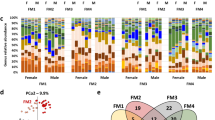
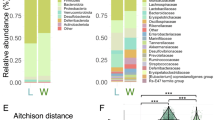
Abstract
Fundamental discoveries in many aspects of mammalian physiology have been made using laboratory mice as research models. These studies have been facilitated by the genetic tractability and inbreeding of such mice, the large set of immunological reagents that are available, and the establishment of environmentally controlled, high-throughput facilities. Such facilities typically include barriers to keep the mouse colonies free of pathogens and the frequent re-derivation of the mice severely limits their commensal flora. Because humans have co-evolved with microorganisms and are exposed to a variety of pathogens, a growing community of researchers posits that preclinical disease research can be improved by studying mice in the context of the microbiota and pathogens that they would encounter in the natural world. Here, we provide a perspective of how these different approaches can be combined and integrated to improve existing mouse models to enhance our understanding of disease mechanisms and develop new therapies for humans. We also propose that the term ‘mice with natural microbiota’ is more appropriate for describing these models than existing terms such as ‘dirty mice’.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dethlefsen, L., McFall-Ngai, M. & Relman, D. A. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449, 811–818 (2007).
Virgin, H. W., Wherry, E. J. & Ahmed, R. Redefining chronic viral infection. Cell 138, 30–50 (2009).
Welsh, R. M., Che, J. W., Brehm, M. A. & Selin, L. K. Heterologous immunity between viruses. Immunol. Rev. 235, 244–266 (2010).
Weldon, L. et al. The gut microbiota of wild mice. PLoS One 10, e0134643 (2015).
Abolins, S. et al. The comparative immunology of wild and laboratory mice, Mus musculus domesticus. Nat. Commun. 8, 14811 (2017).
Abolins, S. et al. The ecology of immune state in a wild mammal, Mus musculus domesticus. PLoS Biol. 16, e2003538 (2018).
Liu, Q. et al. Leveraging dirty mice that have microbial exposure to improve preclinical models of human immune status and disease. Nat. Immunol. 25, 947–950 (2024).
Suzuki, T. A. et al. Codiversification of gut microbiota with humans. Science 377, 1328–1332 (2022).
Sanders, J. G. et al. Widespread extinctions of co-diversified primate gut bacterial symbionts from humans. Nat. Microbiol. 8, 1039–1050 (2023).
Carter, M. M. et al. Ultra-deep sequencing of Hadza hunter-gatherers recovers vanishing gut microbes. Cell 186, 3111–3124.e13 (2023).
Vangay, P. et al. US immigration westernizes the human gut microbiome. Cell 175, 962–972.e10 (2018).
Rothschild, D. et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 (2018).
Vujkovic-Cvijin, I. et al. Host variables confound gut microbiota studies of human disease. Nature 587, 448–454 (2020).
Linz, B. et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 445, 915–918 (2007).
Stacy, A. et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 184, 615–627.e17 (2021).
Fenneman, A. C., Weidner, M., Chen, L. A., Nieuwdorp, M. & Blaser, M. J. Antibiotics in the pathogenesis of diabetes and inflammatory diseases of the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 20, 81–100 (2023).
Levin, D. et al. Diversity and functional landscapes in the microbiota of animals in the wild. Science 372, eabb5352 (2021).
Tung, J. et al. Social networks predict gut microbiome composition in wild baboons. eLife 4, e05224 (2015).
Sarkar, A. et al. Microbial transmission in the social microbiome and host health and disease. Cell 187, 17–43 (2024).
Marsh, K. J. et al. Synchronous seasonality in the gut microbiota of wild mouse populations. Front. Microbiol. 13, 809735 (2022).
Reese, A. T. et al. Effects of domestication on the gut microbiota parallel those of human industrialization. eLife 10, e60197 (2021).
Sheldon, B. C. & Verhulst, S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 (1996).
Babayan, S. A. et al. Wild immunology: converging on the real world. Ann. N. Y. Acad. Sci. 1236, 17–29 (2011).
Flies, A. S. & Wild Comparative Immunology Consortium. Rewilding immunology. Science 369, 37–38 (2020).
