Abstract
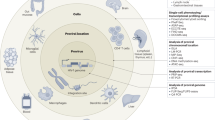
Antiretroviral therapy (ART) has markedly improved the life-expectancy of people living with HIV. However, during both HIV infection of humans and simian immunodeficiency virus infection of macaques, virus replication almost invariably rebounds upon ART interruption, due to the long-term persistency of a pool of latently infected cells harbouring integrated, replication-competent virus (known as the virus reservoir). Solving this ‘HIV reservoir problem’ is the key to achieving a cure (or at least a persistent remission) for HIV infection. Here, we summarize the key scientific evidence supporting the hypothesis that host immune responses, including those mediated by CD8+ T cells, B cells, antibodies and innate immune cells, affect the size, clonality, and cellular, tissue and organ distribution of the HIV reservoir. Importantly, we believe that any solution to the ‘reservoir problem’ must address not only the multifaceted interactions between HIV and the host immune system, but also the complex interplay between the immunobiology of memory CD4+ T helper cells (which form the main virus reservoir) and the molecular mechanisms that regulate HIV latency and reactivation. These concepts provide the rationale to develop new, immune-based approaches to ‘cure’ HIV infection; we review recent efforts to develop such therapies and their efficacy (or lack thereof) in disrupting the establishment and/or persistence of the virus reservoir in preclinical animal models and human clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pino, M., Paiardini, M. & Marconi, V. C. Progress in achieving long-term HIV remission. Curr. Opin. HIV AIDS 13, 435–445 (2018).
Vanhamel, J., Bruggemans, A. & Debyser, Z. Establishment of latent HIV-1 reservoirs: what do we really know? J. Virus Erad. 5, 3–9 (2019).
Whitney, J. B. et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512, 74–77 (2014).
Lian, X. et al. Progressive transformation of the HIV-1 reservoir cell profile over two decades of antiviral therapy. Cell Host Microbe 31, 83–96 (2023).
Kuo, H. H. & Lichterfeld, M. Recent progress in understanding HIV reservoirs. Curr. Opin. HIV AIDS 13, 137–142 (2018).
Landovitz, R. J., Scott, H. & Deeks, S. G. Prevention, treatment and cure of HIV infection. Nat. Rev. Microbiol. 21, 657–670 (2023).
Reeves, D. B. et al. A majority of HIV persistence during antiretroviral therapy is due to infected cell proliferation. Nat. Commun. 9, 4811 (2018).
Immonen, T. T. et al. No evidence for ongoing replication on ART in SIV-infected macaques. Nat. Commun. 15, 5093 (2024).
Kearney, M. F. et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 10, e1004010 (2014).
Evans, D. T. & Desrosiers, R. C. Immune evasion strategies of the primate lentiviruses. Immunol. Rev. 183, 141–158 (2001).
Boutwell, C. L., Rolland, M. M., Herbeck, J. T., Mullins, J. I. & Allen, T. M. Viral evolution and escape during acute HIV-1 infection. J. Infect. Dis. 202, S309–S314 (2010).
Simon, V., Bloch, N. & Landau, N. R. Intrinsic host restrictions to HIV-1 and mechanisms of viral escape. Nat. Immunol. 16, 546–553 (2015).
Garber, D. A., Silvestri, G. & Feinberg, M. B. Prospects for an AIDS vaccine: three big questions, no easy answers. Lancet Infect. Dis. 4, 397–413 (2004).
Maldarelli, F. et al. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183 (2014).
Wagner, T. A. et al. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345, 570–573 (2014).
Chomont, N. et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15, 893–900 (2009).
Margolis, D. M. et al. Curing HIV: seeking to target and clear persistent infection. Cell 181, 189–206 (2020).
Lewin, S. R. & Rasmussen, T. A. Kick and kill for HIV latency. Lancet 395, 844–846 (2020).
Tanaka, K., Kim, Y., Roche, M. & Lewin, S. R. The role of latency reversal in HIV cure strategies. J. Med. Primatol. 51, 278–283 (2022).
Kim, Y., Anderson, J. L. & Lewin, S. R. Getting the “Kill” into “Shock and Kill”: strategies to eliminate latent HIV. Cell Host Microbe 23, 14–26 (2018).
