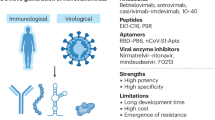
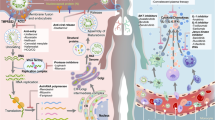
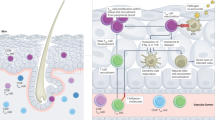
Abstract
The advent of COVID-19 and the persistent threat of infectious diseases such as tuberculosis, malaria, influenza and HIV/AIDS remind us of the marked impact that infections continue to have on public health. Some of the most effective protective measures are vaccines but these have been difficult to develop for some of these infectious diseases even after decades of research. The development of drugs and immunotherapies acting directly against the pathogen can be equally challenging, and such pathogen-directed therapeutics have the potential disadvantage of selecting for resistance. An alternative approach is provided by host-directed therapies, which interfere with host cellular processes required for pathogen survival or replication, or target the host immune response to infection (immunotherapies) to either augment immunity or ameliorate immunopathology. Here, we provide a historical perspective of host-directed immunotherapeutic interventions for viral and bacterial infections and then focus on SARS-CoV-2 and Mycobacterium tuberculosis, two major human pathogens of the current era, to indicate the key lessons learned and discuss candidate immunotherapeutic approaches, with a focus on drugs currently in clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ellis, G. I., Sheppard, N. C. & Riley, J. L. Genetic engineering of T cells for immunotherapy. Nat. Rev. Genet. 22, 427–447 (2021).
Wykes, M. N. & Lewin, S. R. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 18, 91–104 (2018).
Boland, E. W. & Headley, N. E. Effects of cortisone acetate on rheumatoid arthritis. J. Am. Med. Assoc. 141, 301–308 (1949).
Isaacs, A. & Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B. Biol. Sci. 147, 258–267 (1957).
Reichard, O. et al. Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group. Lancet 351, 83–87 (1998).
Heim, M. H. 25 years of interferon-based treatment of chronic hepatitis C: an epoch coming to an end. Nat. Rev. Immunol. 13, 535–542 (2013).
Neesgaard, B., Ruhwald, M. & Weis, N. Inducible protein-10 as a predictive marker of antiviral hepatitis C treatment: a systematic review. World J. Hepatol. 9, 677–688 (2017).
Muir, A. J. et al. A randomized phase 2b study of peginterferon lambda-1a for the treatment of chronic HCV infection. J. Hepatol. 61, 1238–1246 (2014).
Lok, A. S. et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366, 216–224 (2012).
Kaufmann, S. H. E., Dorhoi, A., Hotchkiss, R. S. & Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 17, 35–56 (2018).
Barrat, F. J. & Su, L. A pathogenic role of plasmacytoid dendritic cells in autoimmunity and chronic viral infection. J. Exp. Med. 216, 1974–1985 (2019).
Su, L. Pathogenic role of type i interferons in HIV-induced immune impairments in humanized mice. Curr. HIV/AIDS Rep. 16, 224–229 (2019).
Beilharz, M. W., Cummins, J. M. & Bennett, A. L. Protection from lethal influenza virus challenge by oral type 1 interferon. Biochem. Biophys. Res. Commun. 355, 740–744 (2007).
Davidson, S. et al. IFNlambda is a potent anti-influenza therapeutic without the inflammatory side effects of IFNalpha treatment. EMBO Mol. Med. 8, 1099–1112 (2016).
Davidson, S., Maini, M. K. & Wack, A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J. Interferon Cytokine Res. 35, 252–264 (2015).
Major, J. et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science 369, 712–717 (2020).
Broggi, A. et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 369, 706–712 (2020).
Onwumeh, J., Okwundu, C. I. & Kredo, T. Interleukin-2 as an adjunct to antiretroviral therapy for HIV-positive adults. Cochrane Database Syst. Rev. 5, CD009818 (2017).
Young, C., Walzl, G. & Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 13, 190–204 (2020).