French, S. S., Johnston, G. I. H. & Moore, M. C. Immune activity suppresses reproduction in food-limited female tree lizards Urosaurus ornatus. Funct. Ecol. 21, 1115–1122 (2007).
Stockmaier, S., Dechmann, D. K., Page, R. A. & O’Mara, M. T. No fever and leucocytosis in response to a lipopolysaccharide challenge in an insectivorous bat. Biol. Lett. 11, 20150576 (2015).
Graham, A. L. et al. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science 330, 662–665 (2010).
Leivesley, J. A. et al. Survival costs of reproduction are mediated by parasite infection in wild Soay sheep. Ecol. Lett. 22, 1203–1213 (2019).
Viney, M., Lazarou, L. & Abolins, S. The laboratory mouse and wild immunology. Parasite Immunol. 37, 267–273 (2015).
Link, V. M. et al. Differential peripheral immune signatures elicited by vegan versus ketogenic diets in humans. Nat. Med. 30, 560–572 (2024).
Artwohl, J. E., Cera, L. M., Wright, M. F., Medina, L. V. & Kim, L. J. The efficacy of a dirty bedding sentinel system for detecting Sendai virus infection in mice: a comparison of clinical signs and seroconversion. Lab. Anim. Sci. 44, 73–75 (1994).
Graham, A. L. Naturalizing mouse models for immunology. Nat. Immunol. 22, 111–117 (2021).
Downie, A. E. et al. Spatiotemporal-social association predicts immunological similarity in rewilded mice. Sci. Adv. 9, eadh8310 (2023).
Chen, Y. H. et al. Rewilding of laboratory mice enhances granulopoiesis and immunity through intestinal fungal colonization. Sci. Immunol. 8, eadd6910 (2023).
Arnesen, H. et al. Naturalizing laboratory mice by housing in a farmyard-type habitat confers protection against colorectal carcinogenesis. Gut Microbes 13, 1993581 (2021).
Arnesen, H. et al. Microbial experience through housing in a farmyard-type environment alters intestinal barrier properties in mouse colons. Sci. Rep. 13, 13701 (2023).
Arnesen, H. et al. A model system for feralizing laboratory mice in large farmyard-like pens. Front. Microbiol. 11, 615661 (2020).
Sanders, A. E. et al. Comparison of mouse models of microbial experience reveals differences in microbial diversity and response to vaccination. mSphere 9, e0065423 (2024).
Rosshart, S. P. et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 171, 1015–1028.e3 (2017).
Rosshart, S. P. et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365, eaaw4361 (2019).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Pierson, M., Merley, A. & Hamilton, S. E. Generating mice with diverse microbial experience. Curr. Protoc. 1, e53 (2021).
Burger, S. et al. Natural microbial exposure from the earliest natural time point enhances immune development by expanding immune cell progenitors and mature immune cells. J. Immunol. 210, 1740–1751 (2023).
Tabilas, C. et al. Early microbial exposure shapes adult immunity by altering CD8+ T cell development. Proc. Natl Acad. Sci. USA 119, e2212548119 (2022).
Chandra, S. et al. Transcriptomes and metabolism define mouse and human MAIT cell populations. Sci. Immunol. 8, eabn8531 (2023).
Fay, E. J. et al. Natural rodent model of viral transmission reveals biological features of virus population dynamics. J. Exp. Med. 219, e20211220 (2022).
Hild, B. et al. Neonatal exposure to a wild-derived microbiome protects mice against diet-induced obesity. Nat. Metab. 3, 1042–1057 (2021).
Reese, T. A. et al. Sequential infection with common pathogens promotes human-like immune gene expression and altered vaccine response. Cell Host Microbe 19, 713–719 (2016).
Berton, R. R., Jensen, I. J., Harty, J. T., Griffith, T. S. & Badovinac, V. P. Inflammation controls susceptibility of immune-experienced mice to sepsis. Immunohorizons 6, 528–542 (2022).
Lanzer, K. G. et al. Sequential early-life infections alter peripheral blood transcriptomics in aging female mice but not the response to de novo infection with influenza virus or M. tuberculosis. Immunohorizons 7, 562–576 (2023).
Gray, J. I. & Farber, D. L. Tissue-resident immune cells in humans. Annu. Rev. Immunol. 40, 195–220 (2022).
