Abstract
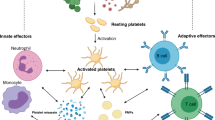
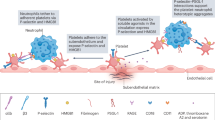
Immune-mediated inflammatory diseases (IMIDs) are characterized by excessive and uncontrolled inflammation and thrombosis, both of which are responsible for organ damage, morbidity and death. Platelets have long been known for their role in primary haemostasis, but they are now also considered to be components of the immune system and to have a central role in the pathogenesis of IMIDs. In patients with IMIDs, platelets are activated by disease-specific factors, and their activation often reflects disease activity. Here we summarize the evidence showing that activated platelets have an active role in the pathogenesis and the progression of IMIDs. Activated platelets produce soluble factors and directly interact with immune cells, thereby promoting an inflammatory phenotype. Furthermore, platelets participate in tissue injury and promote abnormal tissue healing, leading to fibrosis. Targeting platelet activation and targeting the interaction of platelets with the immune system are novel and promising therapeutic strategies in IMIDs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
21 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41577-023-00869-7
References
Scherlinger, M. et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun. Rev. 19, 102531 (2020).
Broen, J. C. A. & van Laar, J. M. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat. Rev. Rheumatol. 16, 167–178 (2020).
Manzi, S. M. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 145, 408–415 (1997).
Conrad, N. et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 400, 733–743 (2022). This large population-based study reports that multiple IMIDs are associated with increased risk of a wide range of cardiovascular diseases.
Gremmel, T., Frelinger, A. & Michelson, A. Platelet physiology. Semin. Thromb. Hemost. 42, 191–204 (2016).
Luo, D. et al. Protective roles for fibrin, tissue factor, plasminogen activator inhibitor-1, and thrombin activatable fibrinolysis inhibitor, but not factor XI, during defense against the gram-negative bacterium Yersinia enterocolitica. J. Immunol. 187, 1866–1876 (2011).
Gaertner, F. et al. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell 171, 1368–1382.e23 (2017). This study shows that platelets actively patrol blood vessels to bind and bundle circulating bacteria, and promote neutrophil activation in infection.
Maouia, A., Rebetz, J., Kapur, R. & Semple, J. W. The immune nature of platelets revisited. Transfus. Med. Rev. 34, 209–220 (2020).
Marcoux, G., Laroche, A., Espinoza Romero, J. & Boilard, E. Role of platelets and megakaryocytes in adaptive immunity. Platelets 32, 340–351 (2021).
Bai, M. et al. Mean platelet volume could reflect disease activity of adult patients with systemic lupus erythematosus. Clin. Lab. 62, 1317–1322 (2016).
Khodashahi, M., Saadati, N., Rezaieyazdi, Z., Sahebari, M. & Saremi, Z. Evaluation of mean platelet volume in patients with rheumatoid arthritis and its relation with severity of disease. Rheumatol. Res. 4, 121–126 (2019).
Masoumi, M. et al. Correlation of clinical signs and symptoms of Behçet’s disease with mean platelet volume (MPV) and red cell distribution width (RDW). Orphanet J. Rare Dis. 15, 297 (2020).
Stenberg, P. E., Shuman, M. A., Levine, S. P. & Bainton, D. F. Redistribution of alpha-granules and their contents in thrombin- stimulated platelets. J. Cell Biol. 98, 748–760 (1984).
Duffau, P. et al. Platelet CD154 potentiates interferon- secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl. Med. 2, 47ra63 (2010). This study shows that platelets interact with pDCs in SLE and promote their production of type I interferon through the CD40L–CD40 axis.
Scherlinger, M. et al. Selectins impair regulatory T cell function and contribute to systemic lupus erythematosus pathogenesis. Sci. Transl. Med. 13, eabi4994 (2021). This study shows that platelets from patients with SLE interact with Treg cells through the P-selectin–PSGL1 axis, which results in FOXP3 downregulation and Treg cell dysfunction.
Puhm, F., Boilard, E. & Machlus, K. R. Platelet extracellular vesicles: beyond the blood. Arterioscler. Thromb. Vasc. Biol. 41, 87–96 (2021).
Tessandier, N. et al. Platelets disseminate extracellular vesicles in lymph in rheumatoid arthritis. Arterioscler. Thromb. Vasc. Biol. 40, 929–942 (2020).
Melki, I. et al. FcγRIIA expression aggravates nephritis and increases platelet activation in systemic lupus erythematosus in mice. Blood https://doi.org/10.1182/blood.2020004974 (2020).
Melki, I. et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci. Transl. Med. 13, eaav5928 (2021). This study reports that activated platelets release mitochondria, either free or embedded in extracellular vesicles, that activate the immune system and participate in immune dysregulation in SLE.
Ohyama, K. et al. Proteomic profiling of antigens in circulating immune complexes associated with each of seven autoimmune diseases. Clin. Biochem. 48, 181–185 (2015).
