Abstract
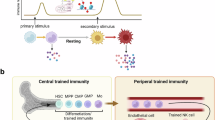
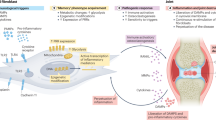
A decade after the term ‘trained immunity’ (TRIM) was coined to reflect the long-lasting hyper-responsiveness of innate immune cells with an epigenetically imprinted ‘memory’ of earlier stimuli, our understanding has broadened to include the potential implications of TRIM in health and disease. Here, after summarizing the well-documented beneficial effects of TRIM against infections, we discuss emerging evidence that TRIM is also a major underlying mechanism in chronic inflammation-related disorders such as periodontitis, rheumatoid arthritis and cardiovascular disease. Furthermore, mounting evidence indicates that the induction of TRIM by certain agonists confers protective antitumour responses. Although the mechanisms underlying TRIM require further study, the current knowledge enables the experimental development of innovative therapeutic approaches to stimulate or inhibit TRIM in a context-appropriate manner, such as the stimulation of TRIM in cancer or its inhibition in inflammatory disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sallusto, F., Lanzavecchia, A., Araki, K. & Ahmed, R. From vaccines to memory and back. Immunity 33, 451–463 (2010).
Benn, C. S., Netea, M. G., Selin, L. K. & Aaby, P. A small jab-a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 34, 431–439 (2013).
Netea, M. G., Quintin, J. & van der Meer, J. W. Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361 (2011).
Reimer-Michalski, E. M. & Conrath, U. Innate immune memory in plants. Semin. Immunol. 28, 319–327 (2016).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Hajishengallis, G., Netea, M. G. & Chavakis, T. Innate immune memory, trained immunity and nomenclature clarification. Nat. Immunol. 24, 1393–1394 (2023).
de Laval, B. et al. C/EBPβ-dependent epigenetic memory induces trained immunity in hematopoietic stem cells. Cell Stem Cell 26, 657–674.e8 (2020).
Jentho, E. et al. Trained innate immunity, long-lasting epigenetic modulation, and skewed myelopoiesis by heme. Proc. Natl Acad. Sci. USA 118, e2102698118 (2021).
Foster, S. L., Hargreaves, D. C. & Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 (2007).
Sun, J. C., Beilke, J. N. & Lanier, L. L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009).
Saeed, S. et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014). References 9–11 are key studies documenting the importance of epigenetic modifications in the development of peripheral TRIM.
Bannister, S. et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci. Adv. 8, eabn4002 (2022).
Cheong, J.-G. et al. Epigenetic memory of coronavirus infection in innate immune cells and their progenitors. Cell 186, 3882–3902.e3824 (2023).
Kleinnijenhuis, J. et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 6, 152–158 (2014).
Mitroulis, I. et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 172, 147–161 e112 (2018).
Kaufmann, E. et al. BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172, 176–190 e119 (2018). References 15 and 16 establish the concept of central TRIM — that inflammation-adapted, long-lived haematopoietic progenitors in the bone marrow represent a persistent reservoir of innate immune memory.
Wendeln, A. C. et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338 (2018).
Yao, Y. et al. Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 175, 1634–1650.e1617 (2018).
Lercher, A. et al. Antiviral innate immune memory in alveolar macrophages following SARS-CoV-2 infection ameliorates secondary influenza A virus disease. Immunity 57, 2530–2546.e13 (2024).
Kleinnijenhuis, J. et al. BCG-induced trained immunity in NK cells: role for non-specific protection to infection. Clin. Immunol. 155, 213–219 (2014).
Moorlag, S. et al. β-Glucan induces protective trained immunity against mycobacterium tuberculosis infection: a key role for IL-1. Cell Rep. 31, 107634 (2020).
Nankabirwa, V., Tumwine, J. K., Mugaba, P. M., Tylleskär, T. & Sommerfelt, H. Child survival and BCG vaccination: a community based prospective cohort study in Uganda. BMC Public. Health 15, 175 (2015).
Buffen, K. et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLOS Pathog. 10, e1004485 (2014).
Arts, R. J. W. et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23, 89–100.e105 (2018).
Kleinnijenhuis, J. et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl Acad. Sci. USA 109, 17537–17542 (2012).
