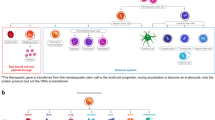
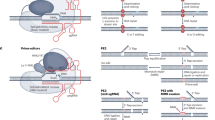
Abstract
Cell and gene therapies using haematopoietic stem cells (HSCs) epitomize the transformative potential of regenerative medicine. Recent clinical successes for gene therapies involving autologous HSC transplantation (HSCT) demonstrate the potential of genetic engineering in this stem cell type for curing disease. With recent advances in CRISPR gene-editing technologies, methodologies for the ex vivo expansion of HSCs and non-genotoxic conditioning protocols, the range of clinical indications for HSC-based gene therapies is expected to significantly expand. However, substantial immunological challenges need to be overcome. These include pre-existing immunity to gene-therapy reagents, immune responses to neoantigens introduced into HSCs by genetic engineering, and unique challenges associated with next-generation and off-the-shelf HSC products. By synthesizing these factors in this Review, we hope to encourage more research to address the immunological issues associated with current and next-generation HSC-based gene therapies to help realize the full potential of this field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wilkinson, A. C., Igarashi, K. J. & Nakauchi, H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet. 21, 541–554 (2020).
Eaves, C. J. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 125, 2605–2613 (2015).
Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Thomas, E. D., Lochte, H. L. Jr., Lu, W. C. & Ferrebee, J. W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 257, 491–496 (1957).
Wahlstrom, J. T., Dvorak, C. C. & Cowan, M. J. Hematopoietic stem cell transplantation for severe combined immunodeficiency. Curr. Pediatr. Rep. 3, 1–10 (2015).
Tiercy, J. M. How to select the best available related or unrelated donor of hematopoietic stem cells? Haematologica 101, 680–687 (2016).
Granot, N. & Storb, R. History of hematopoietic cell transplantation: challenges and progress. Haematologica 105, 2716–2729 (2020).
Gragert, L. et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N. Engl. J. Med. 371, 339–348 (2014).
Hill, G. R., Betts, B. C., Tkachev, V., Kean, L. S. & Blazar, B. R. Current concepts and advances in graft-versus-host disease immunology. Annu. Rev. Immunol. 39, 19–49 (2021).
Morgan, R. A., Gray, D., Lomova, A. & Kohn, D. B. Hematopoietic stem cell gene therapy: progress and lessons learned. Cell Stem Cell 21, 574–590 (2017).
Frangoul, H. et al. CRISPR–Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 384, 252–260 (2021). This work is the first clinical application of the CRISPR–Cas9 system for ex vivo HSC gene therapy.
Thompson, A. A. et al. Gene therapy in patients with transfusion-dependent β-thalassemia. N. Engl. J. Med. 378, 1479–1493 (2018). This paper reports clinical trial results from lentiviral-based gene therapy for β-thalassaemia.
Boztug, K. et al. Stem-cell gene therapy for the Wiskott–Aldrich syndrome. N. Engl. J. Med. 363, 1918–1927 (2010).
Aiuti, A. et al. Correction of ADA–SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 296, 2410–2413 (2002).
Czechowicz, A. et al. Selective hematopoietic stem cell ablation using CD117-antibody-drug-conjugates enables safe and effective transplantation with immunity preservation. Nat. Commun. 10, 617 (2019).
Li, C. et al. In vivo HSC gene therapy using a bi-modular HDAd5/35++ vector cures sickle cell disease in a mouse model. Mol. Ther. 29, 822–837 (2021).
Burns, S. In vivo gene editing of hematopoietic stem and progenitor cells. Intellia https://3o5c4w3neipl16yvhj3nfqam-wpengine.netdna-ssl.com/wp-content/uploads/Keystone_2021_BoneMarrow_10Mar2021-1.pdf (2021). This preclinical data release from Intellia demonstrates in vivo editing of human HSCs in xenografted mice.
Wilkinson, A. C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. Nature 571, 117–121 (2019). This paper is the first to show robust (>100-fold) expansion of HSCs ex vivo.
Piras, F. & Kajaste-Rudnitski, A. Antiviral immunity and nucleic acid sensing in haematopoietic stem cell gene engineering. Gene Ther. 28, 16–28 (2021).
