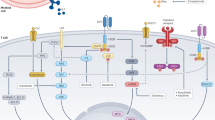
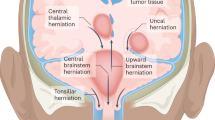
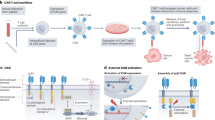
Abstract
A paradigm shift has recently occurred in the field of cancer therapeutics. Traditional anticancer agents, such as chemotherapy, radiotherapy and small-molecule drugs targeting specific signalling pathways, have been joined by cellular immunotherapies based on T cell engineering. The rapid adoption of novel, patient-specific cellular therapies builds on scientific developments in tumour immunology, genetic engineering and cell manufacturing, best illustrated by the curative potential of chimeric antigen receptor (CAR) T cell therapy targeting CD19-expressing malignancies. However, the clinical benefit observed in many patients may come at a cost. In up to one-third of patients, significant toxicities occur that are directly associated with the induction of powerful immune effector responses. The most frequently observed immune-mediated toxicities are cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome. This Review discusses our current understanding of their pathophysiology and clinical features, as well as the development of novel therapeutics for their prevention and/or management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra38 (2013).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013). Together with Brentjens et al. (2013), this article reports early clinical data demonstrating the efficacy of CD19CAR T cells in patients with refractory acute lymphoblastic leukaemia.
Maude, S. L. et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 371, 1507–1517 (2014).
Kochenderfer, J. N. et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 33, 540–549 (2015).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014).
Maher, J. et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002). This article is the first demonstration that fusion receptors containing both T cell receptor and CD28 signalling moieties could redirect and amplify human T cell responses.
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 18, 676–684 (2004).
Lee, D. W. et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124, 188–195 (2014).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Brudno, J. N. & Kochenderfer, J. N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127, 3321–3330 (2016).
Hay, K. A. et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 130, 2295–2306 (2017).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47–62 (2018). This review discusses clinical aspects of CAR T cell-mediated toxicities.
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Gust, J. et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Santomasso, B. D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8, 958–971 (2018).
Locke, F. L., Go, W. Y. & Neelapu, S. S. Development and use of the anti-CD19 chimeric antigen receptor T-cell therapy axicabtagene ciloleucel in large B-cell lymphoma: a review. JAMA Oncol. 6, 281–290 (2019).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 9, 279–286 (2003).
Kowolik, C. M. et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 66, 10995–11004 (2006).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Pennisi, M. et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 4, 676–686 (2020).
Le, R. Q. et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23, 943–947 (2018).
van der Stegen, S. J. C. et al. Preclinical in vivo modeling of cytokine release syndrome induced by ErbB-retargeted human T cells: identifying a window of therapeutic opportunity? J. Immunol. 191, 4589–4598 (2013).
Teachey, D. T. et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 6, 664–679 (2016).
Zhao, Z. et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428 (2015).
Oluwole, O. O. & Davila, M. L. At the bedside: clinical review of chimeric antigen receptor (CAR) T cell therapy for B cell malignancies. J. Leukoc. Biol. 100, 1265–1272 (2016).
Shimabukuro-Vornhagen, A. et al. Cytokine release syndrome. J. Immunother. Cancer 6, 56 (2018).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018). This study reports the role of recipient macrophage-derived IL-1, IL-6 and NO in the pathogenesis of CRS in a mouse model.
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Shah, N. N. et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J. Clin. Oncol. 38, 1938–1950 (2020).
Baird, J. H. et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR-refractory large B-cell lymphoma. Blood https://doi.org/10.1182/blood.2020009432 (2020).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018). This study in humanized mice identifies the roles of monocyte-derived IL-1 and IL-6 as key cytokines mediating CRS and ICANS.
Nijmeijer, B. A., Willemze, R. & Falkenburg, J. H. F. An animal model for human cellular immunotherapy: specific eradication of human acute lymphoblastic leukemia by cytotoxic T lymphocytes in NOD/SCID mice. Blood 100, 654–660 (2002).
Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459 (2018).
Nashleanas, M. & Scott, P. Activated T cells induce macrophages to produce NO and control Leishmania major in the absence of tumor necrosis factor receptor p55. Infect. Immun. 68, 1428–1434 (2000).