Goodridge, H. S. et al. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 16, 392–400 (2016).
Penkov, S., Mitroulis, I., Hajishengallis, G. & Chavakis, T. Immunometabolic crosstalk: an ancestral principle of trained immunity? Trends Immunol. 40, 1–11 (2019).
Kalafati, L. et al. Innate immune training of granulopoiesis promotes anti-tumor activity. Cell 183, 771–785.e712 (2020). This study shows that the beneficial effects of central TRIM go beyond protection from reinfection and include the ability to protect against future cancers.
van Puffelen, J. H. et al. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 17, 513–525 (2020).
Christ, A. et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172, 162–175.e114 (2018).
Bekkering, S. et al. Trained immunity: linking obesity and cardiovascular disease across the life-course? Trends Endocrinol. Metab. 31, 378–389 (2020).
Chavakis, T., Wielockx, B. & Hajishengallis, G. Inflammatory modulation of hematopoiesis: linking trained immunity and clonal hematopoiesis with chronic disorders. Annu. Rev. Physiol. 84, 183–207 (2022).
Chavakis, T., Mitroulis, I. & Hajishengallis, G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat. Immunol. 20, 802–811 (2019).
Li, X. et al. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell 185, 1709–1727.e1718 (2022). This study establishes the concept that maladaptive TRIM is a common mechanistic basis that links various inflammatory comorbidities.
Dong, Z. et al. Myocardial infarction drives trained immunity of monocytes, accelerating atherosclerosis. Eur. Heart J. 45, 669–684 (2023).
Hata, M. et al. Past history of obesity triggers persistent epigenetic changes in innate immunity and exacerbates neuroinflammation. Science 379, 45–62 (2023). This report shows that diet-induced obesity can lead to persistent epigenetic changes in macrophages, even after weight gain and metabolic alterations are normalized, that can thereby aggravate neuroinflammation.
Govindarajah, V. et al. Gestational diabetes in mice induces hematopoietic memory that impacts the long-term health of the offspring. J. Clin. Invest. 134, e169730 (2023).
Simats, A. et al. Innate immune memory after brain injury drives inflammatory cardiac dysfunction. Cell 187, 4637–4655.e26 (2024).
Nakayama, Y. et al. Heart failure promotes multimorbidity through innate immune memory. Sci. Immunol. 9, eade3814 (2024). References 38 and 39 show that cardiac dysfunction can be promoted by acute brain ischaemia or heart failure, respectively, owing to early inflammatory or stress responses that trigger persistent epigenetic rewiring of haematopoietic progenitors.
Blanden, R. V., Lefford, M. J. & Mackaness, G. B. The host response to Calmette-Guérin bacillus infection in mice. J. Exp. Med. 129, 1079–1107 (1969).
Aaby, P. et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 204, 245–252 (2011).
Prentice, S. et al. BCG-induced non-specific effects on heterologous infectious disease in Ugandan neonates: an investigator-blind randomised controlled trial. Lancet Infect. Dis. 21, 993–1003 (2021).
Walk, J. et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 10, 874 (2019).
Naeslund, C. Resultats des experience de vaccination par le BCG poursuivies dans le Norrbotten (Suede) Septembre 1927– Decembre 1931. Vaccination Preventative de la Tuberculose de I’Homme et des Animaux par le BCG: Rapports et Documents Provenant des Divers Pays (Ia France exceptee) 274–281 (1932).
Biering-Sorensen, S. et al. Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin. Infect. Dis. 65, 1183–1190 (2017).
Schaltz-Buchholzer, F. et al. Early BCG vaccination, hospitalizations, and hospital deaths: analysis of a secondary outcome in 3 randomized trials from guinea-bissau. J. Infect. Dis. 219, 624–632 (2019).
Silva, M. V. T. et al. The role of IL-32 in Bacillus Calmette-Guerin (BCG)-induced trained immunity in infections caused by different Leishmania spp. Microb. Pathog. 158, 105088 (2021).
Higgins, J. P. T. et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ 355, i5170 (2016).
Zimmermann, P., Finn, A. & Curtis, N. Does BCG vaccination protect against nontuberculous mycobacterial infection? A systematic review and meta-analysis. J. Infect. Dis. 218, 679–687 (2018).
