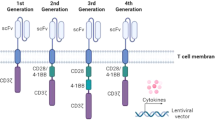
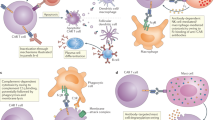
Abstract
Engineered T cells that express chimeric antigen receptors (CARs) have transformed the treatment of haematological cancers. CARs combine the tumour-antigen-binding function of antibodies with the signalling functions of the T cell receptor (TCR) ζ chain and co-stimulatory receptors. The resulting constructs aim to mimic the TCR-based and co-receptor-based activation of T cells. Although these have been successful for some types of cancer, new CAR formats are needed, to limit side effects and broaden their use to solid cancers. Insights into the mechanisms of TCR signalling, including the identification of signalling motifs that are not present in the TCR ζ chain and mechanistic insights in TCR activation, have enabled the development of CAR formats that outcompete the current CARs in preclinical mouse models and clinical trials. In this Perspective, we explore the mechanistic rationale behind new CAR designs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dong, D. et al. Structural basis of assembly of the human T cell receptor–CD3 complex. Nature 573, 546–552 (2019).
Chen, Y. et al. Cholesterol inhibits TCR signaling by directly restricting TCR–CD3 core tunnel motility. Mol. Cell 82, 1278–1287.e5 (2022).
Reth, M. Antigen receptor tail clue. Nature 338, 383–384 (1989).
Love, P. E. & Hayes, S. M. ITAM-mediated signaling by the T-cell antigen receptor. Cold Spring Harb. Persp. Biol. 2, a002485 (2010).
Pitcher, L. A. & van Oers, N. S. C. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 24, 554–560 (2003).
Letourneur, F. & Klausner, R. D. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor ζ family proteins. Proc. Natl Acad. Sci. USA 88, 8905–8909 (1991).
Irving, B. A. & Weiss, A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64, 891–901 (1991).
Romeo, C. & Seed, B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell 64, 1037–1046 (1991).
Rafiq, S., Hackett, C. S. & Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 17, 147–167 (2020).
Majzner, R. G. et al. GD2–CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Huang, J., Huang, X. & Huang, J. CAR-T cell therapy for hematological malignancies: limitations and optimization strategies. Front. Immunol. 13, 1019115 (2022).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 22, 85–96 (2022).
Swamy, M. et al. A cholesterol-based allostery model of T cell receptor phosphorylation. Immunity 44, 1091–1101 (2016).
Courtney, A. H., Lo, W. L. & Weiss, A. TCR signaling: mechanisms of initiation and propagation. Trends Biochem. Sci. 43, 108–123 (2018).
Aivazian, D. & Stern, L. J. Phosphorylation of T cell receptor ζ is regulated by a lipid dependent folding transition. Nat. Struct. Biol. 7, 1023–1026 (2000).
Zhang, H., Cordoba, S.-P., Dushek, O. & van der Merwe, P. A. Basic residues in the T-cell receptor ζ cytoplasmic domain mediate membrane association and modulate signaling. Proc. Natl Acad. Sci. USA 108, 19323–19328 (2011).
Xu, C. et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3ε cytoplasmic tyrosine-based motif. Cell 135, 702–713 (2008).
Li, L. et al. Ionic CD3–Lck interaction regulates the initiation of T-cell receptor signaling. Proc. Natl Acad. Sci. USA 114, E5891–E5899 (2017).
Wu, W. et al. Multiple signaling roles of CD3ε and its application in CAR-T cell therapy. Cell 182, 855–871.e23 (2020).
von Essen, M. et al. The CD3 γ leucine-based receptor-sorting motif is required for efficient ligand-mediated TCR down-regulation. J. Immunol. 168, 4519–4523 (2002).
Janeway, C. A. Ligands for the T-cell receptor: hard times for avidity models. Immunol. Today 16, 223–225 (1995).
Lever, M., Maini, P. K., van der Merwe, P. A. & Dushek, O. Phenotypic models of T cell activation. Nat. Rev. Immunol. 14, 619–629 (2014).
