Abstract
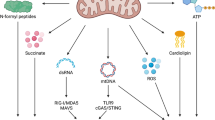
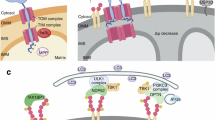
Numerous mitochondrial constituents and metabolic products can function as damage-associated molecular patterns (DAMPs) and promote inflammation when released into the cytosol or extracellular milieu. Several safeguards are normally in place to prevent mitochondria from eliciting detrimental inflammatory reactions, including the autophagic disposal of permeabilized mitochondria. However, when the homeostatic capacity of such systems is exceeded or when such systems are defective, inflammatory reactions elicited by mitochondria can become pathogenic and contribute to the aetiology of human disorders linked to autoreactivity. In addition, inefficient inflammatory pathways induced by mitochondrial DAMPs can be pathogenic as they enable the establishment or progression of infectious and neoplastic disorders. Here we discuss the molecular mechanisms through which mitochondria control inflammatory responses, the cellular pathways that are in place to control mitochondria-driven inflammation and the pathological consequences of dysregulated inflammatory reactions elicited by mitochondrial DAMPs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Roda, G. et al. Crohn’s disease. Nat. Rev. Dis. Prim. 6, 22 (2020).
Tansey, M. G. et al. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-022-00684-6 (2022).
Stark, K. & Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 18, 666–682 (2021).
Basso, P. J., Andrade-Oliveira, V. & Camara, N. O. S. Targeting immune cell metabolism in kidney diseases. Nat. Rev. Nephrol. 17, 465–480 (2021).
Marchi, S., Morroni, G., Pinton, P. & Galluzzi, L. Control of host mitochondria by bacterial pathogens. Trends Microbiol. 30, 452–465 (2022).
Vesely, M. D., Kershaw, M. H., Schreiber, R. D. & Smyth, M. J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 29, 235–271 (2011).
Mantovani, A., Ponzetta, A., Inforzato, A. & Jaillon, S. Innate immunity, inflammation and tumour progression: double-edged swords. J. Intern. Med. 285, 524–532 (2019).
Galluzzi, L., Humeau, J., Buqué, A., Zitvogel, L. & Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 17, 725–741 (2020).
Petroni, G., Buque, A., Coussens, L. M. & Galluzzi, L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat. Rev. Drug Discov. 21, 440–462 (2022).
Rodriguez-Ruiz, M. E., Vitale, I., Harrington, K. J., Melero, I. & Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 21, 120–134 (2020).
Zengeler, K. E. & Lukens, J. R. Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders. Nat. Rev. Immunol. 21, 454–468 (2021).
Kroemer, G., Galassi, C., Zitvogel, L. & Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 23, 487–500 (2022).
Vanpouille-Box, C., Hoffmann, J. A. & Galluzzi, L. Pharmacological modulation of nucleic acid sensors - therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 18, 845–867 (2019).
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009). Elliott et al. (2009) and Ghiringhelli et al. (2009) document the potent chemotactic and immunostimulatory effects of extracellular ATP.
Wein, T. & Sorek, R. Bacterial origins of human cell-autonomous innate immune mechanisms. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-022-00705-4 (2022).
Roger, A. J., Muñoz-Gómez, S. A. & Kamikawa, R. The origin and diversification of mitochondria. Curr. Biol. 27, R1177–R1192 (2017).
Harapas, C. R. et al. Organellar homeostasis and innate immune sensing. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-022-00682-8 (2022).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25, 486–541 (2018).
Galluzzi, L., Yamazaki, T. & Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 19, 731–745 (2018).
Mehta, M. M., Weinberg, S. E. & Chandel, N. S. Mitochondrial control of immunity: beyond ATP. Nat. Rev. Immunol. 17, 608–620 (2017).
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, e108863 (2021).
Galluzzi, L., López-Soto, A., Kumar, S. & Kroemer, G. Caspases connect cell-death signaling to organismal homeostasis. Immunity 44, 221–231 (2016).
Diepstraten, S. T. et al. The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat. Rev. Cancer 22, 45–64 (2022).
Decout, A., Katz, J. D., Venkatraman, S. & Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 21, 548–569 (2021).
Li, X. D. et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341, 1390–1394 (2013).
Civril, F. et al. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 498, 332–337 (2013).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014).
Rongvaux, A. et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014). White et al. (2014) and Rongvaux et al. (2014) show that robust activation of apoptotic caspases downstream of MOMP suppresses cGAS signalling driven by mtDNA.