Huggins, M. A. et al. Microbial exposure enhances immunity to pathogens recognized by TLR2 but increases susceptibility to cytokine storm through TLR4 sensitization. Cell Rep. 28, 1729–1743.e5 (2019).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Sadler, R. et al. Microbiota differences between commercial breeders impacts the post-stroke immune response. Brain Behav. Immun. 66, 23–30 (2017).
Ericsson, A. C. et al. Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS One 10, e0116704 (2015).
Rausch, P. et al. Analysis of factors contributing to variation in the C57BL/6J fecal microbiota across German animal facilities. Int. J. Med. Microbiol. 306, 343–355 (2016).
Villarino, N. F. et al. Composition of the gut microbiota modulates the severity of malaria. Proc. Natl Acad. Sci. USA 113, 2235–2240 (2016).
Servick, K. Of mice and microbes. Science 353, 741–743 (2016).
Stappenbeck, T. S. & Virgin, H. W. Accounting for reciprocal host-microbiome interactions in experimental science. Nature 534, 191–199 (2016).
Threadgill, D. W., Miller, D. R., Churchill, G. A. & de Villena, F. P. The collaborative cross: a recombinant inbred mouse population for the systems genetic era. ILAR J. 52, 24–31 (2011).
Bowie, M. B. et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc. Natl Acad. Sci. USA 104, 5878–5882 (2007).
Wang, J. et al. Fetal and adult progenitors give rise to unique populations of CD8+ T cells. Blood 128, 3073–3082 (2016).
Stolley, J. M. et al. Depleting CD103+ resident memory T cells in vivo reveals immunostimulatory functions in oral mucosa. J. Exp. Med. 220, e20221853 (2023).
Wijeyesinghe, S. & Masopust, D. Resident memory T cells are a Notch above the rest. Nat. Immunol. 17, 1337–1338 (2016).
Beura, L. K. et al. T cells in nonlymphoid tissues give rise to lymph-node-resident memory T cells. Immunity 48, 327–338.e5 (2018).
Li, D. J. et al. Assessment of need for improved identification of a culprit drug in stevens-johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 159, 830–836 (2023).
Divito, S. J. et al. Peripheral host T cells survive hematopoietic stem cell transplantation and promote graft-versus-host disease. J. Clin. Invest. 130, 4624–4636 (2020).
Liu, Q. et al. Crosstalk between skin microbiota and immune system in health and disease. Nat. Immunol. 24, 895–898 (2023).
Neil, J. A. et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nat. Microbiol. 4, 1737–1749 (2019).
Kernbauer, E., Ding, Y. & Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014).
Beura, L. K. et al. Novel lymphocytic choriomeningitis virus strain sustains abundant exhausted progenitor CD8 T cells without systemic viremia. J. Immunol. 209, 1691–1702 (2022).
Kirjavainen, P. V. et al. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat. Med. 25, 1089–1095 (2019).
Block, K. E. et al. Physiological microbial exposure transiently inhibits mouse lung ILC2 responses to allergens. Nat. Immunol. 23, 1703–1713 (2022).
Ma, J. et al. Laboratory mice with a wild microbiota generate strong allergic immune responses. Sci. Immunol. 8, eadf7702 (2023).
Black, G. F. et al. Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi. J. Infect. Dis. 184, 322–329 (2001).
Levine, M. M. Immunogenicity and efficacy of oral vaccines in developing countries: lessons from a live cholera vaccine. BMC Biol. 8, 129 (2010).
Lopman, B. A. et al. Post-licensure experience with rotavirus vaccination in high and middle income countries; 2006 to 2011. Curr. Opin. Virol. 2, 434–442 (2012).
Fiege, J. K. et al. Mice with diverse microbial exposure histories as a model for preclinical vaccine testing. Cell Host Microbe 29, 1815–1827.e6 (2021).
Sjaastad, F. V. et al. Reduced T cell priming in microbially experienced “Dirty” mice results from limited IL-27 production by XCR1+ dendritic cells. J. Immunol. 209, 2149–2159 (2022).
Li, Y. et al. Sequential early-life viral infections modulate the microbiota and adaptive immune responses to systemic and mucosal vaccination. PLoS Pathog. 20, e1012557 (2024).
Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014).