Hubbard, J. J. et al. FcRn is a CD32a coreceptor that determines susceptibility to IgG immune complex-driven autoimmunity. J. Exp. Med. 217, e20200359 (2020).
Qian, K. et al. Functional expression of IgA receptor FcαRI on human platelets. J. Leukoc. Biol. 84, 1492–1500 (2008).
Joseph, M. et al. Expression and functions of the high-affinity IgE receptor on human platelets and megakaryocyte precursors. Eur. J. Immunol. 27, 2212–2218 (1997).
Chalayer, E. et al. Fc receptors gone wrong: a comprehensive review of their roles in autoimmune and inflammatory diseases. Autoimmun. Rev. 21, 103016 (2022).
Love, P. E. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann. Intern. Med. 112, 682 (1990).
Assous, N. et al. Prevalence of antiphospholipid antibodies in systemic sclerosis and association with primitive pulmonary arterial hypertension and endothelial injury. Clin. Exp. Rheumatol. 23, 199–204 (2005).
Fauchais, A. L. et al. Antiphospholipid antibodies in primary Sjögren’s syndrome: prevalence and clinical significance in a series of 74 patients. Lupus 13, 245–248 (2004).
Zhang, W. et al. Anti-β2 glycoprotein I antibodies in complex with β2 glycoprotein I induce platelet activation via two receptors: apolipoprotein E receptor 2′ and glycoprotein I bα. Front. Med. 10, 76–84 (2016).
Müller-Calleja, N. et al. Lipid presentation by the protein C receptor links coagulation with autoimmunity. Science 371, eabc0956 (2021).
Kim, K.-J., Baek, I.-W., Yoon, C.-H., Kim, W.-U. & Cho, C.-S. Elevated levels of soluble CD40 ligand are associated with antiphospholipid antibodies in patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 35, 823–830 (2017).
Patsouras, M. D. et al. Elevated expression of platelet-derived chemokines in patients with antiphospholipid syndrome. J. Autoimmun. 65, 30–37 (2015).
Provan, D. & Semple, J. W. Recent advances in the mechanisms and treatment of immune thrombocytopenia. eBioMedicine 76, 103820 (2022).
Al-Samkari, H. et al. A modern reassessment of glycoprotein-specific direct platelet autoantibody testing in immune thrombocytopenia. Blood Adv. 4, 9–18 (2019).
Kuwana, M., Kaburaki, J., Okazaki, Y., Miyazaki, H. & Ikeda, Y. Two types of autoantibody-mediated thrombocytopenia in patients with systemic lupus erythematosus. Rheumatology 45, 851–854 (2006).
Czirják, L. et al. Anti-platelet antibodies against gpIIb/IIIa in systemic sclerosis. Clin. Exp. Rheumatol. 12, 527–529 (1994).
Hally, K., Fauteux-Daniel, S., Hamzeh-Cognasse, H., Larsen, P. & Cognasse, F. Revisiting platelets and Toll-like receptors (TLRs): at the interface of vascular immunity and thrombosis. Int. J. Mol. Sci. 21, 6150 (2020).
Aslam, R. et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 107, 637–641 (2006).
Andonegui, G. et al. Platelets express functional Toll-like receptor-4. Blood 106, 2417–2423 (2005).
Ward, J. R. et al. Agonists of toll-like receptor (TLR)2 and TLR4 are unable to modulate platelet activation by adenosine diphosphate and platelet activating factor. Thromb. Haemost. 94, 831–838 (2005).
Vogel, S. et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Invest. 125, 4638–4654 (2015).
Koupenova, M. et al. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood 124, 791–802 (2014).
Brown, G. J. et al. TLR7 gain-of-function genetic variation causes human lupus. Nature 605, 349–356 (2022). This study shows that constitutive activation of TLR7 causes early and severe SLE in mice and humans.
Becker, Y. et al. Autoantibodies in systemic lupus erythematosus target mitochondrial RNA. Front. Immunol. 10, 1026 (2019).
Becker, Y. L., Julien, A.-S., Godbout, A., Boilard, É. & Fortin, P. R. Pilot study of anti-mitochondrial antibodies in antiphospholipid syndrome. Lupus 29, 1623–1629 (2020).
Thon, J. N. et al. The functional role of TLR9 in human platelets. Blood 118, 366 (2011).
Boudreau, L. H. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183 (2014).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022).
Draborg, A. H. et al. Reduced response to Epstein-Barr virus antigens by T-cells in systemic lupus erythematosus patients. Lupus Sci. Med. 1, e000015 (2014).
Su, Y. et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 185, 881–895.e20 (2022).
Wood, R. A. et al. Serologic markers of Epstein-Barr virus reactivation are associated with increased disease activity, inflammation, and interferon pathway activation in patients with systemic lupus erythematosus. J. Transl. Autoimmun. 4, 100117 (2021).
Banerjee, M. et al. Platelets endocytose viral particles and are activated via TLR (Toll-like receptor) signaling. Arterioscler. Thromb. Vasc. Biol. 40, 1635–1650 (2020).