Stensballe, L. G. et al. BCG vaccination at birth and early childhood hospitalisation: a randomised clinical multicentre trial. Arch. Dis. Child. 102, 224–231 (2017).
Messina, N. L. et al. Neonatal bacille Calmette-Guérin vaccination and infections in the first year of life: the MIS BAIR randomized controlled trial. J. Infect. Dis. 224, 1115–1127 (2021).
Giamarellos-Bourboulis, E. J. et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell 183, 315–323.e319 (2020).
Netea, M. G. et al. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell 181, 969–977 (2020).
Tsilika, M. et al. ACTIVATE-2: a double-blind randomized trial of BCG vaccination against COVID-19 in individuals at risk. Front. Immunol. 13, 873067 (2022).
Ten Doesschate, T. et al. Bacillus Calmette-Guerin vaccine to reduce healthcare worker absenteeism in COVID-19 pandemic, a randomized controlled trial. Clin. Microbiol. Infect. 28, 1278–1285 (2022).
Moorlag, S. et al. Efficacy of BCG vaccination against respiratory tract infections in older adults during the coronavirus disease 2019 pandemic. Clin. Infect. Dis. 75, e938–e946 (2022).
Upton, C. M. et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: a double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine 48, 101414 (2022).
Aaby, P., Netea, M. G. & Benn, C. S. Beneficial non-specific effects of live vaccines against COVID-19 and other unrelated infections. Lancet Infect. Dis. 23, e34–e42 (2023).
Joosten, S. A. et al. Harnessing donor unrestricted T-cells for new vaccines against tuberculosis. Vaccine 37, 3022–3030 (2019).
Tameris, M. D. et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028 (2013).
Ndiaye, B. P. et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 3, 190–200 (2015).
Verrall, A. J. et al. Early clearance of Mycobacterium tuberculosis is associated with increased innate immune responses. J. Infect. Dis. 221, 1342–1350 (2020).
Suliman, S. et al. Bacillus Calmette-Guérin (BCG) revaccination of adults with latent mycobacterium tuberculosis infection induces long-lived BCG-reactive NK cell responses. J. Immunol. 197, 1100–1110 (2016).
Röring, R. J. et al. MMR vaccination induces trained immunity via functional and metabolic reprogramming of γδ T cells. J. Clin. Invest. 134, e170848 (2024).
Wimmers, F. et al. The single-cell epigenomic and transcriptional landscape of immunity to influenza vaccination. Cell 184, 3915–3935 e3921 (2021).
Debisarun, P. A. et al. Induction of trained immunity by influenza vaccination-impact on COVID-19. PLoS Pathog. 17, e1009928 (2021).
Thomas, M. et al. A cross-sectional study from Qatar on the effect of influenza vaccination on the severity of COVID-19. Medicine 102, e35107 (2023).
Arunachalam, P. S. et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596, 410–416 (2021).
Murphy, D. M. et al. Trained immunity is induced in humans after immunization with an adenoviral vector COVID-19 vaccine. J. Clin. Invest. 133, e162581 (2023).
Benn, C. S., Schaltz-Buchholzer, F., Nielsen, S., Netea, M. G. & Aaby, P. Randomized clinical trials of COVID-19 vaccines: do adenovirus-vector vaccines have beneficial non-specific effects? iScience 26, 106733 (2023).
Joosten, S. A. et al. Mycobacterial growth inhibition is associated with trained innate immunity. J. Clin. Invest. 128, 1837–1851 (2018).
Barton, E. S. et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447, 326–329 (2007).
Ziogas, A. & Netea, M. G. Trained immunity-related vaccines: innate immune memory and heterologous protection against infections. Trends Mol. Med. 28, 497–512 (2022).
Piret, J. & Boivin, G. The impact of trained immunity in respiratory viral infections. Rev. Med. Virol. 34, e2510 (2024).
Dagenais, A., Villalba-Guerrero, C. & Olivier, M. Trained immunity: a “new” weapon in the fight against infectious diseases. Front. Immunol. 14, 1147476 (2023).