Orabona, C. et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat. Immunol. 5, 1134–1142 (2004). This mouse study describes the crucial role of CD80- and CD86-mediated co-stimulation in T cell activation.
Kuhn, N. F. et al. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell 35, 473–488 (2019).
Anderson, M. E. & Siahaan, T. J. Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides 24, 487–501 (2003).
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Tanaka, T. et al. IL-6 in inflammation, immunity and disease. Cold Spring Harb. Perspect. Biol. 6, a016295 (2014).
Mishra, D. et al. Parabrachial interleukin-6 reduces body weight and food intake and increases thermogenesis to regulate energy metabolism. Cell Rep. 26, 3011–3026 (2019).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Ann. Rev. Immunol. 27, 519–550 (2009).
Ricciotti, E. & FitzGerald, G. A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31, 986–1000 (2011).
Garlanda, C., Dinarello, C. A. & Mantovani, A. The interleukin-1 family: back to the future. Immunity 39, 1003–1018 (2013).
Dinarello, C. A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 (2011).
Liu, Y. et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 5, eaax7969 (2020).
Sachdeva, M., Duchateau, P., Depil, S., Poirot, L. & Valton, J. Granulocyte–macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J. Biol. Chem. 294, 5430–5437 (2019).
Manz, M. G. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity 26, 537–541 (2007).
Sterner, R. M. et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 133, 697–709 (2019).
Pennica, D. et al. Human tumor necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature 312, 724–729 (1984).
Li, J. et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci. Transl. Med. 11, eaax8861 (2019).
Lin, C. F. et al. IFN-γ synergizes with LPS to induce nitric oxide biosynthesis through glycogen synthase kinase-3-inhibited IL-10. J. Cell. Biochem. 105, 746–755 (2008).
Tötemeyer, S. et al. IFN-γ enhances production of nitric oxide from macrophages via a mechanism that depends on nucleotide oligomerization domain-2. J. Immunol. 176, 4804–4810 (2006).
Capaldo, C. T. & Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta. 1788, 864–871 (2009).
Rahman, M. T. et al. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood–brain and small intestinal epithelial barriers: relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 507, 274–279 (2018).
Chai, Q., He, W. Q., Zhou, M., Lu, H. & Fu, Z. F. Enhancement of blood–brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J. Virol. 88, 4698–4710 (2014).
Lopez-Ramirez, M. A. et al. Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J. Immunol. 189, 3130–3139 (2012).
Volmar, A. M. The role of atrial natriuretic peptide in the immune system. Peptides 26, 1086–1094 (2005).
Staedtke, V. et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature 564, 273–277 (2018).
Davila, M. L., Kloss, C. C., Gunset, G. & Sadelain, M. CD19 CAR-targeted T cells induce long-term remission and B cell aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS ONE 8, e61338 (2013).
Bondanza, A. et al. Suicide gene therapy of graft versus-host disease induced by central memory human T lymphocytes. Blood 107, 1828–1836 (2006).
Mastaglio, S. et al. NY-ESO-1 TCR single edited stem and central memory T cells to treat multiple myeloma without graft-versus host disease. Blood 130, 606–618 (2017).
Siegler, E. L. & Wang, P. Preclinical models in chimeric antigen receptor engineered T-cell therapy. Hum. Gene Ther. 29, 534–546 (2018).
Locke, F. L. et al. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-hodgkin lymphoma (NHL). Blood 130, 1547 (2017).
Dyson, A. & Singer, M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit. Care Med. 37, S30–S37 (2009).
Page, A. V. & Liles, W. C. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 4, 507–516 (2013).
Schwameis, M., Schörgenhofer, C., Assinger, A., Steiner, M. M. & Jilma, B. VWF excess and ADAMTS13 deficiency: a unifying pathomechanism linking inflammation to thrombosis in DIC, malaria, and TTP. Thromb. Haemost. 113, 708–718 (2015).
Gust, J. et al. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann. Neurol. 86, 42–54 (2019).
Kim, H. J., Tsao, J. W. & Stanfill, A. G. The current state of biomarkers of mild traumatic brain injury. JCI Insight 3, e97105 (2018).
Sofroniew, M. V. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20, 160–172 (2014).
