the gut microbiota drives distinct methylome and transcriptome changes in intestinal epithelial cells during postnatal development. Genome Med. 10, 27 (2018).
Davison, J. M. et al. Microbiota regulate intestinal epithelial gene expression by suppressing the transcription factor hepatocyte nuclear factor 4ɑ. Genome Res. 27, 1195–1206 (2017).
Richards, A. L. et al. Gut microbiota has a widespread and modifiable effect on host gene regulation. mSystems 4, e00323–18 (2019). Together with Davison et al. (2017), this key paper investigates the mechanisms of host gene–microbiome cross-talk.
Semenkovich, N. P. et al. Impact of the gut microbiota on enhancer accessibility in gut intraepithelial lymphocytes. Proc. Natl Acad. Sci. USA 113, 14805–14810 (2016).
Dirksen, P. et al. CeMbio—the Caenorhabditis elegans microbiome resource. G3 10, 3025–3039 (2020).
Yang, W. et al. The inducible response of the nematode Caenorhabditis elegans to members of its natural microbiota across development and adult life. Front. Microbiol. 10, 1793 (2019).
Loomis, K. H. et al. A mixed community of skin microbiome representatives influences cutaneous processes more than individual members. Microbiome 9, 22 (2021).
Brusilovsky, M. et al. Host–microbiota interactions in the esophagus during homeostasis and allergic inflammation. Gastroenterology 162, 521–534.e8 (2022).
Meisel, J. S. et al. Commensal microbiota modulate gene expression in the skin. Microbiome 6, 20 (2018).
Ansari, I. et al. The microbiota programs DNA methylation to control intestinal homeostasis and inflammation. Nat. Microbiol. 5, 610–619 (2020).
Krautkramer, K. A. et al. Diet–microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol. Cell 64, 982–992 (2016).
Kuang, Z. et al. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 365, 1428–1434 (2019).
Ryan, F. J. et al. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat. Commun. 11, 1512 (2020).
Nshanian, M. et al. Short-chain fatty acid metabolites propionate and butyrate are unique epigenetic regulatory elements linking diet, metabolism and gene expression. Nat. Metab. 7, 196–211 (2025).
Muehlbauer, A. L. et al. Interspecies variation in hominid gut microbiota controls host gene regulation. Cell Rep. 37, 110057 (2021).
Qin, Y. et al. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 19, 7 (2018).
Drayman, N., Patel, P., Vistain, L. & Tay, S. HSV-1 single-cell analysis reveals the activation of anti-viral and developmental programs in distinct sub-populations. eLife 8, e46339 (2019).
Lötstedt, B., Stražar, M., Xavier, R., Regev, A. & Vickovic, S. Spatial host–microbiome sequencing reveals niches in the mouse gut. Nat. Biotechnol. 42, 1394–1403 (2024). Together with Galeano Niño et al. (2022), this notable paper describes spatial profiling of host–microbiome interactions.
Massaquoi, M. S. et al. Cell-type-specific responses to the microbiota across all tissues of the larval zebrafish. Cell Rep. 42, 112095 (2023).
Willms, R. J., Jones, L. O., Hocking, J. C. & Foley, E. A cell atlas of microbe-responsive processes in the zebrafish intestine. Cell Rep. 38, 110311 (2022).
Puschhof, J. et al. Intestinal organoid cocultures with microbes. Nat. Protoc. 16, 4633–4649 (2021).
Williamson, I. A. et al. A high-throughput organoid microinjection platform to study gastrointestinal microbiota and luminal physiology. Cell. Mol. Gastroenterol. Hepatol. 6, 301–319 (2018).
Sanna, S., Kurilshikov, A., van der Graaf, A., Fu, J. & Zhernakova, A. Challenges and future directions for studying effects of host genetics on the gut microbiome. Nat. Genet. 54, 100–106 (2022).