Ahmad, A. & Menezes, J. Binding of the Epstein-Barr virus to human platelets causes the release of transforming growth factor-beta. J. Immunol. 159, 3984–3988 (1997).
Swanson, K. V., Deng, M. & Ting, J. P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 (2019).
Murthy, P. et al. The NLRP3 inflammasome and Bruton’s tyrosine kinase in platelets co-regulate platelet activation, aggregation, and in vitro thrombus formation. Biochem. Biophys. Res. Commun. 483, 230–236 (2017).
Vogel, S. et al. The platelet NLRP3 inflammasome is upregulated in sickle cell disease via HMGB1/TLR4 and Bruton tyrosine kinase. Blood Adv. 2, 2672–2680 (2018).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010). This study shows that patients with rheumatoid arthritis have platelet-derived extracellular vesicles in the synovial fluid and inflamed joints, and that these vesicles promote inflammation through IL-1β release.
Chiang, T. M., Takayama, H. & Postlethwaite, A. E. Increase in platelet non-integrin type I collagen receptor in patients with systemic sclerosis. Thromb. Res. 117, 299–306 (2006).
Takahashi, H. & Moroi, M. Antibody against platelet membrane glycoprotein VI in a patient with systemic lupus erythematosus. Am. J. Hematol. 67, 262–267 (2001).
Hechler, B. & Gachet, C. Purinergic receptors in thrombosis and inflammation. Arterioscler. Thromb. Vasc. Biol. 35, 2307–2315 (2015).
Haas, C. B., Lovászi, M., Braganhol, E., Pacher, P. & Haskó, G. Ectonucleotidases in inflammation, immunity, and cancer. J. Immunol. 206, 1983–1990 (2021).
Chaurasia, S. N., Kushwaha, G., Pandey, A. & Dash, D. Human platelets express functional ectonucleotidases that restrict platelet activation signaling. Biochem. Biophys. Res. Commun. 527, 104–109 (2020).
Hesse, J. et al. Profound inhibition of CD73-dependent formation of anti-inflammatory adenosine in B cells of SLE patients. eBioMedicine 73, 103616 (2021).
Han, L. et al. Phenotypical analysis of ectoenzymes CD39/CD73 and adenosine receptor 2A in CD4+CD25highFoxp3+ regulatory T-cells in psoriasis. Australas. J. Dermatol. 59, e31–e38 (2018).
Fuentes, E., Moore-Carrasco, R., de Andrade Paes, A. M. & Trostchansky, A. Role of platelet activation and oxidative stress in the evolution of myocardial infarction. J. Cardiovasc. Pharmacol. Ther. 24, 509–520 (2019).
Praticò, D., Iuliano, L., Ghiselli, A., Alessandri, C. & Violi, F. Hydrogen peroxide as trigger of platelet aggregation. Haemostasis 21, 169–174 (1991).
Polidoro, L. et al. Platelet activation in patients with the Raynaud phenomenon. Intern. Med. J. 42, 531–535 (2012).
Pamuk, G. E. et al. Increased circulating platelet–leucocyte complexes in patients with primary Raynaudʼs phenomenon and Raynaudʼs phenomenon secondary to systemic sclerosis: a comparative study. Blood Coagul. Fibrinolysis 18, 297–302 (2007).
Estevez, B. & Du, X. New concepts and mechanisms of platelet activation signaling. Physiology 32, 162–177 (2017).
Petito, E. et al. A dichotomy in platelet activation: evidence of different functional platelet responses to inflammatory versus haemostatic stimuli. Thromb. Res. 172, 110–118 (2018).
Nagahama, M. et al. Platelet activation markers and soluble adhesion molecules in patients with systemic lupus erythematosus. Autoimmunity 33, 85–94 (2001).
Sheremata, W. A. et al. Evidence of platelet activation in multiple sclerosis. J. Neuroinflammation 5, 27 (2008).
Miao, D., Li, D.-Y., Chen, M. & Zhao, M.-H. Platelets are activated in ANCA-associated vasculitis via thrombin-PARs pathway and can activate the alternative complement pathway. Arthritis Res. Ther. 19, 252 (2017).
Collins, C. E., Cahill, M. R., Newland, A. C. & Rampton, D. S. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology 106, 840–845 (1994).
Linge, P., Fortin, P. R., Lood, C., Bengtsson, A. A. & Boilard, E. The non-haemostatic role of platelets in systemic lupus erythematosus. Nat. Rev. Rheumatol. 14, 195–213 (2018).
Cloutier, N. et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: the microparticle-associated immune complexes: immune complexes contain microparticles. EMBO Mol. Med. 5, 235–249 (2013).
Boilard, E., Blanco, P. & Nigrovic, P. A. Platelets: active players in the pathogenesis of arthritis and SLE. Nat. Rev. Rheumatol. 8, 534–542 (2012).
Nhek, S. et al. Activated platelets induce endothelial cell activation via an interleukin-1β pathway in systemic lupus erythematosus. Arterioscler. Thromb. Vasc. Biol. 37, 707–716 (2017).