Schamel, W. W., Alarcon, B. & Minguet, S. The TCR is an allosterically regulated macromolecular machinery changing its conformation while working. Immunol. Rev. 291, 8–25 (2019).
Minguet, S., Swamy, M., Alarcón, B., Luescher, I. F. & Schamel, W. W. A. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity 26, 43–54 (2007).
Cochran, J. R., Cameron, T. O. & Stern, L. J. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity 12, 241–250 (2000).
Boniface, J. J. et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands [corrected]. Immunity 9, 459–466 (1998).
Davis, S. J. & van der Merwe, P. A. The kinetic-segregation model: TCR triggering and beyond. Nat. Immunol. 7, 803–809 (2006).
Springer, T. A. Adhesion receptors of the immune system. Nature 346, 425–434 (1990).
Cordoba, S.-P. et al. The large ectodomains of CD45 and CD148 regulate their segregation from and inhibition of ligated T-cell receptor. Blood 121, 4295–4302 (2013).
Jung, Y., Wen, L., Altman, A. & Ley, K. CD45 pre-exclusion from the tips of T cell microvilli prior to antigen recognition. Nat. Commun. 12, 3872 (2021).
Acuto, O. T-cell virtuosity in “knowing thyself”. Front. Immunol. 15, 1343575 (2024).
Hartl, F. A. et al. Noncanonical binding of Lck to CD3ε promotes TCR signaling and CAR function. Nat. Immunol. 21, 902–913 (2020).
Gil, D., Schamel, W. W. A., Montoya, M., Sánchez-Madrid, F. & Alarcón, B. Recruitment of Nck by CD3ε reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell 109, 901–912 (2002).
Blanco, R., Borroto, A., Schamel, W., Pereira, P. & Alarcon, B. Conformational changes in the T cell receptor differentially determine T cell subset development in mice. Sci. Signal. 7, ra115 (2014).
Risueño, R. M., van Santen, H. M. & Alarcón, B. A conformational change senses the strength of T cell receptor–ligand interaction during thymic selection. Proc. Natl Acad. Sci. USA 103, 9625–9630 (2006).
Lee, M. S. et al. A mechanical switch couples T cell receptor triggering to the cytoplasmic juxtamembrane regions of CD3ζζ. Immunity 43, 227–239 (2015).
Sušac, L. et al. Structure of a fully assembled tumor-specific T cell receptor ligated by pMHC. Cell 185, 3201–3213.e19 (2022).
Notti, R. Q. et al. The resting state of the human T-cell receptor. Preprint at bioRxiv https://doi.org/10.1101/2023.08.22.554360 (2023).
van Eerden, F. J. et al. TCR binding to a peptide-MHC complex raises a drawbridge for CD3 cross-membrane signaling. preprint at bioRxiv https://doi.org/10.1101/2022.07.27.501668 (2022).
Molnár, E. et al. Cholesterol and sphingomyelin drive ligand-independent T-cell antigen receptor nanoclustering. J. Biol. Chem. 287, 42664–42674 (2012).
Pathan-Chhatbar, S. et al. Direct regulation of the T cell antigen receptor’s activity by cholesterol. Front. Cell Dev. Biol. 8, 615996 (2021).
Shi, X. et al. Ca2+ regulates T-cell receptor activation by modulating the charge property of lipids. Nature 493, 111–115 (2013).
Deford-Watts, L. M. et al. The cytoplasmic tail of the T cell receptor CD3ε subunit contains a phospholipid-binding motif that regulates T cell functions. J. Immunol. 183, 1055–1064 (2009).
Xu, X., Li, H. & Xu, C. Structural understanding of T cell receptor triggering. Cell. Mol. Immunol. 17, 193–202 (2020).
Hem, C. D. et al. T cell specific adaptor protein (TSAd) promotes interaction of Nck with Lck and SLP-76 in T cells. Cell. Commun. Signal. 13, 31 (2015).