Alto, A. et al. The combination of venetoclax and ixazomib selectively and efficiently kills HIV-infected cell lines but has unacceptable toxicity in primary cell models. J. Virol. 95, e00138-21 (2021).
Chandrasekar, A. P., Cummins, N. W. & Badley, A. D. The role of the BCL-2 family of proteins in HIV-1 pathogenesis and persistence. Clin. Microbiol. Rev. 33, e00107–e00119 (2019).
Cummins, N. W. et al. Maintenance of the HIV reservoir is antagonized by selective BCL2 inhibition. J. Virol. 91, e00012–e00017 (2017).
Ren, Y. et al. Selective BCL-XL antagonists eliminate infected cells from a primary-cell model of HIV latency but not from ex vivo reservoirs. J. Virol. 95, e0242520 (2021).
Ren, Y. et al. BCL-2 antagonism sensitizes cytotoxic T cell-resistant HIV reservoirs to elimination ex vivo. J. Clin. Invest. 130, 2542–2559 (2020).
Archin, N. M. et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485 (2012).
Contreras, X. et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J. Biol. Chem. 284, 6782–6789 (2009).
Kulkosky, J. et al. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 98, 3006–3015 (2001).
Vibholm, L. et al. Short-course Toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin. Infect. Dis. 64, 1686–1695 (2017).
Spivak, A. M. et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin. Infect. Dis. 58, 883–890 (2014).
Zerbato, J. M., Purves, H. V., Lewin, S. R. & Rasmussen, T. A. Between a shock and a hard place: challenges and developments in HIV latency reversal. Curr. Opin. Virol. 38, 1–9 (2019).
McBrien, J. B. et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8+ cells. Nature 578, 154–159 (2020).
Mutascio, S. et al. CD8+ T cells promote HIV latency by remodeling CD4+ T cell metabolism to enhance their survival, quiescence, and stemness. Immunity 56, 1132–1147 (2023).
Zanoni, M. et al. Innate, non-cytolytic CD8+ T cell-mediated suppression of HIV replication by MHC-independent inhibition of virus transcription. PLoS Pathog. 16, e1008821 (2020).
Wallace, J., Narasipura, S. D., Sha, B. E., French, A. L. & Al-Harthi, L. Canonical Wnts mediate CD8+ T cell noncytolytic anti-HIV-1 activity and correlate with HIV-1 clinical status. J. Immunol. 205, 2046–2055 (2020).
Crotty, S. Do memory CD4 T cells keep their cell-type programming: plasticity versus fate commitment? Complexities of interpretation due to the heterogeneity of memory CD4 T cells, including T follicular helper cells. Cold Spring Harb. Perspect. Biol. 10, a032102 (2018).
Grydziuszko, E., Phelps, A., Bruton, K., Jordana, M. & Koenig, J. F. E. Heterogeneity, subsets, and plasticity of T follicular helper cells in allergy. J. Allergy Clin. Immunol. 150, 990–998 (2022).
Zhu, J. & Paul, W. E. Heterogeneity and plasticity of T helper cells. Cell Res. 20, 4–12 (2010).
Pinzone, M. R. et al. Naive infection predicts reservoir diversity and is a formidable hurdle to HIV eradication. JCI Insight 6, e150794 (2021).
Dalod, M. et al. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein–Barr virus responses and changes during antiretroviral therapy. J. Virol. 73, 7108–7116 (1999).
Gea-Banacloche, J. C. et al. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165, 1082–1092 (2000).
Betts, M. R. et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75, 11983–11991 (2001).
Batorsky, R., Sergeev, R. A. & Rouzine, I. M. The route of HIV escape from immune response targeting multiple sites is determined by the cost-benefit tradeoff of escape mutations. PLoS Comput. Biol. 10, e1003878 (2014).
Althaus, C. L. & De Boer, R. J. Dynamics of immune escape during HIV/SIV infection. PLoS Comput. Biol. 4, e1000103 (2008).
Roberts, E. R. et al. Collapse of cytolytic potential in SIV-specific CD8+ T cells following acute SIV infection in rhesus macaques. PLoS Pathog. 12, e1006135 (2016).
Buggert, M. et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 10, e1004251 (2014).