Adnot, S., Houssaini, A., Abid, S., Marcos, E. & Amsellem, V. Serotonin transporter and serotonin receptors. Handb. Exp. Pharmacol. 218, 365–380 (2013).
Cloutier, N. et al. Platelets can enhance vascular permeability. Blood 120, 1334–1343 (2012).
Duerschmied, D. et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 121, 1008–1015 (2013).
Leon-Ponte, M., Ahern, G. P. & O’Connell, P. J. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood 109, 3139–3146 (2007).
Yin, J., Albert, R. H., Tretiakova, A. P. & Jameson, B. A. 5-HT1B receptors play a prominent role in the proliferation of T-lymphocytes. J. Neuroimmunol. 181, 68–81 (2006).
Bakogiannis, C., Sachse, M., Stamatelopoulos, K. & Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 122, 154157 (2019).
Du, Y. et al. Chemokines form nanoparticles with DNA and can superinduce TLR-driven immune inflammation. J. Exp. Med. 219, e20212142 (2022).
Krijgsveld, J. et al. Thrombocidins, microbicidal proteins from human blood platelets, are C-terminal deletion products of CXC chemokines. J. Biol. Chem. 275, 20374–20381 (2000).
Valle-Jiménez, X. et al. Human platelets and megakaryocytes express defensin alpha 1. Platelets 31, 344–354 (2020).
Speth, C. et al. Complement and platelets: mutual interference in the immune network. Mol. Immunol. 67, 108–118 (2015).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Mariani, A. et al. Serum calprotectin: review of its usefulness and validity in paediatric rheumatic diseases. Clin. Exp. Rheumatol. 33, 109–114 (2015).
Guo, L. et al. Increased platelet S100A8/S100A9 associated with vasculitis in granulomatosis with polyangiitis (GPA). Blood 138, 3142 (2021).
Lood, C. et al. Platelet-derived S100A8/A9 and cardiovascular disease in systemic lupus erythematosus. Arthritis Rheumatol. 68, 1970–1980 (2016).
Song, N.-P. et al. Plasma calprotectin was associated with platelet activation and no-reflow phenomenon in acute coronary syndrome. BMC Cardiovasc. Disord. 20, 443 (2020).
Guo, Q. et al. Induction of alarmin S100A8/A9 mediates activation of aberrant neutrophils in the pathogenesis of COVID-19. Cell Host Microbe 29, 222–235.e4 (2021).
Colicchia, M. et al. S100A8/A9 drives the formation of procoagulant platelets through GPIbα. Blood https://doi.org/10.1182/blood.2021014966 (2022).
Zhou, H. et al. Platelet HMGB1 is required for efficient bacterial clearance in intra-abdominal bacterial sepsis in mice. Blood Adv. 2, 638–648 (2018).
Maugeri, N. et al. Circulating platelets as a source of the damage-associated molecular pattern HMGB1 in patients with systemic sclerosis. Autoimmunity 45, 584–587 (2012).
Yang, B., Liu, X. & Mei, Q. HMGB1-positive platelet microparticles may be a biomarker of inflammatory bowel disease. Inflamm. Bowel Dis. 26, e10 (2020).
Stark, K. et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 128, 2435–2449 (2016).
Kwak, M. S. et al. Immunological significance of HMGB1 post-translational modification and redox biology. Front. Immunol. 11, 1189 (2020).
Taniguchi, N. et al. High mobility group box chromosomal protein 1 plays a role in the pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis Rheum. 48, 971–981 (2003).
Feng, X. et al. HMGB1 protein promotes glomerular mesangial matrix deposition via TLR2 in lupus nephritis. J. Cell. Physiol. 235, 5111–5119 (2020).
Ek, M., Popovic, K., Harris, H. E., Nauclér, C. S. & Wahren-Herlenius, M. Increased extracellular levels of the novel proinflammatory cytokine high mobility group box chromosomal protein 1 in minor salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 54, 2289–2294 (2006).
Kao, K., Cook, D. & Scornik, J. Quantitative analysis of platelet surface HLA by W6/32 anti-HLA monoclonal antibody. Blood 68, 627–632 (1986).
Chapman, L. M. et al. Platelets present antigen in the context of MHC class I. J. Immunol. 189, 916–923 (2012).
Marcoux, G. et al. Platelet EVs contain an active proteasome involved in protein processing for antigen presentation via MHC-I molecules. Blood 138, 2607–2620 (2021).
Zufferey, A. et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 1, 1773–1785 (2017).
Pariser, D. N. et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Invest. 131, e137377 (2021).
Maître, B. et al. Immature myeloid dendritic cells capture and remove activated platelets from preformed aggregates. J. Thromb. Haemost. 8, 2262–2272 (2010).
Coenen, D. M., Mastenbroek, T. G. & Cosemans, J. M. E. M. Platelet interaction with activated endothelium: mechanistic insights from microfluidics. Blood 130, 2819–2828 (2017).