Johnson, B. J. et al. rhuIL-2 adjunctive therapy in multidrug resistant tuberculosis: a comparison of two treatment regimens and placebo. Tuber. Lung Dis. 78, 195–203 (1997).
Johnson, J. L. et al. Randomized trial of adjunctive interleukin-2 in adults with pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 168, 185–191 (2003).
Larson, R. P., Shafiani, S. & Urdahl, K. B. Foxp3+ regulatory T cells in tuberculosis. Adv. Exp. Med. Biol. 783, 165–180 (2013).
Chen, C. Y. et al. IL-2 simultaneously expands Foxp3+ T regulatory and T effector cells and confers resistance to severe tuberculosis (TB): implicative Treg-T effector cooperation in immunity to TB. J. Immunol. 188, 4278–4288 (2012).
Cooper, A. M. Cell-mediated immune responses in tuberculosis. Annu. Rev. Immunol. 27, 393–422 (2009).
Flynn, J. L. & Chan, J. Immunology of tuberculosis. Annu. Rev. Immunol. 19, 93–129 (2001).
Kristensen, I. A., Veirum, J. E., Moller, B. K. & Christiansen, M. Novel STAT1 alleles in a patient with impaired resistance to mycobacteria. J. Clin. Immunol. 31, 265–271 (2011).
Alangari, A. A. et al. Treatment of disseminated mycobacterial infection with high-dose IFN-gamma in a patient with IL-12Rbeta1 deficiency. Clin. Dev. Immunol. 2011, 691956 (2011).
Condos, R., Rom, W. N. & Schluger, N. W. Treatment of multidrug-resistant pulmonary tuberculosis with interferon- gamma via aerosol. Lancet 349, 1513–1515 (1997).
Wallis, R. S. Lack of a therapeutic role for interferon gamma in patients with tuberculosis. J. Infect. Dis. 209, 627–628 (2014).
Dawson, R. et al. Immunomodulation with recombinant interferon-gamma1b in pulmonary tuberculosis. PLoS One 4, e6984 (2009).
Raju, B. et al. Aerosolized gamma interferon (IFN-gamma) induces expression of the genes encoding the IFN-gamma-inducible 10-kilodalton protein but not inducible nitric oxide synthase in the lung during tuberculosis. Infect. Immun. 72, 1275–1283 (2004).
Guirado, E., Schlesinger, L. S. & Kaplan, G. Macrophages in tuberculosis: friend or foe. Semin. Immunopathol. 35, 563–583 (2013).
Tan, B. H. et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J. Immunol. 177, 1864–1871 (2006).
Gopal, R. et al. S100A8/A9 proteins mediate neutrophilic inflammation and lung pathology during tuberculosis. Am. J. Respir. Crit. Care Med. 188, 1137–1146 (2013).
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Dorhoi, A. et al. Type I IFN signaling triggers immunopathology in tuberculosis-susceptible mice by modulating lung phagocyte dynamics. Eur. J. Immunol. 44, 2380–2393 (2014).
Mayer-Barber, K. D. et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511, 99–103 (2014).
Moreira-Teixeira, L., Mayer-Barber, K., Sher, A. & O’Garra, A. Type I interferons in tuberculosis: foe and occasionally friend. J. Exp. Med. 215, 1273–1285 (2018).
Moreira-Teixeira, L. et al. Type I IFN exacerbates disease in tuberculosis-susceptible mice by inducing neutrophil-mediated lung inflammation and NETosis. Nat. Commun. 11, 5566 (2020).
Moreira-Teixeira, L. et al. Mouse transcriptome reveals potential signatures of protection and pathogenesis in human tuberculosis. Nat. Immunol. 21, 464–476 (2020).
Antonelli, L. R. et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J. Clin. Invest. 120, 1674–1682 (2010).
Romanowski, K. et al. Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 19, 1129–1137 (2019).