Dudek, A. M. & Porteus, M. H. Answered and unanswered questions in early-stage viral vector transduction biology and innate primary cell toxicity for ex-vivo gene editing. Front. Immunol. 12, 660302 (2021).
Rasaiyaah, J. et al. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature 503, 402–405 (2013).
Chiu, Y. L. et al. Tat stimulates cotranscriptional capping of HIV mRNA. Mol. Cell 10, 585–597 (2002).
Piras, F. et al. Lentiviral vectors escape innate sensing but trigger p53 in human hematopoietic stem and progenitor cells. EMBO Mol. Med. 9, 1198–1211 (2017).
Wu, X. et al. Intrinsic immunity shapes viral resistance of stem cells. Cell 172, 423–438.e25 (2018).
Petrillo, C. et al. Cyclosporine H overcomes innate immune restrictions to improve lentiviral transduction and gene editing in human hematopoietic stem cells. Cell Stem Cell 23, 820–832.e9 (2018).
Vaidyanathan, S. et al. Uridine depletion and chemical modification increase Cas9 mRNA activity and reduce immunogenicity without HPLC purification. Mol. Ther. Nucleic Acids 12, 530–542 (2018).
Dowdy, S. F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 35, 222–229 (2017).
Kariko, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Schiroli, G. et al. Preclinical modeling highlights the therapeutic potential of hematopoietic stem cell gene editing for correction of SCID-X1. Sci. Transl. Med. 9, eaan0820 (2017).
Wienert, B., Shin, J., Zelin, E., Pestal, K. & Corn, J. E. In vitro-transcribed guide RNAs trigger an innate immune response via the RIG-I pathway. PLoS Biol. 16, e2005840 (2018).
Hendel, A. et al. Chemically modified guide RNAs enhance CRISPR–Cas genome editing in human primary cells. Nat. Biotechnol. 33, 985–989 (2015). This paper describes the development of chemically modified guides that allow for the first demonstration of CRISPR–Cas9 gene editing in human HSCs.
Zeng, J. et al. Therapeutic base editing of human hematopoietic stem cells. Nat. Med. 26, 535–541 (2020).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019). This paper describes the first demonstration of prime editing, a flexible platform for making gene edits without the use of a double-strand break.
Liao, W., Du, C. & Wang, J. The cGAS–STING pathway in hematopoiesis and its physiopathological significance. Front. Immunol. 11, 573915 (2020).
Dever, D. P. et al. CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature 539, 384–389 (2016).
Genovese, P. et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 510, 235–240 (2014). This work is the first demonstration of gene editing mediated by homology directed repair in HSCs; innate immune signalling of HSCs was overcome using integration-deficient lentiviral vectors.
Lombardo, A. et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 25, 1298–1306 (2007).
Schiroli, G. et al. Precise gene editing preserves hematopoietic stem cell function following transient p53-mediated DNA damage response. Cell Stem Cell 24, 551–565.e8 (2019).
Hosel, M. et al. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology 55, 287–297 (2012).
Zhu, J., Huang, X. & Yang, Y. The TLR9–MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 119, 2388–2398 (2009).
DeWitt, M. A. et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci. Transl. Med. 8, 360ra134 (2016).
Pien, G. C. et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J. Clin. Invest. 119, 1688–1695 (2009).
Li, C. et al. Cytotoxic-T-lymphocyte-mediated elimination of target cells transduced with engineered adeno-associated virus type 2 vector in vivo. J. Virol. 83, 6817–6824 (2009).
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV–factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 (2006). This paper reports adaptive immune responses to gene-therapy reagents as a major barrier to efficacy in clinical trials.
Meadows, A. S., Pineda, R. J., Goodchild, L., Bobo, T. A. & Fu, H. Threshold for pre-existing antibody levels limiting transduction efficiency of systemic rAAV9 gene delivery: relevance for translation. Mol. Ther. Methods Clin. Dev. 13, 453–462 (2019).
Li, A. et al. AAV–CRISPR gene editing is negated by pre-existing immunity to Cas9. Mol. Ther. 28, 1432–1441 (2020).
Long, B. R. et al. The impact of pre-existing immunity on the non-clinical pharmacodynamics of AAV5-based gene therapy. Mol. Ther. Methods Clin. Dev. 13, 440–452 (2019).