Hottz, E. D. et al. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood 122, 3405–3414 (2013).
Truchetet, M.-E. et al. Platelets induce thymic stromal lymphopoietin production by endothelial cells: contribution to fibrosis in human systemic sclerosis. Arthritis Rheumatol. 68, 2784–2794 (2016).
Maugeri, N. et al. Platelet microparticles sustain autophagy-associated activation of neutrophils in systemic sclerosis. Sci. Transl. Med. 10, eaao3089 (2018).
Aslam, R., Speck, E. R., Kim, M., Freedman, J. & Semple, J. W. Transfusion-related immunomodulation by platelets is dependent on their expression of MHC class I molecules and is independent of white cells. Transfusion 48, 1778–1786 (2008).
Malý, P. et al. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86, 643–653 (1996).
Polanowska-Grabowska, R. et al. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler. Thromb. Vasc. Biol. 30, 2392–2399 (2010).
Ehlers, R. et al. Targeting platelet–leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibα. J. Exp. Med. 198, 1077–1088 (2003).
Li, N. et al. Platelet-leukocyte cross talk in whole blood. Arterioscler. Thromb. Vasc. Biol. 20, 2702–2708 (2000).
Schuhmann, M. K. et al. CD84 links T cell and platelet activity in cerebral thrombo-inflammation in acute stroke. Circ. Res. 127, 1023–1035 (2020).
Joseph, J. E., Harrison, P., Mackie, I. J., Isenberg, D. A. & Machin, S. J. Increased circulating platelet–leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br. J. Haematol. 115, 451–459 (2001).
Irving, P. M. et al. Formation of platelet-leukocyte aggregates in inflammatory bowel disease. Inflamm. Bowel Dis. 10, 361–372 (2004).
Popp, S. K. et al. Circulating platelet-neutrophil aggregates characterize the development of type 1 diabetes in humans and NOD mice. JCI Insight 7, e153993 (2022).
Sellam, J. et al. Increased levels of circulating microparticles in primary Sjögren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 11, R156 (2009).
Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. Science 346, 1234–1238 (2014). This study shows that neutrophils scan the circulation for activated platelets through the P-selectin–PSGL1 axis and that this outside-in signalling promotes neutrophil migration and tissue injury in a mouse model of inflammation.
Yeo, E. L., Sheppard, J.-A. I. & Feuerstein, I. A. Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model). Blood 83, 2498–2507 (1994).
Schmitt-Sody, M. et al. Platelet P-selectin is significantly involved in leukocyte-endothelial cell interaction in murine antigen-induced arthritis. Platelets 18, 365–372 (2007).
Elzey, B. D. et al. Platelet-mediated modulation of adaptive immunity: a communication link between innate and adaptive immune compartments. Immunity 19, 9–19 (2003).
Nakabo, S., Romo-Tena, J. & Kaplan, M. J. Neutrophils as drivers of immune dysregulation in autoimmune diseases with skin manifestations. J. Invest. Dermatol. 142, 823–833 (2022).
Wigerblad, G. & Kaplan, M. J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-022-00787-0 (2022).
Li, P. et al. Glutathione peroxidase 4–regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 22, 1107–1117 (2021).
Etulain, J. et al. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 126, 242–246 (2015).
Panicker, S. R. et al. Circulating soluble P-selectin must dimerize to promote inflammation and coagulation in mice. Blood 130, 181–191 (2017).
Nayak, L. et al. A targetable pathway in neutrophils mitigates both arterial and venous thrombosis. Sci. Transl. Med. 14, eabj7465 (2022).
Gros, A. et al. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex–mediated inflammation in mice. Blood 126, 1017–1026 (2015).
Sozzani, S., Del Prete, A. & Bosisio, D. Dendritic cell recruitment and activation in autoimmunity. J. Autoimmun. 85, 126–140 (2017).
Han, P. et al. Platelet P-selectin initiates cross-presentation and dendritic cell differentiation in blood monocytes. Sci. Adv. 6, eaaz1580 (2020). This article shows that platelet P-selectin induces the maturation of monocytes to proinflammatory DCs with increased MHC class II and co-stimulatory molecule expression.
Li, T. et al. Platelets mediate inflammatory monocyte activation by SARS-CoV-2 spike protein. J. Clin. Invest. 132, e150101 (2021).
Saris, A. et al. Inhibition of dendritic cell activation and modulation of T cell polarization by the platelet secretome. Front. Immunol. 12, 631285 (2021).
Kral, J. B., Schrottmaier, W. C., Salzmann, M. & Assinger, A. Platelet interaction with innate immune cells. Transfus. Med. Hemother. 43, 78–88 (2016).
Semple, J. W. & Kapur, R. Platelet immunology from the inside out. ISBT Sci. Ser. 15, 315–319 (2020).
Chen, X. et al. The phosphatase PTEN links platelets with immune regulatory functions of mouse T follicular helper cells. Nat. Commun. 13, 2762 (2022).