Okoye, A. A. & Picker, L. J. CD4+ T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol. Rev. 254, 54–64 (2013).
Petersen, J. L., Morris, C. R. & Solheim, J. C. Virus evasion of MHC class I molecule presentation. J. Immunol. 171, 4473–4478 (2003).
Collins, K. L., Chen, B. K., Kalams, S. A., Walker, B. D. & Baltimore, D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391, 397–401 (1998).
Migueles, S. A. et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl Acad. Sci. USA 97, 2709–2714 (2000).
de Quiros, J. C. et al. Resistance to replication of human immunodeficiency virus challenge in SCID-Hu mice engrafted with peripheral blood mononuclear cells of nonprogressors is mediated by CD8+ T cells and associated with a proliferative response to p24 antigen. J. Virol. 74, 2023–2028 (2000).
Kaslow, R. A. et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2, 405–411 (1996).
Kaseke, C., Tano-Menka, R., Senjobe, F. & Gaiha, G. D. The emerging role for CTL epitope specificity in HIV cure efforts. J. Infect. Dis. 223, 32–37 (2021).
Gaiha, G. D. et al. Structural topology defines protective CD8+ T cell epitopes in the HIV proteome. Science 364, 480–484 (2019).
Martin, M. P. et al. Killer cell immunoglobulin-like receptor 3DL1 variation modifies HLA-B*57 protection against HIV-1. J. Clin. Invest. 128, 1903–1912 (2018).
Pawlak, E. N. & Dikeakos, J. D. HIV-1 Nef: a master manipulator of the membrane trafficking machinery mediating immune evasion. Biochim. Biophys. Acta 1850, 733–741 (2015).
Dikeakos, J. D. et al. An interdomain binding site on HIV-1 Nef interacts with PACS-1 and PACS-2 on endosomes to down-regulate MHC-I. Mol. Biol. Cell 23, 2184–2197 (2012).
Blagoveshchenskaya, A. D., Thomas, L., Feliciangeli, S. F., Hung, C. H. & Thomas, G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111, 853–866 (2002).
Schaefer, M. R., Wonderlich, E. R., Roeth, J. F., Leonard, J. A. & Collins, K. L. HIV-1 Nef targets MHC-I and CD4 for degradation via a final common β-COP-dependent pathway in T cells. PLoS Pathog. 4, e1000131 (2008).
Apps, R. et al. Influence of HLA-C expression level on HIV control. Science 340, 87–91 (2013).
Blais, M. E. et al. High frequency of HIV mutations associated with HLA-C suggests enhanced HLA-C-restricted CTL selective pressure associated with an AIDS-protective polymorphism. J. Immunol. 188, 4663–4670 (2012).
Macatangay, B. J. & Rinaldo, C. R. Preserving HIV-specific T cell responses: does timing of antiretroviral therapy help? Curr. Opin. HIV AIDS 10, 55–60 (2015).
Deng, K. et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517, 381–385 (2015).
Warren, J. A. et al. The HIV-1 latent reservoir is largely sensitive to circulating T cells. eLife 9, e57246 (2020).
Statzu, M. et al. CD8+ lymphocytes do not impact SIV reservoir establishment under ART. Nat. Microbiol. 8, 299–308 (2023).
Huang, S. H. et al. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J. Clin. Invest. 128, 876–889 (2018).
Abrahams, M. R. et al. The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Sci. Transl. Med. 11, eaaw5589 (2019).
Rutishauser, R. L. et al. TCF-1 regulates HIV-specific CD8+ T cell expansion capacity. JCI Insight 6, e136648 (2021).
Strongin, Z. et al. Distinct SIV-specific CD8+ T cells in the lymph node exhibit simultaneous effector and stem-like profiles and are associated with limited SIV persistence. Nat. Immunol. 25, 1245–1256 (2024).
Armani-Tourret, M. et al. Immune targeting of HIV-1 reservoir cells: a path to elimination strategies and cure. Nat. Rev. Microbiol. 22, 328–344 (2024).
Wu, V. H. et al. Profound phenotypic and epigenetic heterogeneity of the HIV-1-infected CD4+ T cell reservoir. Nat. Immunol. 24, 359–370 (2023).
Einkauf, K. B. et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J. Clin. Invest. 129, 988–998 (2019).