Velasco Cárdenas, R. M.-H. et al. Harnessing CD3 diversity to optimize CAR T cells. Nat. Immunol. 24, 2135–2149 (2023).
Horkova, V. et al. Unique roles of co-receptor-bound LCK in helper and cytotoxic T cells. Nat. Immunol. 24, 174–185 (2023).
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Honikel, M. M. & Olejniczak, S. H. Co-stimulatory receptor signaling in CAR-T cells. Biomolecules 12, 1303 (2022).
Hege, K. M. et al. Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer. J. Immunother. Cancer 5, 22 (2017).
Till, B. G. et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 112, 2261–2271 (2008).
Brocker, T. Chimeric Fv-ζ or Fv-ε receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood 96, 1999–2001 (2000).
Asmamaw Dejenie, T. et al. Current updates on generations, approvals, and clinical trials of CAR T-cell therapy. Hum. Vaccin. Immunother. 18, 2114254 (2022).
Wang, H., Song, X., Shen, L., Wang, X. & Xu, C. Exploiting T cell signaling to optimize engineered T cell therapies. Trends Cancer 8, 123–134 (2022).
Ajina, A. & Maher, J. Strategies to address chimeric antigen receptor tonic signaling. Mol. Cancer Ther. 17, 1795–1815 (2018).
Salzer, B. et al. Engineering AvidCARs for combinatorial antigen recognition and reversible control of CAR function. Nat. Commun. 11, 4166 (2020).
Wang, H., Huang, Y. & Xu, C. Charging CAR by electrostatic power. Immunol. Rev. 320, 138–146 (2023).
Nieba, L., Honegger, A., Krebber, C. & Plückthun, A. Disrupting the hydrophobic patches at the antibody variable/constant domain interface: improved in vivo folding and physical characterization of an engineered scFv fragment. Protein Eng. 10, 435–444 (1997).
Atwell, J. L. et al. scFv multimers of the anti-neuraminidase antibody NC10: length of the linker between VH and VL domains dictates precisely the transition between diabodies and triabodies. Protein Eng. 12, 597–604 (1999).
Choudhuri, K., Wiseman, D., Brown, M. H., Gould, K. & van der Merwe, P. A. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature 436, 578–582 (2005).
Srivastava, S. & Riddell, S. R. Engineering CAR-T cells: design concepts. Trends Immunol. 36, 494–502 (2015).
Xiao, Q. et al. Size-dependent activation of CAR-T cells. Sci. Immunol. 7, eabl3995 (2022).
Filby, A. et al. Fyn regulates the duration of TCR engagement needed for commitment to effector function. J. Immunol. 179, 4635–4644 (2007).
Wu, L. et al. CD28–CAR-T cell activation through FYN kinase signaling rather than LCK enhances therapeutic performance. Cell. Rep. Med. 4, 100917 (2023).
Purbhoo, M. A., Irvine, D. J., Huppa, J. B. & Davis, M. M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 5, 524–530 (2004).
Irvine, D. J., Purbhoo, M. A., Krogsgaard, M. & Davis, M. M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Burton, J. et al. Inefficient exploitation of accessory receptors reduces the sensitivity of chimeric antigen receptors. Proc. Natl Acad. Sci. USA 120, e2216352120 (2023).
Gudipati, V. et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat. Immunol. 21, 848–856 (2020).
James, S. E. et al. Mathematical modeling of chimeric TCR triggering predicts the magnitude of target lysis and its impairment by TCR downmodulation. J. Immunol. 184, 4284–4294 (2010).
Harris, D. T. et al. Comparison of T cell activities mediated by human TCRs and CARs that use the same recognition domains. J. Immunol. 200, 1088–1100 (2018).
Mansilla-Soto, J. et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 28, 345–352 (2022).
Walker, A. J. et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 25, 2189–2201 (2017).