Schuster, S. J. et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377, 2545–2554 (2017).
Gutierrez, E. G., Banks, W. A. & Kastin, A. J. Blood-borne interleukin-1 receptor antagonist crosses the blood–brain barrier. J. Neuroimmunol. 55, 153–160 (1994).
Galea, J. et al. Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J. Cereb. Blood Flow. Metab. 31, 439–447 (2011).
Parker, K. R. et al. Single-cell analyses identify brain mural cells expressing CD19 as potential off-tumor targets for CAR-T immunotherapies. Cell 183, 126–142.e17 (2020).
Dorovini-Zis, K. et al. The neuropathology of fatal cerebral malaria in Malawian children. Am. J. Pathol. 178, 2146–2158 (2011).
Olah, M. et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 11, 6129 (2020).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Reu, P. et al. The lifespan and turnover of microglia in the human brain. Cell Rep. 20, 779–784 (2017).
Pluvinage, J. V. et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 568, 187–192 (2019).
DeAngelo, D. J. et al. Clinical outcomes for the phase 2, single-arm, multicenter trial of JCAR015 in adult B-ALL (ROCKET Study) [abstract P217]. SITC 2017 Annual Meeting Abstracts Book https://higherlogicdownload.s3.amazonaws.com/SITCANCER/3bcb5ebf-803a-42fe-83b6-0773bc4eb962/UploadedImages/Annual%20Meeting%202017/SITC_2017_Abstract_Book.pdf (2017).
Mahadeo, K. M. et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat. Rev. Clin. Oncol. 16, 45–63 (2019).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 25, 625–638 (2019).
Maus, M. V. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J. Immunother. Cancer 8, e001511 (2020).
Nishimoto, N. et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 112, 3959–3964 (2008).
Nastoupil, L. J. et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T consortium. J. Clin. Oncol. 38, 3119–3128 (2020).
Strati, P. et al. Prognostic impact of dose, duration, and timing of corticosteroid therapy in patients with large B-cell lymphoma treated with standard of care axicabtagene ciloleucel (Axi-cel). J. Clin. Oncol. 38, 8011 (2020).
Topp, M. V. et al. Earlier steroid use with axicabtagene ciloleucel (Axi-Cel) in patients with relapsed/refractory large B cell lymphoma. Blood 134, 243 (2019).
Siddiqi, T. et al. Patient characteristics and pre-infusion biomarkers of inflammation correlate with clinical outcomes after treatment with the defined composition, CD19-targeted CAR T cell product, JCAR017. Blood 130, 193 (2017).
Jacobson, C. A. et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J. Clin. Oncol. 38, 3095–3106 (2020).
Raje, N. et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N. Engl. J. Med. 380, 1726–1737 (2019).
Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 7, 303ra139 (2015).
Turtle, C. J. et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J. Clin. Oncol. 35, 3010–3020 (2017).
Hirayama, A. V. et al. The response to lymphodepletion impacts PFS in patients with aggressive non-Hodgkin lymphoma treated with CD19 CAR T cells. Blood 133, 1876–1887 (2019).
Kochenderfer, J. N. et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 35, 1803–1813 (2017).
Neelapu, S. S. CAR-T efficacy: is conditioning the key? Blood 133, 1799–1800 (2019).
Neelapu, S. S. et al. Outcomes of older patients in ZUMA-1, a pivotal study of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 135, 2106–2109 (2020).
Ying, Z. et al. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 25, 947–953 (2019). This study reports a novel CD19CAR construct with 4-1BB and CD3ζ co-stimulatory domains that in clinical practice conferred protection against B cell lymphomas without increasing serum cytokine levels or inducing CRS.
Ghorashian, S. et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 25, 1408–1414 (2019). This study reports a modified CD19CAR construct with lower binding affinity, resulting in both reduced CRS and increased expansion of CAR T cell populations in vivo.
Locke, F. L. et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicenter, phase 1-2 trial. Lancet Oncol. 20, 31–42 (2019).
Brudno, J. N. et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat. Med. 26, 270–280 (2020).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020).
Feucht, J. et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 25, 82–88 (2019). This study shows that altering the activation potential of a CAR construct can influence T cell differentiation and the balance between effector and memory T cell phenotypes.