Xu, L., Paterson, A. D., Turpin, W. & Xu, W. Assessment and selection of competing models for zero-inflated microbiome data. PLoS One 10, e0129606 (2015).
Fisher, C. K. & Mehta, P. Identifying keystone species in the human gut microbiome from metagenomic timeseries using sparse linear regression. PLoS One 9, e102451 (2014).
Weissbrod, O., Rothschild, D., Barkan, E. & Segal, E. Host genetics and microbiome associations through the lens of genome wide association studies. Curr. Opin. Microbiol. 44, 9–19 (2018).
Sham, P. C. & Purcell, S. M. Statistical power and significance testing in large-scale genetic studies. Nat. Rev. Genet. 15, 335–346 (2014).
Chetty, A. & Blekhman, R. Multi-omic approaches for host–microbiome data integration. Gut Microbes 16, 2297860 (2024).
Lynch, J. et al. HOMINID: a framework for identifying associations between host genetic variation and microbiome composition. Gigascience 6, 1–7 (2017).
Mills, R. H. et al. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat. Microbiol. 7, 262–276 (2022).
Poretsky, R., Rodriguez-R, L. M., Luo, C., Tsementzi, D. & Konstantinidis, K. T. Strengths and limitations of 16S rRNA gene amplicon sequencing in revealing temporal microbial community dynamics. PLoS One 9, e93827 (2014).
Durazzi, F. et al. Comparison between 16S rRNA and shotgun sequencing data for the taxonomic characterization of the gut microbiota. Sci. Rep. 11, 3030 (2021).
Sharafutdinov, I. et al. A single-nucleotide polymorphism in Helicobacter pylori promotes gastric cancer development. Cell Host Microbe 31, 1345–1358.e6 (2023).
Berthenet, E. et al. A GWAS on Helicobacter pylori strains points to genetic variants associated with gastric cancer risk. BMC Biol. 16, 84 (2018).
Sczyrba, A. et al. Critical assessment of metagenome interpretation—a benchmark of metagenomics software. Nat. Methods 14, 1063–1071 (2017).
Meisel, J. S. et al. Skin microbiome surveys are strongly influenced by experimental design. J. Invest. Dermatol. 136, 947–956 (2016).
Deissová, T. et al. 16S rRNA gene primer choice impacts off-target amplification in human gastrointestinal tract biopsies and microbiome profiling. Sci. Rep. 13, 12577 (2023).
Elie, C. et al. Comparison of DNA extraction methods for 16S rRNA gene sequencing in the analysis of the human gut microbiome. Sci. Rep. 13, 10279 (2023).
Allaband, C. et al. Time of sample collection is critical for the replicability of microbiome analyses. Nat. Metab. 6, 1282–1293 (2024).
Nishijima, S. et al. Fecal microbial load is a major determinant of gut microbiome variation and a confounder for disease associations. Cell 188, 222–236.e15 (2025).
Pallister, T. et al. Food preference patterns in a UK twin cohort. Twin Res. Hum. Genet. 18, 793–805 (2015).
Fildes, A. et al. Nature and nurture in children’s food preferences. Am. J. Clin. Nutr. 99, 911–917 (2014).
Goodrich, J. K., Davenport, E. R., Waters, J. L., Clark, A. G. & Ley, R. E. Cross-species comparisons of host genetic associations with the microbiome. Science 352, 532–535 (2016).
Corrêa, R. O. et al. Inulin diet uncovers complex diet–microbiota–immune cell interactions remodeling the gut epithelium. Microbiome 11, 90 (2023).
Shinn, L. M. et al. Fecal metagenomics to identify biomarkers of food intake in healthy adults: findings from randomized, controlled, nutrition trials. J. Nutr. 154, 271–283 (2024).
Diener, C. et al. Metagenomic estimation of dietary intake from human stool. Nat. Metab. 7, 617–630 (2025).
Zuppinger, C. et al. Performance of the digital dietary assessment tool MyFoodRepo. Nutrients 14, 635 (2022).