Netea, M. G., Schlitzer, A., Placek, K., Joosten, L. A. B. & Schultze, J. L. Innate and adaptive immune memory: an evolutionary continuum in the host’s response to pathogens. Cell Host Microbe 25, 13–26 (2019).
Hajishengallis, G. & Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 21, 426–440 (2021).
Maceiras, A. R., Silvério, D., Gonçalves, R., Cardoso, M. S. & Saraiva, M. Infection with hypervirulent Mycobacterium tuberculosis triggers emergency myelopoiesis but not trained immunity. Front. Immunol. 14, 1211404 (2023).
Takizawa, H. et al. Pathogen-induced TLR4-TRIF innate immune signaling in hematopoietic stem cells promotes proliferation but reduces competitive fitness. Cell Stem Cell 21, 225–240.e225 (2017).
Zhang, H. et al. Sepsis induces hematopoietic stem cell exhaustion and myelosuppression through distinct contributions of TRIF and MYD88. Stem Cell Rep. 6, 940–956 (2016).
Pietras, E. M. et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol. 18, 607–618 (2016).
van Kampen, E., Jaminon, A., van Berkel, T. J. & Van Eck, M. Diet-induced (epigenetic) changes in bone marrow augment atherosclerosis. J. Leukoc. Biol. 96, 833–841 (2014).
Fu, J. & Wu, H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu. Rev. Immunol. 41, 301–316 (2023).
Coppin, E. et al. Peripheral ischemia imprints epigenetic changes in hematopoietic stem cells to propagate inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 43, 889–906 (2023).
Cao, J. et al. DNA-sensing inflammasomes cause recurrent atherosclerotic stroke. Nature 633, 433–441 (2024).
Kain, B. N. et al. Hematopoietic stem and progenitor cells confer cross-protective trained immunity in mouse models. iScience 26, 107596 (2023).
Khan, N. et al. M. tuberculosis reprograms hematopoietic stem cells to limit myelopoiesis and impair trained immunity. Cell 183, 752–770.e722 (2020).
Cottam, M. A., Caslin, H. L., Winn, N. C. & Hasty, A. H. Multiomics reveals persistence of obesity-associated immune cell phenotypes in adipose tissue during weight loss and weight regain in mice. Nat. Commun. 13, 2950 (2022).
Caslin, H. L., Cottam, M. A., Pinon, J. M., Boney, L. Y. & Hasty, A. H. Weight cycling induces innate immune memory in adipose tissue macrophages. Front. Immunol. 13, 984859 (2022).
Edgar, L. et al. Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation 144, 961–982 (2021).
Yan, J. et al. Diabetes impairs wound healing by Dnmt1-dependent dysregulation of hematopoietic stem cells differentiation towards macrophages. Nat. Commun. 9, 33 (2018).
Riksen, N. P., Bekkering, S., Mulder, W. J. M. & Netea, M. G. Trained immunity in atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 20, 799–811 (2023).
Saaoud, F. et al. Cigarette smoke modulates inflammation and immunity via reactive oxygen species-regulated trained immunity and trained tolerance mechanisms. Antioxid. Redox Signal. 38, 1041–1069 (2023).
Dallio, M. et al. Environmental bisphenol A exposure triggers trained immunity-related pathways in monocytes. Front. Immunol. 14, 1270391 (2023).
Movassagh, H. et al. Proinflammatory polarization of monocytes by particulate air pollutants is mediated by induction of trained immunity in pediatric asthma. Allergy 78, 1922–1933 (2023).
van der Heijden, C. D. C. C. et al. Catecholamines induce trained immunity in monocytes in vitro and in vivo. Circ. Res. 127, 269–283 (2020).
Heidt, T. et al. Chronic variable stress activates hematopoietic stem cells. Nat. Med. 20, 754–758 (2014).
McAlpine, C. S. et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 566, 383–387 (2019).
Grigoriou, M. et al. Transcriptome reprogramming and myeloid skewing in haematopoietic stem and progenitor cells in systemic lupus erythematosus. Ann. Rheum. Dis. 79, 242–253 (2020).
Jeljeli, M. et al. Trained immunity modulates inflammation-induced fibrosis. Nat. Commun. 10, 5670 (2019).