West, A. P. et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 520, 553–557 (2015).
Andreeva, L. et al. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature 549, 394–398 (2017).
Zierhut, C. et al. The cytoplasmic DNA sensor cGAS promotes mitotic cell death. Cell 178, 302–315.e323 (2019). This study shows that chromatin is a poor cGAS activator and suppresses cGAS signalling elicited by naked dsDNA.
Michalski, S. et al. Structural basis for sequestration and autoinhibition of cGAS by chromatin. Nature 587, 678–682 (2020).
Zhao, B. et al. The molecular basis of tight nuclear tethering and inactivation of cGAS. Nature 587, 673–677 (2020).
Li, T. et al. Phosphorylation and chromatin tethering prevent cGAS activation during mitosis. Science 371, eabc5386 (2021).
Fuchs, Y. & Steller, H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 16, 329–344 (2015).
Domizio, J. D. et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature 603, 145–151 (2022).
Wiens, K. E. & Ernst, J. D. The mechanism for type I interferon induction by mycobacterium tuberculosis is bacterial strain-dependent. PLoS Pathog. 12, e1005809 (2016).
Sun, B. et al. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci. Rep. 7, 3594 (2017).
Zhou, C. M. et al. Identification of cGAS as an innate immune sensor of extracellular bacterium Pseudomonas aeruginosa. iScience 24, 101928 (2021).
Moriyama, M., Koshiba, T. & Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 10, 4624 (2019).
Danthi, P. Viruses and the diversity of cell death. Annu. Rev. Virol. 3, 533–553 (2016).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, eaao6047 (2018). These authors used super-resolution microscopy to define the roles of BAX and BAK1 in the release of mtDNA by permeabilized mitochondria.
Riley, J. S. et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 37, e99238 (2018).
Bock, F. J. & Tait, S. W. G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 21, 85–100 (2020).
Yamazaki, T. et al. Mitochondrial DNA drives abscopal responses to radiation that are inhibited by autophagy. Nat. Immunol. 21, 1160–1171 (2020). This study shows that mtDNA released by permeabilized mitochondria in the context of limited apoptotic caspase activation underlies the ability of radiotherapy to initiate acute cGAS signalling.
Willemsen, J. et al. TNF leads to mtDNA release and cGAS/STING-dependent interferon responses that support inflammatory arthritis. Cell Rep. 37, 109977 (2021).
Cosentino, K. et al. The interplay between BAX and BAK tunes apoptotic pore growth to control mitochondrial-DNA-mediated inflammation. Mol. Cell 82, 933–949.e939 (2022). The authors elegantly dissect the relative effects of BAX and BAK1 on the kinetics of the assembly of mtDNA-releasing pores at the OMM.
Ichim, G. et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell 57, 860–872 (2015).
Galluzzi, L., Kepp, O., Trojel-Hansen, C. & Kroemer, G. Non-apoptotic functions of apoptosis-regulatory proteins. EMBO Rep. 13, 322–330 (2012).
Cao, K. et al. Mitochondrial dynamics regulate genome stability via control of caspase-dependent DNA damage. Dev. Cell 57, 1211–1225.e1216 (2022).
Brokatzky, D. et al. A non-death function of the mitochondrial apoptosis apparatus in immunity. EMBO J. 38, e100907 (2019).
Tigano, M., Vargas, D. C., Tremblay-Belzile, S., Fu, Y. & Sfeir, A. Nuclear sensing of breaks in mitochondrial DNA enhances immune surveillance. Nature 591, 477–481 (2021). This article delineates a mechanism through which breaks in mtDNA initiate inflammatory responses as a consequence of mtRNA accumulation in the cytosol and RIG-I activation.
Flores-Romero, H. et al. BCL-2-family protein tBID can act as a BAX-like effector of apoptosis. EMBO J. 41, e108690 (2022).
Kim, J. et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science 366, 1531–1536 (2019). These authors suggest a role for VDACs in the pathogenic release of mtDNA that accompanies the development of a lupus-like syndrome in mice.
Bonora, M., Giorgi, C. & Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 23, 266–285 (2022).
Patrushev, M. et al. Mitochondrial permeability transition triggers the release of mtDNA fragments. Cell. Mol. Life Sci. 61, 3100–3103 (2004).
Yu, C. H. et al. TDP-43 triggers mitochondrial DNA release via mPTP to activate cGAS/STING in ALS. Cell 183, 636–649.e618 (2020).