Acharya, U. H. et al. Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy. Exp. Rev. Hematol. 12, 195–205 (2019).
Huarte, E. et al. Itacitinib (INCB039110), a JAK1 inhibitor, reduces cytokines associated with cytokine release syndrome induced by CAR T-cell therapy. Clin. Cancer Res. 26, 6299–6309 (2020).
Dubovsky, J. A. et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a TH1-selective pressure in T lymphocytes. Blood 122, 2539–2549 (2013).
Ruella, M. et al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia 31, 246–248 (2017).
Mestermann, K. et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 11, eaau5907 (2019).
Sun, C. et al. THEMIS-SHP1 recruitment by 4-1BB tunes LCK-mediated priming of chimeric antigen receptor-redirected T cells. Cancer Cell 37, 216–225.e6 (2020).
Strati, P. et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 4, 3123–3127 (2020).
Henter, J. I. et al. Emapalumab in primary hemophagocytic lymphohistiocytosis. N. Engl. J. Med. 383, 596–598 (2020).
Boardman, D. A. et al. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transplant. 17, 931–943 (2017).
Boroughs, A. C. et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight 5, e126194 (2019).
Dawson, N. A. J. et al. Functional effects of chimeric antigen receptor co-receptor signaling domains in human regulatory T cells. Sci. Transl. Med. 12, eaaz3866 (2020).
Rosado-Sánchez, I. & Levings, M. K. Building a CAR-Treg: going from the basic to the luxury model. Cell. Immunol. 358, 104220 (2020).
Jatiani, S. S. et al. Myeloma CAR-T CRS management with IL-1R antagonist anakinra. Clin. Lymphoma Myeloma Leuk. 20, 632–636.e1 (2020).
Voskoboinik, I. et al. Perforin and granzymes: function, dysfunction and human pathology. Nat. Rev. Immunol. 15, 388–400 (2015).
Grom, A. A., Horne, A. & De Benedetti, F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 12, 259–268 (2016).
Gadoury-Levesque, V. et al. Frequency and spectrum of disease-causing variants in 1892 patients with suspected genetic HLH disorders. Blood Adv. 4, 2578–2594 (2020).
Teachey, D. T. et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit ‘ALL’. Nat. Rev. Clin. Oncol. 15, 218 (2018).
Teachey, D. T. et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 121, 5154–5157 (2013).
Ishii, K. et al. Perforin-deficient CAR T cells recapitulate late-onset inflammatory toxicities observed in patients. J. Clin. Invest. 130, 5425–5443 (2020).
Sumegi, J. et al. Gene expression profiling of peripheral blood mononuclear cells from children with active hemophagocytic lymphohistiocytosis. Blood 117, e151–e160 (2011).
Canna, S. W. et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 46, 1140–1146 (2014).
Moore, J. B. & June, C. H. Cytokine release syndrome in severe COVID-19. Science 368, 473–474 (2020).
Ruan, Q., Yang, K., Wang, W., Jiang, L. & Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 46, 846–848 (2020).
Chen, G. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130, 2620–2629 (2020).
Guaraldi, G. et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2, e474–e484 (2020).
Xu, X. et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl Acad. Sci. USA 117, 10970–10975 (2020).
Campochiaro, C. et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-center retrospective cohort study. Eur. J. Intern. Med. 76, 43–49 (2020).
Salama, C. et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N. Engl. J. Med. 384, 20–30 (2021).
Kewan, T. et al. Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine 24, 100418 (2020).
Huet, T. et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2, e393–e400 (2020).
Cavalli, G. et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2, e325–e331 (2020).
Bozzi, G. et al. Anakinra combined with methylprednisolone in patients with severe COVID-19 pneumonia and hyperinflammation: an observational cohort study. J. Allergy Clin. Immunol. 147, 561–566.e4 (2021).
Cauchois, R. et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc. Natl Acad. Sci. USA 117, 18951–18953 (2020).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583, 127–132 (2020).
Dinarello, C. A. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 29, 317–329 (2010).
Voronov, E. et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl Acad. Sci. USA 100, 2645–2650 (2003).
Horikawa, N. et al. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin. Cancer Res. 23, 587–599 (2017).
Voronov, E., Yaron, C. & Apte, R. N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol. 5, 114 (2014).