Patro, S. C. et al. Combined HIV-1 sequence and integration site analysis informs viral dynamics and allows reconstruction of replicating viral ancestors. Proc. Natl Acad. Sci. USA 116, 25891–25899 (2019).
Armani-Tourret, M. et al. Selection of epigenetically privileged HIV-1 proviruses during treatment with panobinostat and interferon-α2a. Cell 187, 1238–1254 (2024).
Blazkova, J. et al. Immunologic and virologic parameters associated with human immunodeficiency virus (HIV) DNA reservoir size in people with HIV receiving antiretroviral therapy. J. Infect. Dis. 229, 1770–1780 (2024).
Sun, W. et al. Footprints of innate immune activity during HIV-1 reservoir cell evolution in early-treated infection. J. Exp. Med. 221, e20241091 (2024).
Khoury, G., Kulpa, D. A. & Parsons, M. S. Potential utility of natural killer cells for eliminating cells harboring reactivated latent HIV-1 following the removal of CD8+ T cell-mediated pro-latency effect(s). Viruses 13, 1451 (2021).
Veenhuis, R. T. et al. Monocyte-derived macrophages contain persistent latent HIV reservoirs. Nat. Microbiol. 8, 833–844 (2023).
Kruize, Z. & Kootstra, N. A. The role of macrophages in HIV-1 persistence and pathogenesis. Front. Microbiol. 10, 2828 (2019).
Groot, F., Welsch, S. & Sattentau, Q. J. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 111, 4660–4663 (2008).
Banga, R. et al. Lymph node dendritic cells harbor inducible replication-competent HIV despite years of suppressive ART. Cell Host Microbe 31, 1714–1731 (2023).
Abrahem, R. et al. The role of dendritic cells in TB and HIV Infection. J. Clin. Med. 9, 2661 (2020).
Miller, C. J. Host and viral factors influencing heterosexual HIV transmission. Rev. Reprod. 3, 42–51 (1998).
Manches, O., Frleta, D. & Bhardwaj, N. Dendritic cells in progression and pathology of HIV infection. Trends Immunol. 35, 114–122 (2014).
Turville, S. G. et al. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3, 975–983 (2002).
Wu, L. & KewalRamani, V. N. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6, 859–868 (2006).
Mikulak, J., Oriolo, F., Zaghi, E., Di Vito, C. & Mavilio, D. Natural killer cells in HIV-1 infection and therapy. AIDS 31, 2317–2330 (2017).
Bastidas-Legarda, L. Y. & Khakoo, S. I. Conserved and variable natural killer cell receptors: diverse approaches to viral infections. Immunology 156, 319–328 (2019).
Yewdell, J. W. & Hill, A. B. Viral interference with antigen presentation. Nat. Immunol. 3, 1019–1025 (2002).
Forthal, D. N. & Finzi, A. Antibody-dependent cellular cytotoxicity in HIV infection. AIDS 32, 2439–2451 (2018).
Ferrari, G., Pollara, J., Tomaras, G. D. & Haynes, B. F. Humoral and innate antiviral immunity as tools to clear persistent HIV infection. J. Infect. Dis. 215, S152–S159 (2017).
Manickam, C., Shah, S. V., Nohara, J., Ferrari, G. & Reeves, R. K. Monkeying around: using non-human primate models to study NK cell biology in HIV infections. Front. Immunol. 10, 1124 (2019).
Vieira, V. A., Herbert, N., Cromhout, G., Adland, E. & Goulder, P. Role of early life cytotoxic T lymphocyte and natural killer cell immunity in paediatric HIV cure/remission in the anti-retroviral therapy era. Front. Immunol. 13, 886562 (2022).
Hartana, C. A. et al. Immune correlates of HIV-1 reservoir cell decline in early-treated infants. Cell Rep. 40, 111126 (2022).
Garcia-Broncano, P. et al. Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Sci. Transl. Med. 11, eaax7350 (2019).
Harper, J. et al. IL-21 and IFNα therapy rescues terminally differentiated NK cells and limits SIV reservoir in ART-treated macaques. Nat. Commun. 12, 2866 (2021).
Nagasawa, M., Spits, H. & Ros, X. R. Innate lymphoid cells (ILCs): cytokine hubs regulating immunity and tissue homeostasis. Cold Spring Harb. Perspect. Biol. 10, a030304 (2018).