Zamora, C. et al. Association of platelet binding to lymphocytes with B cell abnormalities and clinical manifestations in systemic lupus erythematosus. Mediators Inflamm. 2019, e2473164 (2019).
Cognasse, F. et al. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp. Hematol. 35, 1376–1387 (2007).
Viallard, J.-F. et al. Increased soluble and platelet-associated CD40 ligand in essential thrombocythemia and reactive thrombocytosis. Blood 99, 2612–2614 (2002).
Solanilla, A. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood 105, 215–218 (2005).
Sonia D’Souza, C. et al. Platelets drive inflammation and target gray matter and the retina in autoimmune-mediated encephalomyelitis. J. Neuropathol. Exp. Neurol. 77, 567–576 (2018). This study reports that platelets are required for T cell infiltration and, ultimately, tissue damage in a mouse model of inflammatory CNS disease.
Schleicher, R. I. et al. Platelets induce apoptosis via membrane-bound FasL. Blood 126, 1483–1493 (2015).
Kocovski, P. et al. Platelet depletion is effective in ameliorating anxiety-like behavior and reducing the pro-inflammatory environment in the hippocampus in murine experimental autoimmune encephalomyelitis. J. Clin. Med. 8, 162 (2019).
Kronbichler, A. & Mayer, G. Renal involvement in autoimmune connective tissue diseases. BMC Med. 11, 95 (2013).
Ståhl, A. et al. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets via TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood 108, 167–176 (2006).
Kuligowski, M. P., Kitching, A. R. & Hickey, M. J. Leukocyte recruitment to the inflamed glomerulus: a critical role for platelet-derived P-selectin in the absence of rolling. J. Immunol. 176, 6991–6999 (2006).
Finsterbusch, M., Norman, M. U., Hall, P., Kitching, A. R. & Hickey, M. J. Platelet retention in inflamed glomeruli occurs via selective prolongation of interactions with immune cells. Kidney Int. 95, 363–374 (2019).
Dewitte, A. et al. CD154 Induces interleukin-6 secretion by kidney tubular epithelial cells under hypoxic conditions: inhibition by chloroquine. Mediators Inflamm. 2020, e6357046 (2020).
Rigothier, C. et al. CD154 induces matrix metalloproteinase-9 secretion in human podocytes. J. Cell. Biochem. 117, 2737–2747 (2016).
Tanaka, T. Human platelets stimulate mesangial cells to produce monocyte chemoattractant protein-1 via the CD40/CD40 ligand pathway and may amplify glomerular injury. J. Am. Soc. Nephrol. 13, 2488–2496 (2002).
Yuan, Y. et al. Excessive activation of the TLR9/TGF-β1/PDGF-B pathway in the peripheral blood of patients with systemic lupus erythematosus. Arthritis Res. Ther. 19, 70 (2017).
Meng, X., Nikolic-Paterson, D. J. & Lan, H. Y. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325–338 (2016).
Christmann, R. B. et al. Association of interferon- and transforming growth factor β-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol. 66, 714–725 (2014).
Solé, C., Gimenez-Barcons, M., Ferrer, B., Ordi-Ros, J. & Cortés-Hernández, J. Microarray study reveals a transforming growth factor-β-dependent mechanism of fibrosis in discoid lupus erythematosus. Br. J. Dermatol. 175, 302–313 (2016).
Sagonas, I. & Daoussis, D. Serotonin and systemic sclerosis. An emerging player in pathogenesis. Jt. Bone Spine 89, 105309 (2022).
Kowal-Bielecka, O. et al. Beta thromboglobulin and platelet factor 4 in bronchoalveolar lavage fluid of patients with systemic sclerosis. Ann. Rheum. Dis. 64, 484–486 (2005).
Rossaint, J. et al. Platelets orchestrate the resolution of pulmonary inflammation in mice by T reg cell repositioning and macrophage education. J. Exp. Med. 218, e20201353 (2021).
Bonaventura, A. et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 21, 319–329 (2021).
Berger, J. S. et al. Effect of P2Y12 inhibitors on survival free of organ support among non–critically ill hospitalized patients with COVID-19: a randomized clinical trial. JAMA 327, 227 (2022).
Hadjadj, J. et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid-19 patients. Science 369, 718–724 (2020).
Vial, G. et al. The impact of clopidogrel on plasma-soluble CD40 ligand levels in systemic lupus erythematosus patients: the CLOPUS phase I/II pilot study. Jt. Bone Spine 88, 105097 (2021).
Parker, W. A. E. et al. Aspirin, clopidogrel and prasugrel monotherapy in patients with type 2 diabetes mellitus: a double-blind randomised controlled trial of the effects on thrombotic markers and microRNA levels. Cardiovasc. Diabetol. 19, 3 (2020).
Avalos, I. et al. Aspirin therapy and thromboxane biosynthesis in systemic lupus erythematosus. Lupus 16, 981–986 (2007).
Shock, A., Humphreys, D. & Nimmerjahn, F. Dissecting the mechanism of action of intravenous immunoglobulin in human autoimmune disease: lessons from therapeutic modalities targeting Fcγ receptors. J. Allergy Clin. Immunol. 146, 492–500 (2020).