Salter, A. I. et al. Comparative analysis of TCR and CAR signaling informs CAR designs with superior antigen sensitivity and in vivo function. Sci. Signal. 14, eabe2606 (2021).
Harrer, D. C. et al. Fine-tuning the antigen sensitivity of CAR T cells: emerging strategies and current challenges. Front. Immunol. 14, 1321596 (2023).
Qian, D., Griswold-Prenner, I., Rosner, M. R. & Fitch, F. W. Multiple components of the T cell antigen receptor complex become tyrosine-phosphorylated upon activation. J. Biol. Chem. 268, 4488–4493 (1993).
Baniyash, M., Garcia-Morales, P., Luong, E., Samelson, L. E. & Klausner, R. D. The T cell antigen receptor ζ chain is tyrosine phosphorylated upon activation. J. Biol. Chem. 263, 18225–18230 (1988).
Chylek, L. A. et al. Phosphorylation site dynamics of early T-cell receptor signaling. PLoS ONE 9, e104240 (2014).
Holst, J. et al. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat. Immunol. 9, 658–666 (2008).
Pitcher, L. A. et al. The CD3 γε/δε signaling module provides normal T cell functions in the absence of the TCR ζ immunoreceptor tyrosine-based activation motifs. Eur. J. Immunol. 35, 3643–3654 (2005).
Bettini, M. L. et al. Cutting edge: CD3 ITAM diversity is required for optimal TCR signaling and thymocyte development. J. Immunol. 199, 1555–1560 (2017).
Osman, N., Turner, H., Lucas, S., Reif, K. & Cantrell, D. A. The protein interactions of the immunoglobulin receptor family tyrosine-based activation motifs present in the T cell receptor ζ subunits and the CD3 γ, δ and ε chains. Eur. J. Immunol. 26, 1063–1068 (1996).
Sunder-Plassmann, R., Lialios, F., Madsen, M., Koyasu, S. & Reinherz, E. L. Functional analysis of immunoreceptor tyrosine-based activation motif (ITAM)-mediated signal transduction: the two YxxL segments within a single CD3ζ-ITAM are functionally distinct. Eur. J. Immunol. 27, 2001–2009 (1997).
Guirado, M. et al. Phosphorylation of the N-terminal and C-terminal CD3-ε–ITAM tyrosines is differentially regulated in T cells. Biochem. Biophys. Res. Commun. 291, 574–581 (2002).
Paensuwan, P. et al. Nck binds to the T cell antigen receptor using its SH3.1 and SH2 domains in a cooperative manner, promoting TCR functioning. J. Immunol. 196, 448–458 (2016).
Kesti, T. et al. Reciprocal regulation of SH3 and SH2 domain binding via tyrosine phosphorylation of a common site in CD3ε. J. Immunol. 179, 878–885 (2007).
Zhao, Y. et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J. Immunol. 183, 5563–5574 (2009).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
James, J. R. Tuning ITAM multiplicity on T cell receptors can control potency and selectivity to ligand density. Sci. Signal. 11, eaan1088 (2018).
Majzner, R. G. et al. Tuning the antigen density requirement for CAR T-cell activity. Cancer Discov. 10, 702–723 (2020).
Feucht, J. et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 25, 82–88 (2019).
Kochenderfer, J. N., Yu, Z., Frasheri, D., Restifo, N. P. & Rosenberg, S. A. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116, 3875–3886 (2010).
Guo, X. et al. Lipid-dependent conformational dynamics underlie the functional versatility of T-cell receptor. Cell Res. 27, 505–525 (2017).
Gagnon, E., Schubert, D. A., Gordo, S., Chu, H. H. & Wucherpfennig, K. W. Local changes in lipid environment of TCR microclusters regulate membrane binding by the CD3ε cytoplasmic domain. J. Exp. Med. 209, 2423–2439 (2012).
Li, H., Yan, C., Guo, J. & Xu, C. Ionic protein–lipid interactions at the plasma membrane regulate the structure and function of immunoreceptors. Adv. Immunol. 144, 65–85 (2019).