Kruglov, A. G., Kharechkina, E. S., Nikiforova, A. B., Odinokova, I. V. & Kruglova, S. A. Dynamics of the permeability transition pore size in isolated mitochondria and mitoplasts. FASEB J. 35, e21764 (2021).
Heng, J. B. et al. The electromechanics of DNA in a synthetic nanopore. Biophys. J. 90, 1098–1106 (2006).
Neginskaya, M. A. et al. The very low number of calcium-induced permeability transition pores in the single mitochondrion. J. Gen. Physiol. 152, e202012631 (2020).
Ross, C. et al. Inflammatory caspases: toward a unified model for caspase activation by inflammasomes. Annu. Rev. Immunol. 40, 249–269 (2022).
Agostini, L. et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20, 319–325 (2004).
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 (2008).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 (2011). This article reveals the existence of a feedforward loop linking inflammasome activation and mitochondrial dysfunction that is under tonic control by autophagy.
Renaudin, X. Reactive oxygen species and DNA damage response in cancer. Int. Rev. Cell Mol. Biol. 364, 139–161 (2021).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Zhou, R., Tardivel, A., Thorens, B., Choi, I. & Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 (2010).
Saxena, G., Chen, J. & Shalev, A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 285, 3997–4005 (2010).
Iyer, S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013).
Pervaiz, S., Bellot, G. L., Lemoine, A. & Brenner, C. Redox signaling in the pathogenesis of human disease and the regulatory role of autophagy. Int. Rev. Cell Mol. Biol. 352, 189–214 (2020).
Bauernfeind, F. et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187, 613–617 (2011).
Niemi, K. et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J. Immunol. 186, 6119–6128 (2011).
Billingham, L. K. et al. Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat. Immunol. 23, 692–704 (2022).
Zhong, Z. et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 560, 198–203 (2018). The authors elegantly reveal that mtDNA neosynthesis is required for the generation of oxidized mtDNA species that elicit NLRP3 inflammasome activation.
Zhong, Z. et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164, 896–910 (2016).
Kang, D., Kim, S. H. & Hamasaki, N. Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 7, 39–44 (2007).
Xu, Y., Johansson, M. & Karlsson, A. Human UMP-CMP kinase 2, a novel nucleoside monophosphate kinase localized in mitochondria. J. Biol. Chem. 283, 1563–1571 (2008).
Xian, H. et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity 54, 1463–1477.e1411 (2021).
Allam, R. et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 15, 982–990 (2014).
Vince, J. E. et al. The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1β activation. Cell Rep. 25, 2339–2353.e2334 (2018).
Chauhan, D. et al. BAX/BAK-induced apoptosis results in caspase-8-dependent IL-1β maturation in macrophages. Cell Rep. 25, 2354–2368.e2355 (2018).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 (2019).
Platnich, J. M. et al. Shiga toxin/lipopolysaccharide activates caspase-4 and gasdermin D to trigger mitochondrial reactive oxygen species upstream of the NLRP3 inflammasome. Cell Rep. 25, 1525–1536.e1527 (2018).
Guarnieri, J. W. et al. SARS-CoV-2 viroporins activate the NLRP3-inflammasome via the mitochondrial permeability transition pore. Preprint at bioRxiv https://doi.org/10.1101/2022.02.19.481139 (2022).
Dang, E. V., McDonald, J. G., Russell, D. W. & Cyster, J. G. Oxysterol restraint of cholesterol synthesis prevents AIM2 inflammasome activation. Cell 171, 1057–1071.e1011 (2017).
Fernandes-Alnemri, T., Yu, J. W., Datta, P., Wu, J. & Alnemri, E. S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Di Micco, A. et al. AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity. Proc. Natl Acad. Sci. USA 113, E4671–E4680 (2016).
Crane, D. D., Bauler, T. J., Wehrly, T. D. & Bosio, C. M. Mitochondrial ROS potentiates indirect activation of the AIM2 inflammasome. Front. Microbiol. 5, 438 (2014).
Xu, L. et al. Mitochondrial DNA enables AIM2 inflammasome activation and hepatocyte pyroptosis in nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 320, G1034–G1044 (2021).
Jabir, M. S. et al. Mitochondrial damage contributes to Pseudomonas aeruginosa activation of the inflammasome and is downregulated by autophagy. Autophagy 11, 166–182 (2015).