Mingozzi, F. & High, K. A. Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu. Rev. Virol. 4, 511–534 (2017).
Mingozzi, F. et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 13, 419–422 (2007).
Wagner, D. L. et al. High prevalence of Streptococcus pyogenes Cas9-reactive T cells within the adult human population. Nat. Med. 25, 242–248 (2019).
Charlesworth, C. T. et al. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 25, 249–254 (2019).
Boutin, S. et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712 (2010).
Shirley, J. L., de Jong, Y. P., Terhorst, C. & Herzog, R. W. Immune responses to viral gene therapy vectors. Mol. Ther. 28, 709–722 (2020).
George, L. A. et al. Long-term follow-up of the first in human intravascular delivery of AAV for gene transfer: AAV2-hFIX16 for severe hemophilia B. Mol. Ther. 28, 2073–2082 (2020).
Chew, W. L. et al. A multifunctional AAV–CRISPR–Cas9 and its host response. Nat. Methods 13, 868–874 (2016).
Rust, B. J. et al. Envelope-specific adaptive immunity following transplantation of hematopoietic stem cells modified with VSV-G lentivirus. Mol. Ther. Methods Clin. Dev. 19, 438–446 (2020).
Milani, M. et al. Genome editing for scalable production of alloantigen-free lentiviral vectors for in vivo gene therapy. EMBO Mol. Med. 9, 1558–1573 (2017).
Calcedo, R. et al. Class I-restricted T-cell responses to a polymorphic peptide in a gene therapy clinical trial for α-1-antitrypsin deficiency. Proc. Natl Acad. Sci. USA 114, 1655–1659 (2017).
Mendell, J. R. et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 363, 1429–1437 (2010).
Riddell, S. R. et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat. Med. 2, 216–223 (1996). This paper reports the first clinical trial in which gene-modified cells are cleared from the body owing to an adaptive immune response against the introduced transgene.
Uchida, N. et al. Busulfan combined with immunosuppression allows efficient engraftment of gene-modified cells in a rhesus macaque model. Mol. Ther. 27, 1586–1596 (2019).
Uchida, N. et al. Evaluation of engraftment and immunological tolerance after reduced intensity conditioning in a rhesus hematopoietic stem cell gene therapy model. Gene Ther. 21, 148–157 (2014).
Bubnic, S. J., Nagy, A. & Keating, A. Donor hematopoietic cells from transgenic mice that express GFP are immunogenic in immunocompetent recipients. Hematology 10, 289–295 (2005).
Rosenzweig, M. et al. Induction of cytotoxic T lymphocyte and antibody responses to enhanced green fluorescent protein following transplantation of transduced CD34+ hematopoietic cells. Blood 97, 1951–1959 (2001).
Drysdale, C. M., Tisdale, J. F. & Uchida, N. Immunoresponse to gene-modified hematopoietic stem cells. Mol. Ther. Methods Clin. Dev. 16, 42–49 (2020).
Uchida, N. et al. Total body irradiation must be delivered at high dose for efficient engraftment and tolerance in a rhesus stem cell gene therapy model. Mol. Ther. Methods Clin. Dev. 3, 16059 (2016).
Kung, S. K. et al. Induction of transgene-specific immunological tolerance in myeloablated nonhuman primates using lentivirally transduced CD34+ progenitor cells. Mol. Ther. 8, 981–991 (2003).
Manilay, J. O., Pearson, D. A., Sergio, J. J., Swenson, K. G. & Sykes, M. Intrathymic deletion of alloreactive T cells in mixed bone marrow chimeras prepared with a nonmyeloablative conditioning regimen. Transplantation 66, 96–102 (1998).
Styczynski, J. et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transpl. 55, 126–136 (2020).
Ogonek, J. et al. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 7, 507 (2016).
Mikhael, N. L. & Elsorady, M. Clinical significance of T cell receptor excision circle (TREC) quantitation after allogenic HSCT. Blood Res. 54, 274–281 (2019).
Hakim, F. T. et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J. Clin. Invest. 115, 930–939 (2005).
Bhatt, K. H. et al. Short-course rapamycin treatment enables engraftment of immunogenic gene-engineered bone marrow under low-dose irradiation to permit long-term immunological tolerance. Stem Cell. Res. Ther. 8, 57 (2017).