Arman, M. & Krauel, K. Human platelet IgG Fc receptor FcγRIIA in immunity and thrombosis. J. Thromb. Haemost. 13, 893–908 (2015).
Richez, C. et al. Practical management of patients on hydroxychloroquine. Jt. Bone Spine 88, 105316 (2021).
Cornwell, M. G. et al. Hydroxychloroquine is associated with lower platelet activity and improved vascular health in systemic lupus erythematosus. Lupus Sci. Med. 8, e000475 (2021).
Ruiz-Irastorza, G. et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 15, 577–583 (2006).
Neumüller, J., Tohidast-Akrad, M., Fischer, M. & Mayr, W. R. Influence of chloroquine or acid treatment of human platelets on the antigenicity of HLA and the ‘thrombocyte-specific’ glycoproteins Ia/IIa, IIb, and IIb/IIIa. Vox Sang. 65, 223–231 (1993).
Morel, L. Immunometabolism in systemic lupus erythematosus. Nat. Rev. Rheumatol. 13, 280–290 (2017).
Yin, Y. et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci. Transl. Med. 7, 274ra18 (2015).
Sun, F. et al. Safety and efficacy of metformin in systemic lupus erythematosus: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Rheumatol. 2, e210–e216 (2020).
Negrotto, L., Farez, M. F. & Correale, J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA Neurol. 73, 520–528 (2016).
Xin, G. et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Sci. Rep. 6, 36222 (2016).
Lu, D.-Y. et al. Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. BMC Cardiovasc. Disord. 14, 187 (2014).
Xu, H. et al. Colchicine for secondary prevention of coronary artery disease: a meta-analysis of randomised controlled trials. Heart Lung Circ. 31, 685–695 (2022).
Shah, B. et al. Effect of colchicine on platelet-platelet and platelet-leukocyte interactions: a pilot study in healthy subjects. Inflammation 39, 182–189 (2016).
Ito, M. et al. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 6, 7360 (2015).
Ataga, K. I. et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N. Engl. J. Med. 376, 429–439 (2017).
Karki, N. R., Saunders, K. & Kutlar, A. A critical evaluation of crizanlizumab for the treatment of sickle cell disease. Expert. Rev. Hematol. 15, 5–13 (2022).
Zhang, L. et al. P-selectin blockade ameliorates lupus nephritis in MRL/lpr mice through improving renal hypoxia and evaluation using BOLD-MRI. J. Transl. Med. 18, 116 (2020).
Wong, D. J. et al. A PSGL-1 glycomimetic reduces thrombus burden without affecting hemostasis. Blood 138, 1182–1193 (2021).
Furie, R. A. et al. Phase 2, randomized, placebo-controlled trial of dapirolizumab pegol in patients with moderate-to-severe active systemic lupus erythematosus. Rheumatology 60, 5397–5407 (2021).
Jayne, D. et al. POS0687 a randomised dose ranging, placebo-controlled, phase II study assessing the efficacy and safety of BI 655064, an antagonistic anti-CD40 antibody, in patients with lupus nephritis. Ann. Rheum. Dis. 80, 589–590 (2021).
Andrianova, I. A. et al. In systemic lupus erythematosus anti-dsDNA antibodies can promote thrombosis through direct platelet activation. J. Autoimmun. 107, 102355 (2020).
Roberts, D. E., McNicol, A. & Bose, R. Mechanism of collagen activation in human platelets. J. Biol. Chem. 279, 19421–19430 (2004).
Eckly, A. et al. Megakaryocytes use in vivo podosome-like structures working collectively to penetrate the endothelial barrier of bone marrow sinusoids. J. Thromb. Haemost. 18, 2987–3001 (2020).
Lefrançais, E. et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109 (2017).
Valet, C. et al. Sepsis promotes splenic production of a protective platelet pool with high CD40 ligand expression. J. Clin. Invest. 132, e153920 (2022).
Sun, S. et al. Single-cell analysis of ploidy and the transcriptome reveals functional and spatial divergency in murine megakaryopoiesis. Blood 138, 1211–1224 (2021).
Zhang, W. et al. Global characterization of megakaryocytes in bone marrow, peripheral blood, and cord blood by single-cell RNA sequencing. Cancer Gene Ther. 29, 1636–1647 (2022).
Lood, C. et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood 116, 1951–1957 (2010).
Acknowledgements
M.S. is supported by academic sources including the Société Française de Rhumatologie, the Institut pour la Santé et la Recherche Médicale and the Bettencourt-Schueller Fondation. E.B. is recipient of an award from the Fonds de Recherche en Santé du Québec. P.B. is supported by the Fondation pour la Recherche Médicale, Société Française de Rhumatologie, Foundation for Research in Rheumatology (FOREUM) and Fondation Arthritis.