Riley, J. S. & Tait, S. W. Mitochondrial DNA in inflammation and immunity. EMBO Rep. 21, e49799 (2020).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010). This study shows that mitochondrial products that are released into the circulation as a consequence of cell death, including mtDNA and formylated peptides, contribute to the aetiology of systemic inflammatory response syndrome.
Tian, J. et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8, 487–496 (2007).
Mangelinck, A. & Mann, C. DNA methylation and histone variants in aging and cancer. Int. Rev. Cell Mol. Biol. 364, 1–110 (2021).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Lamkanfi, M. et al. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J. Immunol. 185, 4385–4392 (2010).
Liu, Y. et al. Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J. Hepatol. 63, 114–121 (2015).
Little, J. P. et al. Mitochondrial transcription factor A (Tfam) is a pro-inflammatory extracellular signaling molecule recognized by brain microglia. Mol. Cell. Neurosci. 60, 88–96 (2014).
Oka, T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012). The authors show that deletion of a lysosomal nuclease-encoding gene results in inefficient mtDNA disposal by autophagy, culminating in pathogenic TLR9 activation.
De Leo, M. G. et al. Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL. Nat. Cell Biol. 18, 839–850 (2016).
Rabas, N. et al. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. J. Cell Biol. 220, e202006049 (2021).
Todkar, K. et al. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat. Commun. 12, 1971 (2021).
Cadete, V. J. et al. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J. Physiol. 594, 5343–5362 (2016).
Konecna, B. et al. Monocyte exocytosis of mitochondrial danger-associated molecular patterns in sepsis suppresses neutrophil chemotaxis. J. Trauma. Acute Care Surg. 90, 46–53 (2021).
Sansone, P. et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl Acad. Sci. USA 114, E9066–E9075 (2017).
Hur, S. Double-stranded RNA sensors and modulators in innate immunity. Annu. Rev. Immunol. 37, 349–375 (2019).
Dhir, A. et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 560, 238–242 (2018). This study shows that mtRNA can function as a DAMP and initiate MDA5-dependent inflammatory responses in eukaryotic cells.
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, 669–682 (2005).
Coomans de Brachène, A. et al. Endogenous mitochondrial double-stranded RNA is not an activator of the type I interferon response in human pancreatic beta cells. Auto. Immun. Highlights 12, 6 (2021).
Li, S. et al. The mitochondrial protein ERAL1 suppresses RNA virus infection by facilitating RIG-I-like receptor signaling. Cell Rep. 34, 108631 (2021).
Vanpouille-Box, C., Demaria, S., Formenti, S. C. & Galluzzi, L. Cytosolic DNA sensing in organismal tumor control. Cancer Cell 34, 361–378 (2018).
Franchi, L. et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J. Immunol. 193, 4214–4222 (2014).
Galluzzi, L. et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell. Death Differ. 22, 58–73 (2015).
McArthur, K. & Kile, B. T. Apoptotic caspases: multiple or mistaken identities? Trends Cell Biol. 28, 475–493 (2018).
Gyrd-Hansen, M. & Meier, P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat. Rev. Cancer 10, 561–574 (2010).
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131, 682–693 (2007).
Varfolomeev, E. et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 131, 669–681 (2007).
Giampazolias, E. et al. Mitochondrial permeabilization engages NF-kappaB-dependent anti-tumour activity under caspase deficiency. Nat. Cell Biol. 19, 1116–1129 (2017).
Zhang, J. et al. Ubiquitin ligases cIAP1 and cIAP2 limit cell death to prevent inflammation. Cell Rep. 27, 2679–2689.e2673 (2019).
Lecis, D. et al. Smac mimetics induce inflammation and necrotic tumour cell death by modulating macrophage activity. Cell Death Dis. 4, e920 (2013).
Rizk, J. et al. SMAC mimetics promote NIK-dependent inhibition of CD4+ TH17 cell differentiation. Sci. Signal. 12, eaaw3469 (2019).
Boada-Romero, E., Martinez, J., Heckmann, B. L. & Green, D. R. The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol. 21, 398–414 (2020).
Leslie, D. S. et al. Serum lipids regulate dendritic cell CD1 expression and function. Immunology 125, 289–301 (2008).
Dieudé, M. et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted γδ T cells in the normal murine repertoire. J. Immunol. 186, 4771–4781 (2011).
Gouveia, A., Bajwa, E. & Klegeris, A. Extracellular cytochrome c as an intercellular signaling molecule regulating microglial functions. Biochim. Biophys. Acta Gen. Subj. 1861, 2274–2281 (2017).