Beagles, K. E., Peterson, L., Zhang, X., Morris, J. & Kiem, H. P. Cyclosporine inhibits the development of green fluorescent protein (GFP)-specific immune responses after transplantation of GFP-expressing hematopoietic repopulating cells in dogs. Hum. Gene Ther. 16, 725–733 (2005).
Tarantal, A. F. et al. Nonmyeloablative conditioning regimen to increase engraftment of gene-modified hematopoietic stem cells in young rhesus monkeys. Mol. Ther. 20, 1033–1045 (2012).
Swart, J. F. et al. Haematopoietic stem cell transplantation for autoimmune diseases. Nat. Rev. Rheumatol. 13, 244–256 (2017).
Sack, B. K., Herzog, R. W., Terhorst, C. & Markusic, D. M. Development of gene transfer for induction of antigen-specific tolerance. Mol. Ther. Methods Clin. Dev. 1, 14013 (2014).
Coleman, M. A. et al. Tolerance induction with gene-modified stem cells and immune-preserving conditioning in primed mice: restricting antigen to differentiated antigen-presenting cells permits efficacy. Blood 121, 1049–1058 (2013).
Furlan, R. A tolerizing mRNA vaccine against autoimmunity? Mol. Ther. 29, 896–897 (2021).
Weischendorff, S. et al. Reduced plasma amino acid levels during allogeneic hematopoietic stem cell transplantation are associated with systemic inflammation and treatment-related complications. Biol. Blood Marrow Transpl. 25, 1432–1440 (2019).
De La Serna, J. et al. Toxicity and efficacy of busulfan and fludarabine myeloablative conditioning for HLA-identical sibling allogeneic hematopoietic cell transplantation in AML and MDS. Bone Marrow Transplant. 51, 961–966 (2016).
Xun, C. Q., Thompson, J. S., Jennings, C. D., Brown, S. A. & Widmer, M. B. Effect of total body irradiation, busulfan-cyclophosphamide, or cyclophosphamide conditioning on inflammatory cytokine release and development of acute and chronic graft-versus-host disease in H-2-incompatible transplanted SCID mice. Blood 83, 2360–2367 (1994).
Squeri, G. et al. Targeting a pre-existing anti-transgene T cell response for effective gene therapy of MPS-I in the mouse model of the disease. Mol. Ther. 27, 1215–1227 (2019).
Cavazzana-Calvo, M. et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 467, 318–322 (2010).
Gouw, S. C. et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood 119, 2922–2934 (2012).
Ponder, K. P. Immune response hinders therapy for lysosomal storage diseases. J. Clin. Invest. 118, 2686–2689 (2008).
Lutzko, C. et al. Genetically corrected autologous stem cells engraft, but host immune responses limit their utility in canine α-l-iduronidase deficiency. Blood 93, 1895–1905 (1999).
Gentner, B. et al. Hematopoietic stem- and progenitor-cell gene therapy for Hurler syndrome. N. Engl. J. Med. 385, 1929–1940 (2021).
Yin, L. et al. CRISPR–Cas13a inhibits HIV-1 infection. Mol. Ther. Nucleic Acids 21, 147–155 (2020).
Xu, L. et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N. Engl. J. Med. 381, 1240–1247 (2019).
Burke, B. P. et al. Engineering cellular resistance to HIV-1 infection in vivo using a dual therapeutic lentiviral vector. Mol. Ther. Nucleic Acids 4, e236 (2015).
Kim, M. Y. et al. Genetic inactivation of CD33 in hematopoietic stem cells to tnable CAR T cell immunotherapy for acute myeloid leukemia. Cell 173, 1439–1453.e19 (2018).
Voss, J. E. et al. Reprogramming the antigen specificity of B cells using genome-editing technologies. eLife 8, e42995 (2019).
Greiner, V. et al. CRISPR-mediated editing of the B cell receptor in primary human B cells. iScience 12, 369–378 (2019).
De Oliveira, S. N. et al. Modification of hematopoietic stem/progenitor cells with CD19-specific chimeric antigen receptors as a novel approach for cancer immunotherapy. Hum. Gene Ther. 24, 824–839 (2013).
Vatakis, D. N. et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc. Natl Acad. Sci. USA 108, E1408–E1416 (2011).