Moretti, S. et al. HIV-1-host interaction in gut-associated lymphoid tissue (GALT): effects on local environment and comorbidities. Int. J. Mol. Sci. 24, 12193 (2023).
Kloverpris, H. N. et al. Innate lymphoid cells are depleted irreversibly during acute HIV-1 infection in the absence of viral suppression. Immunity 44, 391–405 (2016).
Hutter, G. et al. Long-term control of HIV by CCR5 Δ32/Δ32 stem-cell transplantation. N. Engl. J. Med. 360, 692–698 (2009).
Yukl, S. A. et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 9, e1003347 (2013).
Gupta, R. K. et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568, 244–248 (2019).
Gupta, R. K. et al. Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV 7, e340–e347 (2020).
Hsu, J. et al. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186, 1115–1126 (2023).
Jensen, B. O. et al. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 29, 583–587 (2023).
Hussein, M., Molina, M. A., Berkhout, B. & Herrera-Carrillo, E. A CRISPR-cas cure for HIV/AIDS. Int. J. Mol. Sci. 24, 1563 (2023).
Pauza, C. D., Huang, K. & Bordon, J. Advances in cell and gene therapy for HIV disease: it is good to be specific. Curr. Opin. HIV AIDS 16, 83–87 (2021).
Kitawi, R., Ledger, S., Kelleher, A. D. & Ahlenstiel, C. L. Advances in HIV gene therapy. Int. J. Mol. Sci. 25, 2771 (2024).
Swanstrom, A. E. et al. Antibody-mediated depletion of viral reservoirs is limited in SIV-infected macaques treated early with antiretroviral therapy. J. Clin. Invest. 131, e142421 (2021).
Veenhuis, R. T. et al. CD4+ depletion results in robust CNS rebound and larger macrophage reservoirs [CROI Abstract 129]. Abstracts from CROI 2022 Conference on Retroviruses and Opportunistic Infections (2022).
Clark, I. C. et al. HIV silencing and cell survival signatures in infected T cell reservoirs. Nature 614, 318–325 (2023).
Sun, W. et al. Phenotypic signatures of immune selection in HIV-1 reservoir cells. Nature 614, 309–317 (2023).
Harper, J. et al. CTLA-4 and PD-1 dual blockade induces SIV reactivation without control of rebound after antiretroviral therapy interruption. Nat. Med. 26, 519–528 (2020).
Rahman, S. A. et al. PD-1 blockade and vaccination provide therapeutic benefit against SIV by inducing broad and functional CD8+ T cells in lymphoid tissue. Sci. Immunol. 6, eabh3034 (2021).
Dashti, A. et al. AZD5582 plus SIV-specific antibodies reduce lymph node viral reservoirs in antiretroviral therapy-suppressed macaques. Nat. Med. 29, 2535–2546 (2023).
Lim, S. Y. et al. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci. Transl Med. 10, eaao4521 (2018).
Bekerman, E. et al. PD-1 blockade and TLR7 activation lack therapeutic benefit in chronic simian immunodeficiency virus-infected macaques on antiretroviral therapy. Antimicrob. Agents Chemother. 63, e01163–19 (2019).
Del Prete, G. Q. et al. TLR7 agonist administration to SIV-infected macaques receiving early initiated cART does not induce plasma viremia. JCI Insight 4, e127717 (2019).
Miller, J. S. et al. Safety and virologic impact of the IL-15 superagonist N-803 in people living with HIV: a phase 1 trial. Nat. Med. 28, 392–400 (2022).
Ho, Y. C. et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551 (2013).
Siliciano, J. D. & Siliciano, R. F. Low inducibility of latent human immunodeficiency virus type 1 proviruses as a major barrier to cure. J. Infect. Dis. 223, 13–21 (2021).
Henrich, T. J. et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann. Intern. Med. 161, 319–327 (2014).
Frattari, G. S., Caskey, M. & Sogaard, O. S. Broadly neutralizing antibodies for HIV treatment and cure approaches. Curr. Opin. HIV AIDS 18, 157–163 (2023).