Baeuerle, P. A. et al. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 10, 2087 (2019).
Gomes-Silva, D. et al. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell. Rep. 21, 17–26 (2017).
DeFord-Watts, L. M., Young, J. A., Pitcher, L. A. & van Oers, N. S. C. The membrane-proximal portion of CD3 ε associates with the serine/threonine kinase GRK2. J. Biol. Chem. 282, 16126–16134 (2007).
Dobbins, J. et al. Binding of the cytoplasmic domain of CD28 to the plasma membrane inhibits Lck recruitment and signaling. Sci. Signal. 9, ra75 (2016).
Yang, W. et al. Dynamic regulation of CD28 conformation and signaling by charged lipids and ions. Nat. Struct. Mol. Biol. 24, 1081–1092 (2017).
Hartl, F. A. et al. Cooperative interaction of nck and lck orchestrates optimal TCR signaling. Cells 10, 834 (2021).
Tailor, P. et al. The proline-rich sequence of CD3ε as an amplifier of low-avidity TCR signaling. J. Immunol. 181, 243–255 (2008).
Borroto, A. et al. First-in-class inhibitor of the T cell receptor for the treatment of autoimmune diseases. Sci. Transl. Med. 8, 370ra184 (2016).
Borroto, A. et al. Nck recruitment to the TCR required for ZAP70 activation during thymic development. J. Immunol. 190, 1103–1112 (2013).
Martin-Blanco, N. et al. CD3ε recruits Numb to promote TCR degradation. Int. Immunol. 28, 127–137 (2016).
Mingueneau, M. et al. The proline-rich sequence of CD3ε controls T cell antigen receptor expression on and signaling potency in preselection CD4+CD8+ thymocytes. Nat. Immunol. 9, 522–532 (2008).
Borroto, A. et al. Relevance of Nck–CD3ε interaction for T cell activation in vivo. J. Immunol. 192, 2042–2053 (2014).
Szymczak, A. L. et al. The CD3ε proline-rich sequence, and its interaction with Nck, is not required for T cell development and function. J. Immunol. 175, 270–275 (2005).
Göbel, T. W. & Dangy, J. P. Evidence for a stepwise evolution of the CD3 family. J. Immunol. 164, 879–883 (2000).
Dietrich, J., Hou, X., Wegener, A. M. & Geisler, C. CD3 γ contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 13, 2156–2166 (1994).
Boding, L. et al. TCR down-regulation controls T cell homeostasis. J. Immunol. 183, 4994–5005 (2009).
Bonefeld, C. M. et al. TCR down-regulation controls virus-specific CD8+ T cell responses. J. Immunol. 181, 7786–7799 (2008).
Kolanus, W., Romeo, C. & Seed, B. T cell activation by clustered tyrosine kinases. Cell 74, 171–183 (1993).
Fitzer-Attas, C. J., Schindler, D. G., Waks, T. & Eshhar, Z. Harnessing Syk family tyrosine kinases as signaling domains for chimeric single chain of the variable domain receptors: optimal design for T cell activation. J. Immunol. 160, 145–154 (1998).
Tousley, A. M. et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 615, 507–516 (2023).
Balagopalan, L. et al. Generation of antitumor chimeric antigen receptors incorporating T cell signaling motifs. Sci. Signal. 17, eadp8569 (2024).
Liu, Y. et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Sci. Transl. Med. 13, eabb5191 (2021).
Xu, Y. et al. A novel antibody-TCR (AbTCR) platform combines Fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 4, 62 (2018).
Helsen, C. W. et al. The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity. Nat. Commun. 9, 3049 (2018).
Schamel, W. W. A. et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J. Exp. Med. 202, 493–503 (2005).
Minguet, S. et al. The extracellular part of ζ is buried in the T cell antigen receptor complex. Immunol. Lett. 116, 203–210 (2008).