Willcox, P. A. & Ferguson, A. D. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir. Med. 83, 195–198 (1989).
Youssef, J., Novosad, S. A. & Winthrop, K. L. Infection risk and safety of corticosteroid use. Rheum. Dis. Clin. North. Am. 42, 157–176 (2016).
Barnard, C. Tuberculous meningitis; cortisone treatment as an adjunct to the antibiotics; the effect on the clinical features and the cerebrospinal fluid. S. Afr. Med. J. 27, 219–220 (1953).
Cochran, J. B. Cortisone in the treatment of pulmonary tuberculosis. Edinb. Med. J. 61, 238–249 (1954).
Dooley, D. P., Carpenter, J. L. & Rademacher, S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin. Infect. Dis. 25, 872–887 (1997).
Critchley, J. A., Young, F., Orton, L. & Garner, P. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 13, 223–237 (2013).
Meintjes, G. et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS 24, 2381–2390 (2010).
Meintjes, G. et al. Prednisone for the prevention of paradoxical tuberculosis-associated IRIS. N. Engl. J. Med. 379, 1915–1925 (2018).
Wallis, R. S. Corticosteroid effects on sputum culture in pulmonary tuberculosis: a meta-regression analysis. Open Forum Infect. Dis. 1, ofu020 (2014).
The National Institutes of Health-University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia. Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. N. Engl. J. Med. 323, 1500–1504 (1990).
Fujikura, Y., Manabe, T., Kawana, A. & Kohno, S. Adjunctive corticosteroids for Pneumocystis jirovecii pneumonia in non-HIV-infected patients: a systematic review and meta-analysis of observational studies. Arch. Bronconeumol. 53, 55–61 (2017).
Ewald, H. et al. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane Database Syst. Rev. 2015, CD006150 (2015).
de Jong, M. D. et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12, 1203–1207 (2006).
Hui, D. S., Lee, N., Chan, P. K. & Beigel, J. H. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antivir. Res. 150, 202–216 (2018).
Zhou, Y. et al. Use of corticosteroids in influenza-associated acute respiratory distress syndrome and severe pneumonia: a systemic review and meta-analysis. Sci. Rep. 10, 3044 (2020).
Ni, Y. N., Chen, G., Sun, J., Liang, B. M. & Liang, Z. A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit. Care 23, 99 (2019).
Wong, L. R. & Perlman, S. Immune dysregulation and immunopathology induced by SARS-CoV-2 and related coronaviruses - are we our own worst enemy? Nat. Rev. Immunol. 22, 47–56 (2022).
Diamond, M. S. & Kanneganti, T. D. Innate immunity: the first line of defense against SARS-CoV-2. Nat. Immunol. 23, 165–176 (2022).
Zhang, Q. et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020).
Bastard, P. et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020).
Park, A. & Iwasaki, A. Type I and type III interferons- induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 27, 870–878 (2020).
Laing, A. G. et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 26, 1623–1635 (2020).
Lucas, C. et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469 (2020).
Galani, I. E. et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 22, 32–40 (2021).
Zhou, Z. et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe 27, 883–890.e2 (2020).
Wang, N. et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe 28, 455–464.e2 (2020).
Arabi, Y. M. et al. Interferon beta-1b and Lopinavir-Ritonavir for middle east respiratory syndrome. N. Engl. J. Med. 383, 1645–1656 (2020).
WHO Solidarity Trial Consortium. et al. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N. Engl. J. Med. 384, 497–511 (2021).
Monk, P. D. et al. Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 9, 196–206 (2021).
Hung, I. F. et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet 395, 1695–1704 (2020).
Feld, J. J. et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a phase 2, placebo-controlled randomised trial. Lancet Respir. Med. 9, 498–510 (2021).
Channappanavar, R. et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe 19, 181–193 (2016).