Bello, M. G. D., Knight, R., Gilbert, J. A. & Blaser, M. J. Preserving microbial diversity. Science 362, 33–34 (2018).
Constantinides, M. G. et al. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science 366, eaax6624 (2019).
Ito, E. et al. Sulfated bile acid is a host-derived ligand for MAIT cells. Sci. Immunol. 9, eade6924 (2024).
Martin, M. D. et al. CD115+ monocytes protect microbially experienced mice against E. coli-induced sepsis. Cell Rep. 42, 113345 (2023).
Sun, D. G. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 12, 3049–3062 (2022).
Beyersdorf, N., Hanke, T., Kerkau, T. & Hunig, T. CD28 superagonists put a break on autoimmunity by preferentially activating CD4+CD25+ regulatory T cells. Autoimmun. Rev. 5, 40–45 (2006).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Lo, B. C. et al. Microbiota-dependent activation of CD4+ T cells induces CTLA-4 blockade-associated colitis via Fcγ receptors. Science 383, 62–70 (2024).
Leung, J. M. et al. Rapid environmental effects on gut nematode susceptibility in rewilded mice. PLoS Biol. 16, e2004108 (2018).
Lin, J. D. et al. Rewilding Nod2 and Atg16l1 mutant mice uncovers genetic and environmental contributions to microbial responses and immune cell composition. Cell Host Microbe 27, 830–840.e4 (2020).
Yeung, F. et al. Altered immunity of laboratory mice in the natural environment is associated with fungal colonization. Cell Host Microbe 27, 809–822.e6 (2020).
Camell, C. D. et al. Senolytics reduce coronavirus-related mortality in old mice. Science 373, eabe4832 (2021).
Oyesola, O. et al. Genetic and environmental interactions contribute to immune variation in rewilded mice. Nat. Immunol. 25, 1270–1282 (2024).
Labuda, J. C., Fong, K. D. & McSorley, S. J. Cohousing with dirty mice increases the frequency of memory T cells and has variable effects on intracellular bacterial infection. Immunohorizons 6, 184–190 (2022).
Zarek, C. M. et al. Preexisting helminth challenge exacerbates infection and reactivation of gammaherpesvirus in tissue resident macrophages. PLoS Pathog. 19, e1011691 (2023).
Cardilli, A. et al. Impact of high salt-intake on a natural gut ecosystem in wildling mice. Nutrients 15, 1565 (2023).
Takeda, A. J. et al. Human PI3Kγ deficiency and its microbiota-dependent mouse model reveal immunodeficiency and tissue immunopathology. Nat. Commun. 10, 4364 (2019).
Masopust, D., Sivula, C. P. & Jameson, S. C. Of mice, dirty mice, and men: using mice to understand human immunology. J. Immunol. 199, 383–388 (2017).
Acknowledgements
We thank Dr. Qian Liu, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) for comments. This work was supported by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases, NIH (to B.R.), National Science Foundation grant DEB-2153923 and funds from the State of New Jersey (to A.L.G.), NIH grant R01 AI150600 (to D.M.) and NIH grant R01AI155468 (to S.E.H.).
Author information
Authors and Affiliations
Contributions
B.R. conceptualized the review. B.R. and S.E.H. wrote the main body with additions by A.L.G. and constructive comments from D.M. B.R. prepared the figure. All authors contributed to editing of the manuscript, researching data for the tables and providing references. All authors approved the submitted version of the article.
Corresponding author
Ethics declarations
Competing interests
B.R. discloses that the National Institute of Diabetes and Digestive and Kidney Diseases granted a license on the WildR mice to Taconic Biosciences and has a Collaborative Research Agreement and Development Award with Genentech. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks S. Rosshart and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rehermann, B., Graham, A.L., Masopust, D. et al. Integrating natural commensals and pathogens into preclinical mouse models. Nat Rev Immunol 25, 385–397 (2025). https://doi.org/10.1038/s41577-024-01108-3
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-024-01108-3
This article is cited by
-
Microbiota in inflammatory bowel disease: mechanisms of disease and therapeutic opportunities
Nature Reviews Microbiology (2025)
-
Physiological microbial exposure normalizes memory T cell surveillance of the brain and modifies host seizure outcomes
Nature Immunology (2025)