Abdill, R. J., Adamowicz, E. M. & Blekhman, R. Public human microbiome data are dominated by highly developed countries. PLoS Biol. 20, e3001536 (2022).
Sirugo, G., Williams, S. M. & Tishkoff, S. A. The missing diversity in human genetic studies. Cell 177, 1080 (2019).
McCarthy, M. I. et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet. 9, 356–369 (2008).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Yang, J. et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 43, 519–525 (2011).
Boyle, E. A., Li, Y. I. & Pritchard, J. K. An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177–1186 (2017).
Xu, F. et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome 8, 145 (2020).
Lloyd-Price, J. et al. Strains, functions and dynamics in the expanded human microbiome project. Nature 550, 61–66 (2017).
Weiss, S. et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 5, 27 (2017).
Luca, F., Kupfer, S. S., Knights, D., Khoruts, A. & Blekhman, R. Functional genomics of host–microbiome interactions in humans. Trends Genet. 34, 30–40 (2018).
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012).
Gloor, G. B., Macklaim, J. M., Pawlowsky-Glahn, V. & Egozcue, J. J. Microbiome datasets are compositional: and this is not optional. Front. Microbiol. 8, 2224 (2017).
Franzosa, E. A. et al. Species-level functional profiling of metagenomes and metatranscriptomes. Nat. Methods 15, 962–968 (2018).
Acknowledgements
The authors thank T. Vilgalys, R. Abdill and members of the Blekhman laboratory for their valuable feedback. R.B. acknowledges funding from National Institutes of Health (NIH) grants R35-GM128716 and R01-HD109830. K.J. acknowledges funding from NIH grants K99-HD113834 and R01-HD109830. S.P. acknowledges funding from the Duchossois Family Institute Fellowship (Chicago Fellows programme) at the University of Chicago.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Peer review
Peer review information
Nature Reviews Genetics thanks Andre Franke, John Rawls and Rafael Valdes-Mas for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- 16S rRNA gene amplicon sequencing
-
A targeted sequencing approach that amplifies and sequences regions of the 16S ribosomal RNA gene, which is present in the genomes of all bacteria. This method enables taxonomic profiling of microbial communities without sequencing entire genomes, allowing for a cost-effective way to characterize the taxonomic composition of microbiome samples.
- Alpha diversity
-
A measure of microbial community complexity within a single sample, quantifying the number of different species and their relative abundances. Alpha-diversity metrics can be used to assess how factors such as genetics, diet and disease affect the ecological structure of microbial communities.
- Beta diversity
-
A measure of the difference in microbial community composition between two or more samples. Beta-diversity metrics quantify how samples differ from each other in terms of which microorganisms are present and their relative abundances, allowing the quantification of microbiome variation across conditions, environments and host factors.
- Heritability
-
The proportion of phenotypic variation in a population that can be attributed to genetic differences; heritability is expressed as a value between 0 and 1, where 0 indicates that none of the observed variation is due to genetic factors and 1 indicates that all variation is genetic.
- Quantitative trait locus
-
A specific region of DNA that contributes to variation in a measurable characteristic or quantitative trait, such as height, blood pressure or disease susceptibility. In microbiome research, quantitative trait locus mapping identifies host genetic regions that influence microbial community features, revealing how host genetic variation shapes microbiome composition.
- Shotgun metagenomic sequencing
-
A comprehensive sequencing technique that sequences all DNA found in a microbiome sample, which enables the capture of genomic information from bacteria, archaea, viruses and fungi found in a microbial community. Unlike 16S rRNA amplicon sequencing, shotgun metagenomic sequencing provides information on not only the taxonomic composition but also the functional potential, gene composition and genetic variation within microbial communities.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ferretti, P., Johnson, K., Priya, S. et al. Genomics of host–microbiome interactions in humans. Nat Rev Genet (2025). https://doi.org/10.1038/s41576-025-00849-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41576-025-00849-8