Garcia-Del-Barco, D., Risco-Acevedo, D., Berlanga-Acosta, J., Martos-Benitez, F. D. & Guillen-Nieto, G. Revisiting pleiotropic effects of type I interferons: rationale for its prophylactic and therapeutic use against SARS-CoV-2. Front. Immunol. 12, 655528 (2021).
Prokunina-Olsson, L. et al. COVID-19 and emerging viral infections: the case for interferon lambda. J. Exp. Med. 217, e20200653 (2020).
Davidson, S., Crotta, S., McCabe, T. M. & Wack, A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat. Commun. 5, 3864 (2014).
Angus, D. C. et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA 324, 1317–1329 (2020).
Recovery Collaborative Group. et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384, 693–704 (2021).
Leisman, D. E. et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 8, 1233–1244 (2020).
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Le, R. Q. et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23, 943–947 (2018).
Rosas, I. O. et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N. Engl. J. Med. 384, 1503–1516 (2021).
Recovery Collaborative Group. et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397, 1637–1645 (2021).
Remap-Cap Investigators. et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N. Engl. J. Med. 384, 1491–1502 (2021).
Stone, J. H. et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N. Engl. J. Med. 383, 2333–2344 (2020).
Rubin, E. J., Longo, D. L. & Baden, L. R. Interleukin-6 receptor inhibition in Covid-19-cooling the inflammatory soup. N. Engl. J. Med. 384, 1564–1565 (2021).
Cremer, P. C. et al. Mavrilimumab in patients with severe COVID-19 pneumonia and systemic hyperinflammation (MASH-COVID): an investigator initiated, multicentre, double-blind, randomised, placebo-controlled trial. Lancet Rheumatol. 3, e410–e418 (2021).
De Luca, G. et al. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. Lancet Rheumatol. 2, e465–e473 (2020).
Cavalli, G. et al. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol. 3, e253–e261 (2021).
Cakarova, L. et al. Macrophage tumor necrosis factor-alpha induces epithelial expression of granulocyte-macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am. J. Respir. Crit. Care Med. 180, 521–532 (2009).
Rosler, B. & Herold, S. Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia-a new therapeutic strategy? Mol. Cell. Pediatr. 3, 29 (2016).
Mehta, P., Chambers, R. C. & Dagna, L. Granulocyte-macrophage colony stimulating factor in COVID-19: friend or foe? Lancet Rheumatol. 3, e394–e395 (2021).
Kremer, J. M. et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 60, 1895–1905 (2009).
Kalil, A. C. et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 384, 795–807 (2021).
Guimaraes, P. O. et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N. Engl. J. Med. 385, 406–415 (2021).
Stebbing, J. et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci. Adv. 7, eabe4724 (2021).
Seftel, D. & Boulware, D. R. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect. Dis. 8, ofab050 (2021).
Lenze, E. J. et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 324, 2292–2300 (2020).
Rosen, D. A. et al. Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 11, eaau5266 (2019).
Bonaventura, A. et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 21, 319–329 (2021).
Gusev, E., Sarapultsev, A., Solomatina, L. & Chereshnev, V. SARS-CoV-2-Specific immune response and the pathogenesis of COVID-19. Int. J. Mol. Sci. 23, 1716 (2022).
Acanfora, D. et al. The cross-talk between thrombosis and inflammatory storm in acute and long-COVID-19: therapeutic targets and clinical cases. Viruses 13, 1904 (2021).
Middleton, E. A. et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136, 1169–1179 (2020).
Weber, A. G., Chau, A. S., Egeblad, M., Barnes, B. J. & Janowitz, T. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: a case series. Mol. Med. 26, 91 (2020).
Zumla, A. et al. Towards host-directed therapies for tuberculosis. Nat. Rev. Drug Discov. 14, 511–512 (2015).
Musser, J. M., Amin, A. & Ramaswamy, S. Negligible genetic diversity of mycobacterium tuberculosis host immune system protein targets: evidence of limited selective pressure. Genetics 155, 7–16 (2000).