Pullerits, R., Bokarewa, M., Jonsson, I. M., Verdrengh, M. & Tarkowski, A. Extracellular cytochrome c, a mitochondrial apoptosis-related protein, induces arthritis. Rheumatology 44, 32–39 (2005).
Kepp, O. et al. ATP and cancer immunosurveillance. EMBO J. 40, e108130 (2021).
Bolivar, B. E. et al. Noncanonical roles of caspase-4 and caspase-5 in heme-driven IL-1beta release and cell death. J. Immunol. 206, 1878–1889 (2021).
Wang, Y. et al. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 9, 1624–1625 (2013).
Ma, Y. et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 38, 729–741 (2013).
Lin, S. et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J. Neuroinflamm. 9, 46 (2012).
May, O. et al. The receptor for advanced glycation end products is a sensor for cell-free heme. FEBS J. 288, 3448–3464 (2021).
Belcher, J. D. et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123, 377–390 (2014).
Han, C. et al. Tumor cells suppress radiation-induced immunity by hijacking caspase 9 signaling. Nat. Immunol. 21, 546–554 (2020).
Rodriguez-Ruiz, M. E. et al. Apoptotic caspases inhibit abscopal responses to radiation and identify a new prognostic biomarker for breast cancer patients. Oncoimmunology 8, e1655964 (2019).
Ning, X. et al. Apoptotic caspases suppress type I interferon production via the cleavage of cGAS, MAVS, and IRF3. Mol. Cell 74, 19–31.e17 (2019).
Segawa, K. et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 344, 1164–1168 (2014).
Fadok, V. A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 (1992).
Buqué, A., Rodriguez-Ruiz, M. E., Fucikova, J. & Galluzzi, L. Apoptotic caspases cut down the immunogenicity of radiation. Oncoimmunology 8, e1655364 (2019).
Bernardini, J. P. et al. Parkin inhibits BAK and BAX apoptotic function by distinct mechanisms during mitophagy. EMBO J. 38, e99916 (2019).
Lindqvist, L. M. et al. Autophagy induced during apoptosis degrades mitochondria and inhibits type I interferon secretion. Cell Death Differ. 25, 784–796 (2018).
Galluzzi, L. et al. Molecular definitions of autophagy and related processes. EMBO J. 36, 1811–1836 (2017).
Sliter, D. A. et al. Parkin and PINK1 mitigate STING-induced inflammation. Nature 561, 258–262 (2018). This study shows that PINK1- and PRKN-dependent mitophagy has a major role in restricting STING1 signalling by clearing permeabilized, mtDNA-releasing mitochondria.
Mouton-Liger, F. et al. Parkin deficiency modulates NLRP3 inflammasome activation by attenuating an A20-dependent negative feedback loop. Glia 66, 1736–1751 (2018).
Zhang, N. P., Liu, X. J., Xie, L., Shen, X. Z. & Wu, J. Impaired mitophagy triggers NLRP3 inflammasome activation during the progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Lab. Invest. 99, 749–763 (2019).
Shi, C. S. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13, 255–263 (2012).
Richter, B. et al. Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc. Natl Acad. Sci. USA 113, 4039–4044 (2016).
Di Rita, A. et al. HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKα. Nat. Commun. 9, 3755 (2018).
Yu, J. et al. Inflammasome activation leads to caspase-1-dependent mitochondrial damage and block of mitophagy. Proc. Natl Acad. Sci. USA 111, 15514–15519 (2014).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011).
Wang, Y. et al. Inflammasome activation triggers caspase-1-mediated cleavage of cGAS to regulate responses to DNA virus infection. Immunity 46, 393–404 (2017).
Corrales, L. et al. Antagonism of the STING pathway via activation of the AIM2 inflammasome by intracellular DNA. J. Immunol. 196, 3191–3198 (2016).
Melki, I. et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci. Transl. Med. 13, eaav5928 (2021).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016).
Caielli, S. et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 184, 4464–4479.e4419 (2021). This is an elegant demonstration of the links between defective mitophagy in erythroid precursors and the formation of mitochondria-containing erythrocytes that drive pathogenic type I interferon responses in SLE.
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
Khaloian, S. et al. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn’s disease recurrence. Gut 69, 1939–1951 (2020).
Jackson, D. N. et al. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut 69, 1928–1938 (2020).
Benmerzoug, S. et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 9, 5226 (2018).
Wiegman, C. H. et al. Oxidative stress-induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 136, 769–780 (2015).