Irving, A. T., Ahn, M., Goh, G., Anderson, D. E. & Wang, L. F. Lessons from the host defences of bats, a unique viral reservoir. Nature 589, 363–370 (2021).
Bai, T. et al. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat. Med. 25, 1566–1575 (2019).
Fares, I. et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 345, 1509–1512 (2014).
Boitano, A. E. et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348 (2010).
Demirci, S., Leonard, A. & Tisdale, J. F. Hematopoietic stem cells from pluripotent stem cells: clinical potential, challenges, and future perspectives. Stem Cell Transl. Med. 9, 1549–1557 (2020).
Sandler, V. M. et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature 511, 312–318 (2014).
Riddell, J. et al. Reprogramming committed murine blood cells to induced hematopoietic stem cells with defined factors. Cell 157, 549–564 (2014). This paper is the first to demonstrate trans-differentiation of cells to HSCs.
Dendrou, C. A., Petersen, J., Rossjohn, J. & Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 18, 325–339 (2018).
Robinson, J., Soormally, A. R., Hayhurst, J. D. & Marsh, S. G. E. The IPD-IMGT/HLA Database — new developments in reporting HLA variation. Hum. Immunol. 77, 233–237 (2016).
Xu, H. et al. Targeted disruption of HLA genes via CRISPR–Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell 24, 566–578.e7 (2019).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Gornalusse, G. G. et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35, 765–772 (2017). This paper reports that hypoimmune cells allow for allogeneic transplantation without rejection or HLA matching.
Deuse, T. et al. Hypoimmune induced pluripotent stem cell-derived cell therapeutics treat cardiovascular and pulmonary diseases in immunocompetent allogeneic mice. Proc. Natl Acad. Sci. USA 118, e2022091118 (2021).
Liu, X. et al. Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining activation of the kinase Btk. Nat. Immunol. 12, 416–424 (2011).
Madsen, L. et al. Mice lacking all conventional MHC class II genes. Proc. Natl Acad. Sci. USA 96, 10338–10343 (1999).
Grusby, M. J. et al. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl Acad. Sci. USA 90, 3913–3917 (1993).
Burger, P., Hilarius-Stokman, P., de Korte, D., van den Berg, T. K. & van Bruggen, R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 119, 5512–5521 (2012).
Noyan, F. et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am. J. Transplant. 17, 917–930 (2017).
Todo, S. et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology 64, 632–643 (2016).
MacDonald, K. G. et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 126, 1413–1424 (2016).
Akbarpour, M. et al. Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci. Transl. Med. 7, 289ra281 (2015).
Cunningham, E. C. et al. Gene therapy for tolerance: high-level expression of donor major histocompatibility complex in the liver overcomes naive and memory alloresponses to skin grafts. Transplantation 95, 70–77 (2013).
Calne, R. Y. et al. Induction of immunological tolerance by porcine liver allografts. Nature 223, 472–476 (1969).
Bernardo, M. E. & Aiuti, A. The role of conditioning in hematopoietic stem-cell gene therapy. Hum. Gene Ther. 27, 741–748 (2016).
Chanut, F. J. A. et al. Conditioning regimens in long-term pre-clinical studies to support development of ex vivo gene therapy: review of nonproliferative and proliferative changes. Hum. Gene Ther. 32, 66–76 (2021).
Agarwal, R. et al. First report of non-genotoxic conditioning with JSP191 (anti-CD117) and hematopoietic stem cell transplantation in a newly diagnosed patient with severe combined immune deficiency. Blood 136, 10–10 (2020).
Myburgh, R. et al. Anti-human CD117 CAR T-cells efficiently eliminate healthy and malignant CD117-expressing hematopoietic cells. Leukemia 34, 2688–2703 (2020).
Chhabra, A. et al. Hematopoietic stem cell transplantation in immunocompetent hosts without radiation or chemotherapy. Sci. Transl. Med. 8, 351ra105 (2016).
George, B. M. et al. Antibody conditioning enables MHC-mismatched hematopoietic stem cell transplants and organ graft tolerance. Cell Stem Cell 25, 185–192.e3 (2019). This paper reports that antibody-mediated conditioning allows for HSCT between completely MHC-mismatched donor and recipient mice.
Taya, Y. et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science 354, 1152–1155 (2016).