Ding, J. et al. Mesothelin-targeting T cells bearing a novel T cell receptor fusion construct (TRuC) exhibit potent antitumor efficacy against solid tumors. Oncoimmunology 12, 2182058 (2023).
Rana, J. et al. CAR- and TRuC-redirected regulatory T cells differ in capacity to control adaptive immunity to FVIII. Mol. Ther. 29, 2660–2676 (2021).
Sun, Y. et al. Chimeric anti-GPC3 sFv-CD3ε receptor-modified T cells with IL7 co-expression for the treatment of solid tumors. Mol. Ther. Oncolyt. 25, 160–173 (2022).
Zhang, Z. et al. Treating solid tumors with TCR-based chimeric antigen receptor targeting extra domain B-containing fibronectin. J. Immunother. Cancer 11, e007199 (2023).
Birtel, M. et al. A TCR-like CAR promotes sensitive antigen recognition and controlled T-cell expansion upon mRNA vaccination. Cancer Res. Commun. 2, 827–841 (2022).
Chen, Z. et al. Antibody-based binding domain fused to TCRγ chain facilitates T cell cytotoxicity for potent anti-tumor response. Oncogenesis 12, 33 (2023).
Lesch, S. et al. PD-1-CD28 fusion protein strengthens mesothelin-specific TRuC T cells in preclinical solid tumor models. Cell. Oncol. 46, 227–235 (2023).
Juraske, C. et al. Reprogramming of human γδ T cells by expression of an anti-CD19 TCR fusion construct (εTRuC) to enhance tumor killing. J. Leuk. Biol. 115, 293–305 (2024).
Li, C. et al. Novel CD19-specific γ/δ TCR-T cells in relapsed or refractory diffuse large B-cell lymphoma. J. Hematol. Oncol. 16, 5 (2023).
Kuwana, Y. et al. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 149, 960–968 (1987).
Wang, J. et al. A novel adoptive synthetic TCR and antigen receptor (STAR) T-Cell therapy for B-cell acute lymphoblastic leukemia. Am. J. Hematol. 97, 992–1004 (2022).
Hassan, R. et al. Mesothelin-targeting T cell receptor fusion construct cell therapy in refractory solid tumors: phase 1/2 trial interim results. Nat. Med. 29, 2099–2109 (2023).
He, P. et al. A novel antibody-TCR (AbTCR) T-cell therapy is safe and effective against CD19-positive relapsed/refractory B-cell lymphoma. J. Cancer Res. Clin. Oncol. 149, 2757–2769 (2023).
Singh, N., Perazzelli, J., Grupp, S. A. & Barrett, D. M. Early memory phenotypes drive T cell proliferation in patients with pediatric malignancies. Sci. Transl. Med. 8, 320ra3 (2016).
Fraietta, J. A. et al. Biomarkers of response to anti-CD19 chimeric antigen receptor (CAR) T-cell therapy in patients with chronic lymphocytic leukemia. Blood 128, 57–57 (2016).
Frigault, M. J. et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol. Res. 3, 356–367 (2015).
Feucht, J. & Sadelain, M. Function and evolution of the prototypic CD28ζ and 4-1BBζ chimeric antigen receptors. Immunooncol. Technol. 8, 2–11 (2020).
Chen, J. et al. Tuning charge density of chimeric antigen receptor optimizes tonic signaling and CAR-T cell fitness. Cell Res. 33, 341–354 (2023).
Sarén, T. et al. Complementarity-determining region clustering may cause CAR-T cell dysfunction. Nat. Commun. 14, 4732 (2023).
Hudecek, M. et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol. Res. 3, 125–135 (2015).
Hombach, A., Hombach, A. A. & Abken, H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 17, 1206–1213 (2010).
Watanabe, N. et al. Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology 5, e1253656 (2016).
Myers, D. R., Zikherman, J. & Roose, J. P. Tonic signals: why do lymphocytes bother? Trends Immunol. 38, 844–857 (2017).
Stefanová, I. et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat. Immunol. 4, 248–254 (2003).