Caskey, M., Klein, F. & Nussenzweig, M. C. Broadly neutralizing antibodies for HIV-1 prevention or immunotherapy. N. Engl. J. Med. 375, 2019–2021 (2016).
Gaebler, C. et al. Prolonged viral suppression with anti-HIV-1 antibody therapy. Nature 606, 368–374 (2022).
Sneller, M. C. et al. Combination anti-HIV antibodies provide sustained virological suppression. Nature 606, 375–381 (2022).
Corey, L. et al. Two randomized trials of neutralizing antibodies to prevent HIV-1 acquisition. N. Engl. J. Med. 384, 1003–1014 (2021).
Gilbert, P. B. et al. Neutralization titer biomarker for antibody-mediated prevention of HIV-1 acquisition. Nat. Med. 28, 1924–1932 (2022).
Pegu, A. et al. A meta-analysis of passive immunization studies shows that serum-neutralizing antibody titer associates with protection against SHIV challenge. Cell Host Microbe 26, 336–346 (2019).
Niessl, J. et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat. Med. 26, 222–227 (2020).
Nishimura, Y. et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543, 559–563 (2017).
Gunst, J. D. et al. Early intervention with 3BNC117 and romidepsin at antiretroviral treatment initiation in people with HIV-1: a phase 1b/2a, randomized trial. Nat. Med. 28, 2424–2435 (2022).
Rosas-Umbert, M. et al. Administration of broadly neutralizing anti-HIV-1 antibodies at ART initiation maintains long-term CD8+ T cell immunity. Nat. Commun. 13, 6473 (2022).
Reece, J. et al. An “escape clock” for estimating the turnover of SIV DNA in resting CD4+ T cells. PLoS Pathog. 8, e1002615 (2012).
Barouch, D. H. et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503, 224–228 (2013).
Borducchi, E. N. et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364 (2018).
Shingai, M. et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J. Exp. Med. 211, 2061–2074 (2014).
Julg, B. et al. Safety and antiviral effect of a triple combination of HIV-1 broadly neutralizing antibodies: a phase 1/2a trial. Nat. Med. 30, 3534–3543 (2024).
Bar, K. J. et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N. Engl. J. Med. 375, 2037–2050 (2016).
Cohen, Y. Z. et al. Relationship between latent and rebound viruses in a clinical trial of anti-HIV-1 antibody 3BNC117. J. Exp. Med. 215, 2311–2324 (2018).
Riddler, S. A. et al. Randomized clinical trial to assess the impact of the broadly neutralizing HIV-1 monoclonal antibody VRC01 on HIV-1 persistence in individuals on effective ART. Open Forum Infect. Dis. 5, ofy242 (2018).
Wagh, K. & Seaman, M. S. Divide and conquer: broadly neutralizing antibody combinations for improved HIV-1 viral coverage. Curr. Opin. HIV AIDS 18, 164–170 (2023).
Julg, B. et al. Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial. Nat. Med. 28, 1288–1296 (2022).
Mendoza, P. et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484 (2018).
Parikh, U. M., Bacheler, L., Koontz, D. & Mellors, J. W. The K65R mutation in human immunodeficiency virus type 1 reverse transcriptase exhibits bidirectional phenotypic antagonism with thymidine analog mutations. J. Virol. 80, 4971–4977 (2006).
Rajashekar, J. K. et al. Modulating HIV-1 envelope glycoprotein conformation to decrease the HIV-1 reservoir. Cell Host Microbe 29, 904–916 (2021).
Sekaly, R. et al. Dual blockade of IL-10 and PD-1 leads to control of SIV viral rebound following analytical treatment interruption. Nat. Immunol. 25, 1900–1912 (2023).
Lim, S. Y. et al. Induction of durable remission by dual immunotherapy in SHIV-infected ART-suppressed macaques. Science 383, 1104–1111 (2024).
Chandrasekar, A. P. et al. The BCL-2 inhibitor venetoclax augments immune effector function mediated by fas ligand, TRAIL, and perforin/granzyme B, resulting in reduced plasma viremia and decreased HIV reservoir size during acute HIV infection in a humanized mouse model. J. Virol. 96, e0173022 (2022).
Cummins, N. W. et al. Prime, shock, and kill: priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J. Virol. 90, 4032–4048 (2016).