Lechner, A. et al. Macrophages acquire a TNF-dependent inflammatory memory in allergic asthma. J. Allergy Clin. Immunol. 149, 2078–2090 (2022).
Martinez-Gonzalez, I. et al. Allergen-experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity 45, 198–208 (2016).
Wang, B. et al. Sepsis induces non-classic innate immune memory in granulocytes. Cell Rep. 42, 113044 (2023).
Bhattarai, S. et al. TLR4 is a regulator of trained immunity in a murine model of Duchenne muscular dystrophy. Nat. Commun. 13, 879 (2022).
Petrof, B. J., Podolsky, T., Bhattarai, S., Tan, J. & Ding, J. Trained immunity as a potential target for therapeutic immunomodulation in Duchenne muscular dystrophy. Front. Immunol. 14, 1183066 (2023).
Koelwyn, G. J. et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat. Med. 26, 1452–1458 (2020). This study shows that maladaptive trained myelopoiesis is not necessarily associated with increased immune responsiveness but may also be linked to immune suppression, such as when myocardial infarction leads to epigenetic reprogramming of bone marrow progenitors and progeny monocytes that acquire an immunosuppressive phenotype and promote cancer progression.
Noz, M. P. et al. Reprogramming of bone marrow myeloid progenitor cells in patients with severe coronary artery disease. eLife 9, e60939 (2020).
Ling, M. R., Chapple, I. L. & Matthews, J. B. Peripheral blood neutrophil cytokine hyper-reactivity in chronic periodontitis. Innate Immun. 21, 714–725 (2015).
Radvar, M., Tavakkol-Afshari, J., Bajestan, M. N., Naseh, M. R. & Arab, H. R. The effect of periodontal treatment on IL-6 production of peripheral blood monocytes in aggressive periodontitis and chronic periodontitis patients. Iran. J. Immunol. 5, 100–106 (2008).
Gustafsson, A., Ito, H., Asman, B. & Bergstrom, K. Hyper-reactive mononuclear cells and neutrophils in chronic periodontitis. J. Clin. Periodontol. 33, 126–129 (2006).
Fokkema, S. J., Loos, B. G., Hart, A. A. M. & Van Der Velden, U. Long-term effect of full-mouth tooth extraction on the responsiveness of peripheral blood monocytes. J. Clin. Periodontol. 30, 756–760 (2003).
Matthews, J. B. et al. Neutrophil hyper-responsiveness in periodontitis. J. Dent. Res. 86, 718–722 (2007).
Johnstone, A. M., Koh, A., Goldberg, M. B. & Glogauer, M. A hyperactive neutrophil phenotype in patients with refractory periodontitis. J. Periodontol. 78, 1788–1794 (2007).
Figueredo, C. M., Gustafsson, A., Asman, B. & Bergstrom, K. Expression of intracellular elastase activity in peripheral neutrophils from patients with adult periodontitis. J. Clin. Periodontol. 27, 572–577 (2000).
Cirovic, B. et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe 28, 322–334.e325 (2020). This study provides human relevance to earlier studies in mice that established the concept of central TRIM by showing that BCG vaccination imprints a persistent myeloid-differentiation bias on human haematopoietic progenitors, which in turn give rise to epigenetically reprogrammed and trained myeloid progeny.
Ishai, A. et al. Periodontal disease associates with arterial inflammation via potentiation of a hematopoietic-arterial axis. JACC Cardiovasc. Imaging 12, 2271–2273 (2019).
Van Dyke, T. E. et al. Inflammation of the periodontium associates with risk of future cardiovascular events. J. Periodontol. 92, 348–358 (2021).
Bernelot Moens, S. J. et al. Unexpected arterial wall and cellular inflammation in patients with rheumatoid arthritis in remission using biological therapy: a cross-sectional study. Arthritis Res. Ther. 18, 115 (2016).
Bekkering, S. et al. Treatment with statins does not revert trained immunity in patients with familial hypercholesterolemia. Cell Metab. 30, 1–2 (2019).
Fok, E. T. et al. A chromatin-regulated biphasic circuit coordinates IL-1β-mediated inflammation. Nat. Gen. 56, 85–99 (2023).
Rong, H. et al. Association between IL1B polymorphisms and the risk of rheumatoid arthritis. Int. Immunopharmacol. 83, 106401 (2020).