Ernst, J. D. Antigenic variation and immune escape in the MTBC. Adv. Exp. Med. Biol. 1019, 171–190 (2017).
Drain, P. K. et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin. Microbiol. Rev. 31, e00021-18 (2018).
Ottenhoff, T. H. et al. Genome-wide expression profiling identifies type 1 interferon response pathways in active tuberculosis. PLoS One 7, e45839 (2012).
Scriba, T. J. et al. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. 13, e1006687 (2017).
Singhania, A. et al. A modular transcriptional signature identifies phenotypic heterogeneity of human tuberculosis infection. Nat. Commun. 9, 2308 (2018).
Bloom, C. I. et al. Detectable changes in the blood transcriptome are present after two weeks of antituberculosis therapy. PLoS One 7, e46191 (2012).
Cliff, J. M. et al. Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J. Infect. Dis. 207, 18–29 (2013).
Zhang, X. et al. Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature 517, 89–93 (2015).
Gideon, H. P., Skinner, J. A., Baldwin, N., Flynn, J. L. & Lin, P. L. Early whole blood transcriptional signatures are associated with severity of lung inflammation in cynomolgus macaques with mycobacterium tuberculosis infection. J. Immunol. 197, 4817–4828 (2016).
Bogunovic, D. et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science 337, 1684–1688 (2012).
Ji, D. X. et al. Type I interferon-driven susceptibility to Mycobacterium tuberculosis is mediated by IL-1Ra. Nat. Microbiol. 4, 2128–2135 (2019).
Manca, C. et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc. Natl Acad. Sci. USA 98, 5752–5757 (2001).
Manca, C. et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J. Interferon Cytokine Res. 25, 694–701 (2005).
McNab, F. W. et al. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J. Immunol. 191, 1732–1743 (2013).
Ordway, D. et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179, 522–531 (2007).
Redford, P. S. et al. Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway. J. Infect. Dis. 209, 270–274 (2014).
Stanley, S. A., Johndrow, J. E., Manzanillo, P. & Cox, J. S. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178, 3143–3152 (2007).
Zhang, L., Jiang, X., Pfau, D., Ling, Y. & Nathan, C. F. Type I interferon signaling mediates Mycobacterium tuberculosis-induced macrophage death. J. Exp. Med. 218, e20200887 (2021).
Cooper, A. M., Pearl, J. E., Brooks, J. V., Ehlers, S. & Orme, I. M. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect. Immun. 68, 6879–6882 (2000).
Moreira-Teixeira, L. et al. Type I IFN inhibits alternative macrophage activation during Mycobacterium tuberculosis infection and leads to enhanced protection in the absence of IFN-gamma signaling. J. Immunol. 197, 4714–4726 (2016).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Maiga, M. et al. Risk of tuberculosis reactivation with tofacitinib (CP-690550). J. Infect. Dis. 205, 1705–1708 (2012).
Maiga, M. et al. Efficacy of adjunctive tofacitinib therapy in mouse models of tuberculosis. EBioMedicine 2, 868–873 (2015).
Vilcheze, C. & Jacobs, W. R. Jr The promises and limitations of N-acetylcysteine as a potentiator of first-line and second-line tuberculosis drugs. Antimicrob. Agents Chemother. 65, e01703-20 (2021).
Amaral et al. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp Med. 216, 556–570 (2019).
Mai, N. T. et al. A randomised double blind placebo controlled phase 2 trial of adjunctive aspirin for tuberculous meningitis in HIV-uninfected adults. eLife 7, e33478 (2018).
Misra, U. K., Kalita, J. & Nair, P. P. Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J. Neurol. Sci. 293, 12–17 (2010).
Schoeman, J. F., Janse van Rensburg, A., Laubscher, J. A. & Springer, P. The role of aspirin in childhood tuberculous meningitis. J. Child Neurol. 26, 956–962 (2011).