Szaniawski, M. A. & Spivak, A. M. Senotherapeutics for HIV and aging. Curr. Opin. HIV AIDS 15, 83–93 (2020).
Sanchez-Diaz, L., Espinosa-Sanchez, A., Blanco, J. R. & Carnero, A. Senotherapeutics in cancer and HIV. Cells 11, 1222 (2022).
Blackbourn, D. J. et al. Suppression of HIV replication by lymphoid tissue CD8+ cells correlates with the clinical state of HIV-infected individuals. Proc. Natl Acad. Sci. USA 93, 13125–13130 (1996).
Blazek, D., Teque, F., Mackewicz, C., Peterlin, M. & Levy, J. A. The CD8+ cell non-cytotoxic antiviral response affects RNA polymerase II-mediated human immunodeficiency virus transcription in infected CD4+ cells. J. Gen. Virol. 97, 220–224 (2016).
Levy, J. A., Mackewicz, C. E. & Barker, E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol. Today 17, 217–224 (1996).
Mackewicz, C. & Levy, J. A. CD8+ cell anti-HIV activity: nonlytic suppression of virus replication. AIDS Res. Hum. Retroviruses 8, 1039–1050 (1992).
Mackewicz, C. E., Blackbourn, D. J. & Levy, J. A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc. Natl Acad. Sci. USA 92, 2308–2312 (1995).
Mackewicz, C. E., Craik, C. S. & Levy, J. A. The CD8+ cell noncytotoxic anti-HIV response can be blocked by protease inhibitors. Proc. Natl Acad. Sci. USA 100, 3433–3438 (2003).
Mackewicz, C. E., Garovoy, M. R. & Levy, J. A. HLA compatibility requirements for CD8+-T-cell-mediated suppression of human immunodeficiency virus replication. J. Virol. 72, 10165–10170 (1998).
Mackewicz, C. E., Ortega, H. & Levy, J. A. Effect of cytokines on HIV replication in CD4+ lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 153, 329–343 (1994).
Mackewicz, C. E., Patterson, B. K., Lee, S. A. & Levy, J. A. CD8+ cell noncytotoxic anti-human immunodeficiency virus response inhibits expression of viral RNA but not reverse transcription or provirus integration. J. Gen. Virol. 81, 1261–1264 (2000).
Mackewicz, C. E. et al. Lack of the CD8+ cell anti-HIV factor in CD8+ cell granules. Blood 102, 180–183 (2003).
Yang, O. O. et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71, 3120–3128 (1997).
Klatt, N. R. et al. CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog. 6, e1000747 (2010).
Wong, J. K. et al. In vivo CD8+ T-cell suppression of siv viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog. 6, e1000748 (2010).
Elemans, M. et al. Why don’t CD8+ T cells reduce the lifespan of SIV-infected cells in vivo? PLoS Comput. Biol. 7, e1002200 (2011).
Seich Al Basatena, N. K. et al. Can non-lytic CD8+ T cells drive HIV-1 escape? PLoS Pathog. 9, e1003656 (2013).
Cartwright, E. K. et al. CD8+ lymphocytes are required for maintaining viral suppression in SIV-infected macaques treated with short-term antiretroviral therapy. Immunity 45, 656–668 (2016).
Mavigner, M. et al. CD8 lymphocyte depletion enhances the latency reversal activity of the SMAC mimetic AZD5582 in ART-suppressed SIV-infected rhesus macaques. J. Virol. 95, e01429-20 (2021).
Acknowledgements
This work was supported by NIH grants R01-AI125064 to G.S., R01-AI143414 to D.A.K. and UM1 164562 to M.P. The authors thank A. Chahroudi, D. Margolis, R. Sekaly, F. Simonetti and C. Van Lint for helpful discussions.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kulpa, D.A., Paiardini, M. & Silvestri, G. Immune-mediated strategies to solving the HIV reservoir problem. Nat Rev Immunol 25, 542–553 (2025). https://doi.org/10.1038/s41577-025-01136-7
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-025-01136-7
This article is cited by
-
Mathematical modeling and mechanisms of HIV latency for personalized anti latency therapies
npj Systems Biology and Applications (2025)
-
A new era in CAR-NK cell therapy: from technological innovations to clinical applications
World Journal of Pediatrics (2025)