Ochi, K., Morita, M., Wilkinson, A. C., Iwama, A. & Yamazaki, S. Non-conditioned bone marrow chimeric mouse generation using culture-based enrichment of hematopoietic stem and progenitor cells. Nat. Commun. 12, 3568 (2021).
Shimoto, M., Sugiyama, T. & Nagasawa, T. Numerous niches for hematopoietic stem cells remain empty during homeostasis. Blood 129, 2124–2131 (2017). This work is the first demonstration of robust HSC engraftment without conditioning in immunocompetent mice.
Rio, P. et al. Successful engraftment of gene-corrected hematopoietic stem cells in non-conditioned patients with Fanconi anemia. Nat. Med. 25, 1396–1401 (2019).
Wilkinson, A. C. et al. Cas9-AAV6 gene correction of β-globin in autologous HSCs improves sickle cell disease erythropoiesis in mice. Nat. Commun. 12, 686 (2021).
Ginn, S. L. et al. Limiting thymic precursor supply increases the risk of lymphoid malignancy in murine X-linked severe combined immunodeficiency. Mol. Ther. Nucleic Acids 6, 1–14 (2017).
Pasi, K. J. et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for hemophilia A. N. Engl. J. Med. 382, 29–40 (2020).
Jacobson, S. G. et al. Improvement and decline in vision with gene therapy in childhood blindness. N. Engl. J. Med. 372, 1920–1926 (2015).
Goldstein, J. M. et al. In situ modification of tissue stem and progenitor cell genomes. Cell Rep. 27, 1254–1264.e7 (2019).
Humbert, O. et al. Rapid immune reconstitution of SCID-X1 canines after G-CSF/AMD3100 mobilization and in vivo gene therapy. Blood Adv. 2, 987–999 (2018).
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021). This work is the first clinical trial to use the CRISPR–Cas9 system in vivo.
Krishnamurthy, S. et al. Engineered amphiphilic peptides enable delivery of proteins and CRISPR-associated nucleases to airway epithelia. Nat. Commun. 10, 4906 (2019).
Staahl, B. T. et al. Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nat. Biotechnol. 35, 431–434 (2017).
[No authors listed.] High-dose AAV gene therapy deaths. Nat. Biotechnol. 38, 910 (2020).
Sibbald, B. Death but one unintended consequence of gene-therapy trial. CMAJ 164, 1612 (2001).
Rothgangl, T. et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 39, 949–957 (2021).
Brown, B. D., Venneri, M. A., Zingale, A., Sergi Sergi, L. & Naldini, L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 12, 585–591 (2006).
Dunleavy, K. With the pricing situation ‘untenable’ in Europe, Bluebird will wind down its operations in the ‘broken’ market. Fierce Pharma https://www.fiercepharma.com/pharma/situation-untenable-bluebird-will-wind-down-its-operations-broken-europe (2021).
Ferrari, G., Thrasher, A. J. & Aiuti, A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 22, 216–234 (2021).
Cavazzana, M., Bushman, F. D., Miccio, A., Andre-Schmutz, I. & Six, E. Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat. Rev. Drug Discov. 18, 447–462 (2019).
Aiuti, A., Roncarolo, M. G. & Naldini, L. Gene therapy for ADA–SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol. Med. 9, 737–740 (2017).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370, 901–910 (2014).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Yla-Herttuala, S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol. Ther. 20, 1831–1832 (2012).
Porteus, M. H. & Baltimore, D. Chimeric nucleases stimulate gene targeting in human cells. Science 300, 763 (2003).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Blaese, R. M. et al. T lymphocyte-directed gene therapy for ADA–SCID: initial trial results after 4 years. Science 270, 475–480 (1995).
Civin, C. I. et al. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J. Immunol. 133, 157–165 (1984).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Gyurkocza, B. & Sandmaier, B. M. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 124, 344–353 (2014).
Acknowledgements
C.T.C. is supported by the National Science Foundation (NSF). A.C.W. acknowledges support from the Medical Research Council (MRC) and the Kay Kendall Leukaemia Fund. H.N. is supported by the National Institutes of Health (NIH) (grants R01DK116944; R01HL147124) and the Virginia and D.K. Ludwig Fund for Cancer Research (D.K. Ludwig Fund).
Author information
Authors and Affiliations
Contributions
All authors contributed to conceiving, writing and editing this Review.