Kaplanov, I. et al. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc. Natl Acad. Sci. USA 116, 1361–1369 (2019).
Carey, A. et al. Identification of interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 18, 3204–3218 (2017).
Ridker, P. M. et al. Anti-inflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Litmanovich, A., Khazim, K. & Cohen, I. The role of interleukin-1 in the pathogenesis of cancer and its potential as a therapeutic target in clinical practice. Oncol. Ther. 6, 109–127 (2018).
Acknowledgements
E.C.M. was supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
M.S. and T.G. are listed as inventors in a Memorial Sloan Kettering-held patent application related to treatment of cytokine release syndrome: ‘Methods and compositions for alleviating cytokine release syndrome’ (WO2019099993A1). E.C.M. is scientific co-founder of Quell Therapeutics Ltd, which is developing chimeric antigen receptor (CAR)-modified regulatory T cells, and has served as a consultant to Kite, a Gilead company. S.S.N. served as consultant to Kite, a Gilead Company, Merck, Bristol-Myers Squibb, Novartis, Celgene, Pfizer, Allogene Therapeutics, Cell Medica/Kuur, Incyte, Precision Biosciences, Legend Biotech, Adicet Bio, Calibr and Unum Therapeutics; received research support from Kite, a Gilead Company, Bristol-Myers Squibb, Merck, Poseida, Cellectis, Celgene, Karus Therapeutics, Unum Therapeutics, Allogene Therapeutics, Precision Biosciences and Acerta; received royalties from Takeda Pharmaceuticals; and has intellectual property related to cell therapy.
Additional information
Peer review information
Nature Reviews Immunology thanks A. Bondanza, S. Grupp and C. Jacobson for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Glossary
- Poor-risk malignant disease
-
Subtypes of malignancy (cancer) that have a poor prognosis with conventional therapies.
- Blood–brain barrier
-
(BBB). A network of blood vessels and tissues that form a functional barrier to prevent harmful substances from entering the brain.
- Activation-induced cell death
-
In peripheral T cells, death that is often the result of engagement of cell death pathways (for example, CD95–CD95L) that are upregulated during immune activation.
- CD19+ lymphoma xenograft model
-
A lymphoma model in which immunodeficient mice are engrafted with human lymphoma cells positive for the CD19 surface antigen.
- Humanized NSG mice
-
An immunodeficient mouse strain (NSG) reconstituted with human immune cells; in some iterations of this model, mice are engineered with transgenes encoding human haematopoietic cytokines.
- Acute phase response
-
An early-onset, innate, systemic inflammatory reaction that results from various insults such as infection and tissue injury.
- SCID–beige xenograft model
-
Severe combined immunodeficient (SCID)–beige mice lack mature B cells and T cells and exhibit defective natural killer cell responses, which makes them permissive to engraftment with human tumour tissue.
- Human PBMC xenograft model
-
An immunodeficient mouse strain with an immune system reconstituted with human peripheral blood mononuclear cells (PBMCs) that can be engrafted with human tumour tissue.
- Disseminated intravascular coagulation
-
A rare condition in which abnormal blood clotting occurs throughout the body’s blood vessels in response to various triggers that activate coagulation pathways.
- Conditioning therapy
-
Chemotherapy and/or radiotherapy delivered prior to the infusion of chimeric antigen receptor (CAR) T cells, with the aim of increasing the expansion of the infused T cell population.
Rights and permissions
About this article
Cite this article
Morris, E.C., Neelapu, S.S., Giavridis, T. et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol 22, 85–96 (2022). https://doi.org/10.1038/s41577-021-00547-6
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-021-00547-6
This article is cited by
-
Cytokine storm
Nature Reviews Disease Primers (2026)
-
The dawn of in vivo immune cell engineering in oncology
Nature Biotechnology (2026)
-
Revitalizing T cells: breakthroughs and challenges in overcoming T cell exhaustion
Signal Transduction and Targeted Therapy (2026)
-
Obstacles and improvement strategies for CAR-T cell therapy in solid tumors
Discover Oncology (2026)
-
Quantitative systems pharmacology models: unleashing their potential in cancer immunotherapy
Journal of Pharmaceutical Investigation (2026)