Giordano, L. et al. Extracellular release of mitochondrial DNA: triggered by cigarette smoke and detected in COPD. Cells 11, 369 (2022).
Maekawa, H. et al. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 29, 1261–1273.e1266 (2019).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799.e785 (2019).
Chen, K. et al. Optineurin inhibits NLRP3 inflammasome activation by enhancing mitophagy of renal tubular cells in diabetic nephropathy. FASEB J. 33, 4571–4585 (2019).
Tang, C. et al. PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 14, 880–897 (2018).
Kasten, M. et al. Genotype-phenotype relations for the Parkinson’s disease genes Parkin, PINK1, DJ1: MDSGene systematic review. Mov. Disord. 33, 730–741 (2018).
Borsche, M. et al. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain 143, 3041–3051 (2020).
Noda, S. et al. Loss of Parkin contributes to mitochondrial turnover and dopaminergic neuronal loss in aged mice. Neurobiol. Dis. 136, 104717 (2020).
Wilkins, H. M. et al. Mitochondrial lysates induce inflammation and Alzheimer’s disease-relevant changes in microglial and neuronal cells. J. Alzheimers Dis. 45, 305–318 (2015).
Fang, E. F. et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 22, 401–412 (2019).
Roca-Agujetas, V. et al. Cholesterol alters mitophagy by impairing optineurin recruitment and lysosomal clearance in Alzheimer’s disease. Mol. Neurodegener. 16, 15 (2021).
Bellenguez, C. et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 54, 412–436 (2022).
King, K. R. et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 23, 1481–1487 (2017).
Kwon, W. Y. et al. Circulating mitochondrial N-formyl peptides contribute to secondary nosocomial infection in patients with septic shock. Proc. Natl Acad. Sci. USA 118, e2018538118 (2021).
Marques, P. E. et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56, 1971–1982 (2012).
Bai, J. et al. DsbA-L prevents obesity-induced inflammation and insulin resistance by suppressing the mtDNA release-activated cGAS-cGAMP-STING pathway. Proc. Natl Acad. Sci. USA 114, 12196–12201 (2017).
Christensen, M. H. et al. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression. EMBO J. 35, 1385–1399 (2016).
Saffran, H. A., Pare, J. M., Corcoran, J. A., Weller, S. K. & Smiley, J. R. Herpes simplex virus eliminates host mitochondrial DNA. EMBO Rep. 8, 188–193 (2007).
Chen, H. et al. HBx inhibits DNA sensing signaling pathway via ubiquitination and autophagy of cGAS. Virol. J. 19, 55 (2022).
Yum, S., Li, M., Fang, Y. & Chen, Z. J. TBK1 recruitment to STING activates both IRF3 and NF-kappaB that mediate immune defense against tumors and viral infections. Proc. Natl Acad. Sci. USA 118, e2100225118 (2021).
Thomsen, M. K. et al. Lack of immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. Hepatology 64, 746–759 (2016).
Galluzzi, L. et al. Autophagy in malignant transformation and cancer progression. EMBO J. 34, 856–880 (2015).
Buque, A. et al. Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat. Commun. 11, 3819 (2020).
Li, S. et al. SFTSV infection induces BAK/BAX-dependent mitochondrial DNA release to trigger NLRP3 inflammasome activation. Cell Rep. 30, 4370–4385.e4377 (2020).
Costa, T. J. et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vasc. Pharmacol. 142, 106946 (2022).
Neufeldt, C. J. et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. Commun. Biol. 5, 45 (2022).
Kuo, C. L. et al. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 474, 138–150 (2020).
Singel, K. L. et al. Mitochondrial DNA in the tumour microenvironment activates neutrophils and is associated with worse outcomes in patients with advanced epithelial ovarian cancer. Br. J. Cancer 120, 207–217 (2019).
Bao, D. et al. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene 38, 5007–5020 (2019).
Li, C. et al. PINK1 and PARK2 suppress pancreatic tumorigenesis through control of mitochondrial iron-mediated immunometabolism. Dev. Cell 46, 441–455.e448 (2018).
Dorflinger, B. et al. Mitochondria supply sub-lethal signals for cytokine secretion and DNA-damage in H. pylori infection. Cell Death Differ. https://doi.org/10.1038/s41418-022-01009-9 (2022).
Schmidts, A., Wehrli, M. & Maus, M. V. Toward better understanding and management of CAR-T cell-associated toxicity. Annu. Rev. Med. 72, 365–382 (2021).