Huang, X.-L. et al. Association of interleukin-1 family cytokines single nucleotide polymorphisms with susceptibility to systemic sclerosis: an independent case–control study and a meta-analysis. Immunol. Res. 64, 1041–1052 (2016).
Mooney, R. E. et al. Association of TGFB1 rs1800469 and BCMO1 rs6564851 with coronary heart disease and IL1B rs16944 with all-cause mortality in men from the Northern Ireland PRIME study. PLoS ONE 17, e0273333 (2022).
Wu, X. et al. Association of interleukin-1 gene variations with moderate to severe chronic periodontitis in multiple ethnicities. J. Periodontal Res. 50, 52–61 (2015).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Weber, B. N., Giles, J. T. & Liao, K. P. Shared inflammatory pathways of rheumatoid arthritis and atherosclerotic cardiovascular disease. Nat. Rev. Rheumatol. 19, 417–428 (2023).
Cappuccio, F. P., Cooper, D., D’Elia, L., Strazzullo, P. & Miller, M. A. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur. Heart J. 32, 1484–1492 (2011).
Gozal, D. & Kheirandish-Gozal, L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am. J. Respir. Crit. Care Med. 177, 369–375 (2008).
Hinterdobler, J., Schunkert, H., Kessler, T. & Sager, H. B. Impact of acute and chronic psychosocial stress on vascular inflammation. Antioxid. Redox Signal. 35, 1531–1550 (2021).
Schloss, M. J., Swirski, F. K. & Nahrendorf, M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ. Res. 126, 1242–1259 (2020).
Kalafati, L., Hatzioannou, A., Hajishengallis, G. & Chavakis, T. The role of neutrophils in trained immunity. Immunol. Rev. 314, 142–157 (2023).
Axelrad, J. E., Lichtiger, S. & Yajnik, V. Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 22, 4794–4801 (2016).
Greten, F. R. & Grivennikov, S. I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 (2019).
Lega, I. C. & Lipscombe, L. L. Review: diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr. Rev. 41, bnz014 (2020).
McAllister, S. S. & Weinberg, R. A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 16, 717–727 (2014).
Fridman, W. H., Zitvogel, L., Sautès-Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Ng, M. S. F. et al. Deterministic reprogramming of neutrophils within tumors. Science 383, eadf6493 (2024).
Zhou, J. et al. Tumor-associated macrophages: recent insights and therapies. Front. Oncol. 10, 188 (2020).
Wildes, T. J., DiVita Dean, B. & Flores, C. T. Myelopoiesis during solid cancers and strategies for immunotherapy. Cells 10, 968 (2021).
Wu, C., Hua, Q. & Zheng, L. Generation of myeloid cells in cancer: the spleen matters. Front. Immunol. 11, 1126 (2020).
Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021).
Casbon, A. J. et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc. Natl Acad. Sci. USA 112, E566–E575 (2015).
Aliazis, K., Yenyuwadee, S., Phikulsod, P. & Boussiotis, V. A. Emergency myelopoiesis in solid cancers. Br. J. Haematol. 205, 798–811 (2024).
Bui, T. M. et al. Tissue-specific reprogramming leads to angiogenic neutrophil specialization and tumor vascularization in colorectal cancer. J. Clin. Invest. 134, e174545 (2024).
LaMarche, N. M. et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature 625, 166–174 (2024). This report shows that tumours can reprogramme bone marrow myelopoiesis and shift myeloid cells towards an immunosuppressive phenotype, driven by locally produced IL-4.
Zilio, S., Bicciato, S., Weed, D. & Serafini, P. CCR1 and CCR5 mediate cancer-induced myelopoiesis and differentiation of myeloid cells in the tumor. J. Immunother. Cancer 10, e003131 (2022).
Atallah, A. et al. Systemic versus localized Bacillus Calmette Guérin immunotherapy of bladder cancer promotes an anti-tumoral microenvironment: novel role of trained immunity. Int. J. Cancer 155, 352–364 (2024).
Han, J., Gu, X., Li, Y. & Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 129, 110393 (2020).