Corresponding authors
Ethics declarations
Competing interests
H.N. is a co-founder and shareholder in Megakaryon, Century Therapeutic and Celaid Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks P. Genovese and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Glossary
- Sickle cell disease
-
A disease caused by a specific point mutation in the haemoglobin-β (HBB) gene, which leads to the formation of haemoglobin tetramers that can polymerize with each other and that cause red blood cells to become highly fragile and adopt a characteristic sickle shape.
- Conditioning regimens
-
Treatments given prior to haematopoietic stem cell transplantation (HSCT) to ablate components of the haematopoietic system in the recipient in order to ensure engraftment of transplanted cells.
- Autoimmune haemolytic anaemia
-
An autoimmune disease whereby patients produce autoantibodies that target red blood cells, causing them to lyse prematurely and thus leading to anaemia.
- cGAS–STING pathway
-
A cytosolic DNA-sensing signalling pathway, in which binding of cGAS to double-stranded DNA (dsDNA) in the cytoplasm leads to the downstream activation of STING and, subsequently, the activation of an inflammatory transcriptional programme in cells.
- Adenosine deaminase deficiency–severe combined immunodeficiency
-
(ADA–SCID). A disease caused by mutations in the adenosine deaminase (ADA) gene. ADA is an essential enzyme in the purine salvage pathway, deficiency of which prevents the maturation of B cells, T cells and natural killer cells.
- β-Thalassaemia
-
A disease caused by mutations in the haemoglobin-β (HBB) gene that prevent functional HBB expression, leading to an inability of red blood cells to form haemoglobin tetramers.
- CRISPR–Cas9
-
A CRISPR–Cas gene-editing platform adapted from bacteria that can be directed to make double-strand breaks at specific sequences of DNA.
- Base editors
-
Gene-editing platforms that allow for the alteration of single nucleotides in the genome without requiring a double-strand break in DNA, through fusion of a catalytically dead DNA endonuclease Cas9 to a deaminase enzyme.
- Prime editing
-
A gene-editing platform that allows for the modification of small sequences (up to ~40 bp) in the genome through fusion of a catalytically dead DNA endonuclease Cas9 to a reverse transcriptase.
- Homology directed repair
-
A DNA repair pathway that corrects double-strand breaks using a homologous DNA sequence. This pathway may be used to change specific sequences in the genome or to introduce transgenes in specific locations in the genome.
- Mucopolysaccharidosis type 1
-
(MPS1). A disease caused by mutations in α-l-iduronidase (IDUA), which lead to a build-up of glycosaminoglycan in lysosomes.
- Small interfering RNAs
-
Small, 20–27 bp, double-stranded RNA molecules that bind endogenous mRNAs, leading to their downregulation through the RNA-induced silencing complex (RISC) pathway.
- CRISPR–Cas13
-
A CRISPR–Cas gene-editing platform adapted from bacteria that can be used to target mRNAs for cleavage, preventing their translation within cells.
- Chimeric antigen receptor T cell
-
(CAR T cell). A T cell that has been genetically modified to express a chimeric receptor that consists of the intracellular portion of the T cell receptor (TCR) fused to an extracellular domain that can bind an antigen of interest, causing the T cell to become activated.
Rights and permissions
About this article
Cite this article
Charlesworth, C.T., Hsu, I., Wilkinson, A.C. et al. Immunological barriers to haematopoietic stem cell gene therapy. Nat Rev Immunol 22, 719–733 (2022). https://doi.org/10.1038/s41577-022-00698-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-022-00698-0
This article is cited by
-
Heparan sulfate binding protein treatment ameliorates neuropathology and behavioral abnormalities in mucopolysaccharidosis IIIB mice
Cell Death Discovery (2025)
-
CRISPR-based therapeutic genome editing for inherited blood disorders
Nature Reviews Drug Discovery (2025)
-
Identification of potential molecular targets of rhaponticin in the treatment of periodontitis using bioinformatics tools
Scientific Reports (2025)
-
Role of Neurotransmitters in Steady State Hematopoiesis, Aging, and Leukemia
Stem Cell Reviews and Reports (2025)
-
Base Editors-Mediated Gene Therapy in Hematopoietic Stem Cells for Hematologic Diseases
Stem Cell Reviews and Reports (2024)