Le Naour, J., Zitvogel, L., Galluzzi, L., Vacchelli, E. & Kroemer, G. Trial watch: STING agonists in cancer therapy. Oncoimmunology 9, 1777624 (2020).
Glab, J. A., Cao, Z. & Puthalakath, H. Bcl-2 family proteins, beyond the veil. Int. Rev. Cell Mol. Biol. 351, 1–22 (2020).
Mason, K. D. et al. Programmed anuclear cell death delimits platelet life span. Cell 128, 1173–1186 (2007).
Galluzzi, L., Zitvogel, L. & Kroemer, G. Immunological mechanisms underneath the efficacy of cancer therapy. Cancer Immunol. Res. 4, 895–902 (2016).
Lopez, A. et al. Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer. Nat. Commun. 13, 1199 (2022).
Reyna, D. E. et al. Direct activation of BAX by BTSA1 overcomes apoptosis resistance in acute myeloid leukemia. Cancer Cell 32, 490–505.e410 (2017).
Spitz, A. Z., Zacharioudakis, E., Reyna, D. E., Garner, T. P. & Gavathiotis, E. Eltrombopag directly inhibits BAX and prevents cell death. Nat. Commun. 12, 1134 (2021).
Amgalan, D. et al. A small-molecule allosteric inhibitor of BAX protects against doxorubicin-induced cardiomyopathy. Nat. Cancer 1, 315–328 (2020).
Yamazaki, T. & Galluzzi, L. BAX and BAK dynamics control mitochondrial DNA release during apoptosis. Cell Death Differ. 29, 1296–1298 (2022).
Swanson, S. K. et al. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc. Natl Acad. Sci. USA 89, 3741–3745 (1992).
Galluzzi, L., Bravo-San Pedro, J. M., Levine, B., Green, D. R. & Kroemer, G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 16, 487–511 (2017).
Lekakis, V. & Cholongitas, E. The impact of emricasan on chronic liver diseases: current data. Clin. J. Gastroenterol. 15, 271–285 (2022).
Galluzzi, L., Vanpouille-Box, C., Bakhoum, S. F. & Demaria, S. SnapShot: CGAS-STING signaling. Cell 173, 276–276.e271 (2018).
Yatim, N. et al. RIPK1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells. Science 350, 328–334 (2015).
Bakhoum, S. F. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472 (2018).
Li, H., Zimmerman, S. E. & Weyemi, U. Genomic instability and metabolism in cancer. Int. Rev. Cell Mol. Biol. 364, 241–265 (2021).
Acknowledgements
S.M. is supported by the Italian Ministry of Health (GR-2016-02364602), Nanoblend and local funds from Marche Polytechnic University (Ancona, Italy). S.W.G.T. is supported by funding from Cancer Research UK, Prostate Cancer UK, Tenovus Scotland and the Swiss National Science Foundation. The laboratory of L.G. (as a principal investigator unless otherwise indicated) is or has been supported by two Breakthrough Level 2 grants from the US Department of Defense Breast Cancer Research Program (BC180476P1 and BC210945), by a Transformative Breast Cancer Consortium Grant from the US Department of Defense Breast Cancer Research Program (W81XWH2120034; principal investigator Formenti), by a U54 grant from the NCI of the NIH (CA274291; principal investigators Deasy, Formenti and Weichselbaum), by the 2019 Laura Ziskin Prize in Translational Research (ZP-6177; principal investigator Formenti) from Stand Up to Cancer, by a Mantle Cell Lymphoma Research Initiative grant from the Leukaemia and Lymphoma Society (principal investigator Chen-Kiang), by a Rapid Response Grant from the Functional Genomics Initiative (New York, USA), by start-up funds from the Department of Radiation Oncology at Weill Cornell Medicine (New York, USA), by industrial collaborations with Lytix Biopharma (Oslo, Norway) and Promontory (New York, USA), and by donations from Promontory (New York, USA), the Luke Heller TECPR2 Foundation (Boston, USA), Sotio a.s. (Prague, Czech Republic), Lytix Biopharma (Oslo, Norway), Onxeo (Paris, France), Ricerchiamo (Brescia, Italy) and Noxopharm (Chatswood, Australia).