Graham, C. H. et al. Innate immune memory is associated with increased disease-free survival in bladder cancer patients treated with bacillus Calmette-Guérin. Can. Urol. Assoc. J. 15, E412–E417 (2021).
Singh, A. K. et al. Re-engineered BCG overexpressing cyclic di-AMP augments trained immunity and exhibits improved efficacy against bladder cancer. Nat. Commun. 13, 878 (2022).
Engel-Riedel, W. et al. A randomized, controlled trial evaluating the efficacy and safety of BTH1677 in combination with bevacizumab, carboplatin, and paclitaxel in first-line treatment of advanced non-small cell lung cancer. J. Immunother. Cancer 6, 16 (2018).
Steimbach, L. et al. Fungal beta-glucans as adjuvants for treating cancer patients - a systematic review of clinical trials. Clin. Nutr. 40, 3104–3113 (2021).
Broquet, A. et al. Sepsis-trained macrophages promote antitumoral tissue-resident T cells. Nat. Immunol. 25, 802–819 (2024). This study shows that induction of peripheral TRIM in tissue-resident alveolar macrophages by experimental sepsis leads to the accumulation of CXCR6+ tissue-resident T cells with antitumour activity, and provides evidence that survivors of sepsis have reduced incidence of malignancies.
Geller, A. E. et al. The induction of peripheral trained immunity in the pancreas incites anti-tumor activity to control pancreatic cancer progression. Nat. Commun. 13, 759 (2022).
Vuscan, P. et al. Potent induction of trained immunity by Saccharomyces cerevisiae β-glucans. Front. Immunol. 15, 1323333 (2024).
Ding, C. et al. Inducing trained immunity in pro-metastatic macrophages to control tumor metastasis. Nat. Immunol. 24, 239–254 (2023). This study shows that β-glucan-induced training of bone marrow-derived lung interstitial macrophages, mediated by the metabolite S1P, leads to inhibition of tumour metastasis to the lung.
Bekkering, S. et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 172, 135–146.e139 (2018).
Liu, Y. et al. Squalene-epoxidase-catalyzed 24(S),25-epoxycholesterol synthesis promotes trained-immunity-mediated antitumor activity. Cell Rep. 43, 114094 (2024).
Dos Santos, J. C. et al. Leishmania braziliensis enhances monocyte responses to promote anti-tumor activity. Cell Rep. 43, 113932 (2024).
Wang, T. et al. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs. Nat. Immunol. 24, 423–438 (2023). This study shows IFNγ-mediated induction of peripheral TRIM in alveolar macrophages by prior influenza A virus infection of the lung; the trained macrophages have increased phagocytic and tumoricidal activity despite the immunosuppressive TME and thereby block tumour metastasis to the lung.
Aegerter, H. et al. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat. Immunol. 21, 145–157 (2020).
Iliakis, C. S. et al. The role of recruitment versus training in influenza-induced lasting changes to alveolar macrophage function. Nat. Immunol. 24, 1639–1641 (2023).
Liu, G. et al. Bacteria-derived nanovesicles enhance tumour vaccination by trained immunity. Nat. Nanotechnol. 19, 387–398 (2024).
Liang, J. et al. Outer membrane vesicle-based nanohybrids target tumor-associated macrophages to enhance trained immunity-related vaccine-generated antitumor activity. Adv. Mater. 35, e2306158 (2023).
Chen, Z. et al. Engineered probiotic-based personalized cancer vaccine potentiates antitumor immunity through initiating trained immunity. Adv. Sci. 11, e2305081 (2024).
Priem, B. et al. Trained immunity-promoting nanobiologic therapy suppresses tumor growth and potentiates checkpoint inhibition. Cell 183, 786–801.e719 (2020).
Zent, C. S. et al. Early treatment of high risk chronic lymphocytic leukemia with alemtuzumab, rituximab and poly-(1–6)-β-glucotriosyl-(1-3)-β-glucopyranose beta-glucan is well tolerated and achieves high complete remission rates. Leuk. Lymphoma 56, 2373–2378 (2015).
Chan, A. S. H. et al. Imprime PGG enhances anti-tumor effects of tumor-targeting, anti-angiogenic, and immune checkpoint inhibitor antibodies. Front. Oncol. 12, 869078 (2022).