Dustin, M. L. & Choudhuri, K. Signaling and polarized communication across the T cell immunological synapse. Annu. Rev. Cell Dev. Biol. 32, 303–325 (2016).
Davenport, A. J. et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc. Natl Acad. Sci. USA 115, E2068–E2076 (2018).
Gross, G., Waks, T. & Eshhar, Z. Expression of immunoglobulin-T-cell receptor 960 chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989).
Becker, M. L. et al. Expression of a hybrid immunoglobulin-T cell receptor protein in 963 transgenic mice. Cell 58, 911–921 (1989).
Goverman, J. et al. Chimeric immunoglobulin-T cell receptor proteins form functional 965 receptors: implications for T cell receptor complex formation and activation. Cell 60, 966 929–39 (1990).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of 968 cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding 969 domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993).
McGuinness, R. P. et al. Anti-tumor activity of human T cells expressing the CC49-972 ζ chimeric immune receptor. Hum. Gene Ther. 10, 165–173 (1999).
Maher, J., Brentjens, R. J., Gunset, G., Rivière, I. & Sadelain, M. Human T980 lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ 981 /CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen 977 receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–33 978 (2011).
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18, 676–684 (2004).
He, C. et al. CD19 CAR antigen engagement mechanisms and affinity tuning. Sci. Immunol. 8, eadf1426 (2023).
Ghorashian, S. et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat. Med. 25, 1408–1414 (2019).
Valitutti, S., Müller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature 375, 148–151 (1995).
Watanabe, K. et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 ζ chimeric antigen receptor-modified effector CD8+ T cells. J. Immunol. 194, 911–920 (2015).
Feng, Y. et al. Mechanosensing drives acuity of αβ T-cell recognition. Proc. Natl Acad. Sci. USA 114, E8204–E8213 (2017).
Acknowledgements
The research groups of S.M. and W.W.S. are supported by the German Research Foundation (DFG) under Germany’s Excellence Strategy (EXC-2189 project 390939984) and under the Excellence Initiative of the German Federal and State Governments (EXC-294) and in part by the Ministry for Science, Research and Arts of the State of Baden-Württemberg. Further support is given by the DFG under FOR2799 (SCHA976/8-2 to W.W.S. and MI1942/3-1 to S.M.), SFB1381 (project 403222702-A9 to W.W.S.), SFB1479 (project 441891347-P15 to S.M.), SFB1160 (project 256073931-B01 to S.M.), and projects MI1942/4-1 (project 501418856 to S.M.) and MI1942/5-1 (project 501436442 to S.M.).
Author information
Authors and Affiliations
Contributions
S.M. and W.W.S. contributed equally to all aspects of the article. All authors wrote, reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
S.M. and W.W.S. are patent holders on ‘Lck-binding motif in CD3e’ (European patent application number 20711971.0 and US patent application number 20230070126). M.V.M. is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital and the University of Pennsylvania (some licensed to Novartis); holds equity in 2Seventy Bio, Genocea, Oncternal and Neximmune; and serves on the board of directors of 2Seventy Bio. W.W.S. and M.V.M. have served as consultants for multiple companies involved in cell therapies. The interests of M.V.M. were reviewed and are managed by Massachusetts General Hospital, and Massachusetts General Brigham in accordance with their conflict-of-interest policies.
Peer review
Peer review information
Nature Reviews Immunology thanks Philip Anton van der Merwe, Daniel Abate-Daga and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Minguet, S., Maus, M.V. & Schamel, W.W. From TCR fundamental research to innovative chimeric antigen receptor design. Nat Rev Immunol 25, 212–224 (2025). https://doi.org/10.1038/s41577-024-01093-7
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41577-024-01093-7
This article is cited by
-
In vivo CAR engineering for immunotherapy
Nature Reviews Immunology (2025)
-
Bispecific chimeric T cell receptors tackle tumor heterogeneity
Nature Cancer (2025)