Author information
Authors and Affiliations
Contributions
T.Y. and L.G. conceived the idea for the Review. S.M. and E.G. prepared the first version of the manuscript, with constructive input from S.W.G.T. and under the supervision of T.Y. and L.G. E.G. prepared display items under the supervision of T.Y. and L.G. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
L.G. has held research contracts with Lytix Biopharma and Promontory, has received consulting/advisory honoraria from Boehringer Ingelheim, AstraZeneca, OmniSEQ, Onxeo, The Longevity Labs, Inzen, Sotio, Promontory, Noxopharm, EduCom and the Luke Heller TECPR2 Foundation, and holds Promontory stock options. All other authors declare no conflicts of interest.
Peer review
Peer review information
Nature Reviews Immunology thanks N. Chandel, C. Hauser and A. García-Sáez for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Cancer immunosurveillance
-
A process through which the immune system recognizes and eliminates the majority of newly formed cancer cell precursors, hence suppressing oncogenesis.
- Type II cells
-
Mammalian cells that (in contrast to type I cells) require mitochondrial outer membrane permeabilization to enable death receptors to induce full activation of executioner caspases during apoptosis.
- Regulated cell death
-
(RCD). A type of cell death that depends on a genetically encoded machinery and hence can be modulated by pharmacological or genetic interventions.
- Mitochondrial outer membrane permeabilization
-
(MOMP). A key event in apoptotic cell death, culminating in the release of mitochondrial components into the cytosol and activation of proapoptotic caspases.
- Autophagy
-
An evolutionarily conserved, lysosome-dependent mechanism through which eukaryotic cells clear the cytoplasm of potentially cytotoxic or superfluous material to preserve homeostasis.
- Caspase
-
A member of a family of cysteine-dependent proteases that regulate the timing and immunological effects of various forms of cell death.
- Programmed cell death
-
A variant of regulated cell death that is triggered as a part of physiological programmes (such as embryonic development or preservation of adult tissue homeostasis) and not as a consequence of failing adaptation to stress.
- Mitophagy
-
A type of autophagy response that preferentially degrades permeabilized or otherwise dysfunctional mitochondria.
- Mitochondrial permeability transition
-
(MPT). A regulated process resulting in the abrupt loss of the impermeability of the inner mitochondrial membrane to solutes and water, resulting in osmotic swelling of the mitochondrial matrix and, ultimately, cell death.
- Inhibitor of apoptosis protein
-
(IAP). A member of a protein family that inhibits apoptosis by antagonizing the catalytic activity of caspases and functioning as a ubiquitin ligase to control upstream apoptotic signal transduction.
- Ripoptosome
-
A supramolecular complex containing receptor-interacting serine/threonine kinase 1 (RIPK1) and RIPK3 that promotes cell death coupled to inflammatory responses in various stress conditions (for example, exposure to genotoxins and allergens).
- Non-canonical NF-κB signalling
-
A transcriptional response generally initiated by the NIK-driven activation of NF-κB heterodimers composed of RELB and p52.
- Canonical NF-κB signalling
-
A transcriptional response generally initiated by the TAK1-driven activation of NF-κB heterodimers composed of RELA and p50.
- Immunogenic cell death
-
A variant of regulated cell death that is sufficient, in immunocompetent and syngeneic settings, to elicit an adaptive immune response to dead cell-associated antigens.
- BH3 mimetics
-
Pharmacological agents that mimic the ability of natural BH3-only proteins to directly or indirectly promote mitochondrial outer membrane permeabilization.
- Neutrophil extracellular traps
-
Networks of extracellular fibres enriched in DNA and proteins that are released by neutrophils in response to activating stimuli (such as pathogens).
Rights and permissions
About this article
Cite this article
Marchi, S., Guilbaud, E., Tait, S.W.G. et al. Mitochondrial control of inflammation. Nat Rev Immunol 23, 159–173 (2023). https://doi.org/10.1038/s41577-022-00760-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-022-00760-x
This article is cited by
-
Immune remodeling via mitochondria-dependent STING activation enhances cabozantinib response in hepatocellular carcinoma
Journal of Experimental & Clinical Cancer Research (2026)
-
MitoCommun: a database for decoding mitochondrial communication networks
BMC Genomics (2026)
-
UBA5 deficiency disrupts mitochondrial autophagy via the PINK1–parkin pathway and impairs myoblast proliferation
Biological Research (2026)
-
Mitophagy in the pathogenesis and management of disease
Cell Research (2026)
-
Mitochondrial DNA drives NLRP3-IL-1β axis activation in microglia by binding to NLRP3, leading to neurodegeneration in Parkinson’s disease models
Cell Death & Disease (2026)