Author information
Authors and Affiliations
Contributions
M.S. wrote the first draft and conceptualized the figures. E.B., G.C.T., C.R. and P.B. edited the manuscript and suggested additions. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
C.R. has received consulting or speaker fees from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Biogen, Eli Lilly, GlaxoSmithKline, Janssen Novartis and Pfizer and grants from Biogen, Eli Lilly and Nordic Pharma, all unrelated to this work. M.S. has received consulting fees from Sandoz, Amgen and Nordic Pharma, all unrelated to this work. The other authors report no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks S. Massberg, J. Semple, S. Vogel and A. Zarbock for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Antineutrophil cytoplasmic antibody
-
A type of autoantibody that targets neutrophil components and is characteristic of antineutrophil cytoplasmic antibody-associated vasculitis.
- Antiphospholipid syndrome
-
(APS). An autoimmune disorder characterized by antiphospholipid antibodies responsible for arterial and venous thrombosis and obstetric issues.
- Clopidogrel
-
An anti-aggregation therapy that targets the platelet purine receptor P2Y12, which is widely prescribed for the prevention of cardiovascular disease in patients at high risk (such as patients with a history of myocardial infarction).
- Disseminated intravascular coagulation
-
A critical medical condition characterized by widespread and dysregulated clotting throughout the blood vessels.
- Extracellular vesicles
-
Small (10–1,000 nm) vesicles surrounded by a lipid bilayer that are released from a cell (often after activation) and that contain soluble factors and surface molecules from that cell.
- Ferroptosis
-
A form of programmed cell death that is dependent on intracellular iron, characterized by the intracellular accumulation of lipid peroxides.
- Immune thrombocytopenia
-
(ITP). An autoimmune disorder characterized by severe thrombocytopenia secondary to immune destruction of platelets that is responsible for increased bleeding risk.
- Inflammatory bowel disease
-
(IBD). A group of immune-mediated inflammatory diseases such as Crohn’s disease or ulcerative colitis characterized by inflammation of the bowel wall.
- Ischaemia–reperfusion injury
-
A condition in which tissue damage occurs after blood flow is restored to an ischaemic tissue owing to the local release of toxic factors after reperfusion.
- Mesangial cells
-
Mesenchymal cells of the kidney that are responsible for the architecture of the glomeruli.
- NETosis
-
A form of programmed cell death by which neutrophils release the contents of their cytoplasm and nucleus (including DNA and histones) into the extracellular milieu, often in the context of infection or autoimmunity.
- Primary haemostasis
-
The primary response to ensure blood vessel integrity upon injury, which involves platelets, subendothelial matrix tissue factor and circulating fibrinogen.
- Podocytes
-
Specialized epithelial cells that form the finely fenestrated lining of the kidney glomeruli and are responsible for blood filtration with minimal loss of protein in the urine.
- Psoriasis
-
A frequent skin inflammatory disease characterized by widespread scaly and itchy patches, which can be associated with inflammatory arthritis (psoriatic arthritis).
- Raynaud phenomenon
-
A condition in which the blood vessels in the extremities constrict upon exposure to cold, resulting in skin colour changes (typically white, blue then red) and pain. The condition may be benign (Raynaud disease) or associated with an autoimmune disease (secondary Raynaud phenomenon).
- Rheumatoid arthritis
-
A non-rare (prevalence of 0.5% in high-income countries) autoimmune disease characterized by widespread inflammation and destruction of joints, as well as involvement of other organs (lungs and eyes).
- Sjögren syndrome
-
A connective tissue disorder characterized by the involvement of exocrine glands, causing dry eyes and/or mouth, as well as systemic symptoms.
- Systemic lupus erythematosus
-
(SLE). A prototypical systemic autoimmune disease characterized by the production of antibodies to DNA and the involvement of a wide range of organs.
- Systemic sclerosis
-
A connective tissue disorder characterized by vasculopathy, autoimmunity and excessive fibrosis.
- von Willebrand factor
-
A multimeric blood glycoprotein involved in platelet adhesion to a wound and which is necessary for the initial steps of blood clotting.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Scherlinger, M., Richez, C., Tsokos, G.C. et al. The role of platelets in immune-mediated inflammatory diseases. Nat Rev Immunol 23, 495–510 (2023). https://doi.org/10.1038/s41577-023-00834-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-023-00834-4
This article is cited by
-
Impact of an eight-week isocaloric vegan dietary intervention on hemogram parameters and lymphocyte subsets: a randomized-controlled trial
BMC Medicine (2026)
-
Platelet-derived non-coding RNAs as emerging contributors to autoimmune inflammation
Naunyn-Schmiedeberg's Archives of Pharmacology (2026)
-
Explainable AI-based analysis of human pancreas sections identifies traits of type 2 diabetes
Nature Communications (2026)
-
Platelet glycoprotein VI promotes folic acid-induced acute kidney injury through interaction with tubular epithelial cell-derived galectin-3
Cell Communication and Signaling (2025)
-
Association of non-insulin-based insulin resistance indices, mean platelet volume and prostate cancer: a cross-sectional study
BMC Cancer (2025)