Skerry, C. et al. Simvastatin increases the in vivo activity of the first-line tuberculosis regimen. J. Antimicrob. Chemother. 69, 2453–2457 (2014).
Dutta, N. K. et al. Statin adjunctive therapy shortens the duration of TB treatment in mice. J. Antimicrob. Chemother. 71, 1570–1577 (2016).
Ralph, A. P. et al. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena. PLoS One 8, e80302 (2013).
Meghji, J., Simpson, H., Squire, S. B. & Mortimer, K. A systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLoS One 11, e0161176 (2016).
Ross, J., Ehrlich, R. I., Hnizdo, E., White, N. & Churchyard, G. J. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax 65, 1010–1015 (2010).
Ehrlich, R. I. et al. Predictors of chronic bronchitis in South African adults. Int. J. Tuberc. Lung Dis. 8, 369–376 (2004).
Byrne, A. L., Marais, B. J., Mitnick, C. D., Lecca, L. & Marks, G. B. Tuberculosis and chronic respiratory disease: a systematic review. Int. J. Infect. Dis. 32, 138–146 (2015).
Subbian, S. et al. Pharmacologic inhibition of host phosphodiesterase-4 improves isoniazid-mediated clearance of Mycobacterium tuberculosis. Front. Immunol. 7, 238 (2016).
Subbian, S. et al. Adjunctive phosphodiesterase-4 inhibitor therapy improves antibiotic response to pulmonary tuberculosis in a rabbit model. EBioMedicine 4, 104–114 (2016).
Wallis, R. S. et al. Adjunctive host-directed therapies for pulmonary tuberculosis: a prospective, open-label, phase 2, randomised controlled trial. Lancet Respir. Med. 9, 897–908 (2021).
Napier, R. J. et al. Low doses of imatinib induce myelopoiesis and enhance host anti-microbial immunity. PLoS Pathog. 11, e1004770 (2015).
Napier, R. J. et al. Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe 10, 475–485 (2011).
Han, Q., Lin, L., Zhao, B., Wang, N. & Liu, X. Inhibition of mTOR ameliorates bleomycin-induced pulmonary fibrosis by regulating epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 500, 839–845 (2018).
Cabahug, V. L. O., Uy, H. S., Yu-Keh, E. & Sapno, K. J. D. Outcomes of treatment with sirolimus for non-infectious uveitis: a meta-analysis and systematic review. Clin. Ophthalmol. 13, 649–669 (2019).
Singhal, A. et al. Metformin as adjunct anti-tuberculosis therapy. Sci. Transl. Med. 6, 263ra159 (2014).
Pan, S. W. et al. The risk of TB in patients with type 2 diabetes initiating metformin vs sulfonylurea treatment. Chest 153, 1347–1357 (2018).
Lin, S. Y. et al. Metformin is associated with a lower risk of active tuberculosis in patients with type 2 diabetes. Respirology 23, 1063–1073 (2018).
Marupuru, S. et al. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Braz. J. Infect. Dis. 21, 312–316 (2017).
Degner, N. R., Wang, J. Y., Golub, J. E. & Karakousis, P. C. Metformin use reverses the increased mortality associated with diabetes mellitus during tuberculosis treatment. Clin. Infect. Dis. 66, 198–205 (2018).
Ma, Y. et al. Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up. Eur. J. Clin. Microbiol. Infect. Dis. 37, 1259–1263 (2018).
Padmapriydarsini, C. et al. Randomized trial of metformin with Anti-tuberculosis drugs for early sputum conversion in adults with pulmonary tuberculosis. Clin. Infect. Dis. https://doi.org/10.1093/cid/ciab964 (2021).
Badri, M., Wilson, D. & Wood, R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 359, 2059–2064 (2002).
Tezera, L. B. et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-alpha. eLife 9, e52668 (2020).
Barber, D. L. et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci. Transl. Med. 11, eaat2702 (2019).