O’Day, S. J. et al. IMPRIME 1 (NCT02981303): a novel phase 2 study in second-line+, metastatic triple negative breast cancer patients shows promising clinical benefit for the combination of the immune checkpoint inhibitor, pembrolizumab (pembro), with the novel innate immune activator, Imprime PGG. Cancer Res. 80, Abstr. CT073 (2020).
Hajishengallis, G. & Chavakis, T. Central trained immunity and its impact on chronic inflammatory and autoimmune diseases. J. Allergy Clin. Immunol. 154, 1113–1116 (2024).
Bulut, O., Kilic, G., Domínguez-Andrés, J. & Netea, M. G. Overcoming immune dysfunction in the elderly: trained immunity as a novel approach. Int. Immunol. 32, 741–753 (2020).
Berendsen, M. L. T. et al. Bacillus Calmette-Guerin vaccination induces a trained innate immunity phenotype in adults over 50 years of age: a randomized trial in Guinea-Bissau. Vaccine 42, 126439 (2024).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Wang, H. et al. Clonal hematopoiesis driven by mutated DNMT3A promotes inflammatory bone loss. Cell 187, 3690–3711.e3619 (2024).
Mills, T. S. et al. A distinct metabolic and epigenetic state drives trained immunity in HSC-derived macrophages from autoimmune mice. Cell Stem Cell 31, 1630–1649.e8 (2024).
Ziogas, A., Bruno, M., van der Meel, R., Mulder, W. J. M. & Netea, M. G. Trained immunity: target for prophylaxis and therapy. Cell Host Microbe 31, 1776–1791 (2023).
Eskan, M. A. et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol. 13, 465–473 (2012).
Dutzan, N. et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 10, eaat0797 (2018).
Tsukasaki, M. et al. Host defense against oral microbiota by bone-damaging T cells. Nat. Commun. 9, 701 (2018).
Mitroulis, I., Hajishengallis, G. & Chavakis, T. Bone marrow inflammatory memory in cardiometabolic disease and inflammatory comorbidities. Cardiovasc. Res. 119, 2801–2812 (2023).
Herre, J., Gordon, S. & Brown, G. D. Dectin-1 and its role in the recognition of β-glucans by macrophages. Mol. Immunol. 40, 869–876 (2004).
Larsen, S. B. et al. Establishment, maintenance, and recall of inflammatory memory. Cell Stem Cell 28, 1758–1774.e1758 (2021).
Acknowledgements
The authors’ research is supported by grants from the NIH (DE031206 and DE033643 to G.H.), the Deutsche Forschungsgemeinschaft (SFB-TRR 332, project B4 and SFB-TRR369, project C3 to T.C.), the ‘Exzellenzförderprogramm für etablierte Wissenschaftlerinnen und Wissenschaftler’ of the ‘Deutsche Krebshilfe’ (to T.C.), the Saxon State Ministry of Science, Culture, and Tourism-SMWK (Sonderzuweisung zur Unterstützung profilbestimmender Struktureinheiten der TUD to T.C.) and Spinoza grant of the Netherlands Organization for Scientific Research (to M.G.N.). The figures in the submitted version of the manuscript were created using BioRender.com.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
M.G.N. is scientific founder of Lemba, TTxD and Biotrip. G.H. and T.C. declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Arthur Liesz, Michael Sieweke and Fred Sheedy for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hajishengallis, G., Netea, M.G. & Chavakis, T. Trained immunity in chronic inflammatory diseases and cancer. Nat Rev Immunol 25, 497–514 (2025). https://doi.org/10.1038/s41577-025-01132-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-025-01132-x
This article is cited by
-
Lung cancer vaccines to enhance immune checkpoint inhibitor therapy: evidence and future perspectives
Journal of Hematology & Oncology (2026)
-
Deciphering cholangiocarcinoma heterogeneity and specific progenitor cell niche of extrahepatic cholangiocarcinoma at single-cell resolution
Journal of Hematology & Oncology (2025)
-
Hematopoietic stem cell state and fate in trained immunity
Cell Communication and Signaling (2025)
-
Immune Memory: A New Frontier in Treating Recurrent Inflammatory Skin Diseases
Clinical Reviews in Allergy & Immunology (2025)