Fujita, K., Terashima, T. & Mio, T. Anti-PD1 antibody treatment and the development of acute pulmonary tuberculosis. J. Thorac. Oncol. 11, 2238–2240 (2016).
Tzelepis, F. et al. Mitochondrial cyclophilin D regulates T cell metabolic responses and disease tolerance to tuberculosis. Sci. Immunol. 3, eaar4135 (2018).
DiNardo, A. R. et al. Tuberculosis endotypes to guide stratified host-directed therapy. Med 2, 217–232 (2021).
Manion, M. et al. To induce immune reconstitution inflammatory syndrome or suppress it: the spectrum of Mycobacterium genavense in the antiretroviral era. Clin. Infect. Dis. 72, 315–318 (2020).
National Tuberculosis Association. Diagnostic Standards and Classification of Tuberculosis (NTA, 1940).
Albright, F. S., Orlando, P., Pavia, A. T., Jackson, G. G. & Cannon Albright, L. A. Evidence for a heritable predisposition to death due to influenza. J. Infect. Dis. 197, 18–24 (2008).
Tadokoro, T. et al. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc. Natl Acad. Sci. USA 111, E3641–E3649 (2014).
Garbers, C., Heink, S., Korn, T. & Rose-John, S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 17, 395–412 (2018).
Arunachalam, P. S. et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 369, 1210–1220 (2020).
Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 10, a028415 (2017).
Schnepf, D. et al. Selective Janus kinase inhibition preserves interferon-lambda-mediated antiviral responses. Sci. Immunol. 6, eabd5318 (2021).
Allwood, B. W. et al. Post-tuberculosis lung health: perspectives from the first international symposium. Int. J. Tuberc. Lung Dis. 24, 820–828 (2020).
Davis, G. L. et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. N. Engl. J. Med. 321, 1501–1506 (1989).
Gillis, S., Ferm, M. M., Ou, W. & Smith, K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J. Immunol. 120, 2027–2032 (1978).
Kovacs, J. A. et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. A preliminary study. N. Engl. J. Med. 332, 567–575 (1995).
Acknowledgements
The authors thank E. Wall and W. Blanchett for helpful suggestions on the manuscript. The contribution of R.S.W. was partially supported by Horizon 2020 grant 847465 for Research and Innovation (DRTB-HDT) and project RIA2019AMR-2647 (panTB-HM) of the EDCTP2 programme of the European Union. The contribution of A.W. was funded by The Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001206), the UK Medical Research Council (FC001206) and the Wellcome Trust (FC001206). A.O’G. is funded by The Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001126), the UK Medical Research Council (FC001126) and the Wellcome Trust (FC001126). A.S. is supported by the intramural research program of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks T. Decker, R. Mahon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
EDCTP: https://www.edctp.org/
PACTR: https://pactr.samrc.ac.za/
Rights and permissions
About this article
Cite this article
Wallis, R.S., O’Garra, A., Sher, A. et al. Host-directed immunotherapy of viral and bacterial infections: past, present and future. Nat Rev Immunol 23, 121–133 (2023). https://doi.org/10.1038/s41577-022-00734-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-022-00734-z
This article is cited by
-
Granulomas microenvironment-guided sono-immunotherapy to treat and prevent recurrence of tuberculosis
Nature Communications (2026)
-
Treatment outcomes and associated influencing factors among elderly patients with rifampicin-resistant tuberculosis: a multicenter, retrospective, cohort study in China
BMC Infectious Diseases (2025)
-
Genetic and infectious determinants of puerperal fever: a study of viral and bacterial coinfections in postpartum women
Egyptian Journal of Medical Human Genetics (2025)
-
Mycobacterium tuberculosis biology, pathogenicity and interaction with the host
Nature Reviews Microbiology (2025)
-
Systemic cytokines drive conserved severity-associated myeloid responses across bacterial and viral infections
Communications Biology (2025)